Abstract
Background
Patients with Chronic Granulomatous Disease (CGD) suffer immunodeficiency due to defects in the phagocyte NADPH oxidase (NOX2) and concomitant reduction in reactive oxygen intermediates. This may result in a reduction in atherosclerotic injury.
Methods and Results
We prospectively assessed the prevalence of cardiovascular risk factors, biomarkers of inflammation and neutrophil activation, and the presence of MRI and CT quantified subclinical atherosclerosis in the carotid and coronary arteries of 41 CGD patients and 25 healthy controls in the same age range. Uni- and multivariable associations between risk factors, inflammatory markers and atherosclerosis burden were assessed. CGD patients had significant elevations in traditional risk factors and inflammatory markers compared with controls, including; hypertension, hsCRP, oxidized LDL, and low HDL. Despite this, CGD patients had a 22% lower internal carotid artery wall volume compared with controls (361.3 ± 76.4 mm3 vs. 463.5 ± 104.7 mm3, p<0.001). This difference was comparable in p47phox and gp91phox deficient subtypes of CGD, and independent of risk factors in multivariate regression analysis. In contrast, prevalence of coronary arterial calcification was similar between CGD patients and controls (14.6%, CGD, and 6.3%, controls, p=0.39).
Conclusions
The observation by MRI of reduced carotid but not coronary artery atherosclerosis in CGD patients despite the high prevalence of traditional risk factors raises questions about the role of NOX2 in the pathogenesis of clinically significant atherosclerosis. Additional high-resolution studies in multiple vascular beds are required to address the therapeutic potential of NOX-inhibition in cardiovascular diseases.
Clinical Trial Registration Information
clinicaltrials.gov. Identifier: NCT01063309.
Keywords: atherosclerosis, inflammation, carotid arteries, coronary arteries, immune system
INTRODUCTION
Chronic Granulomatous Disease (CGD) is an inherited immunodeficiency caused by mutations in genes encoding the main components of the phagocyte NADPH oxidase (NOX2), gp91phox, p22phox, p47phox, p67phox, and rarely p40phox, resulting in impaired production of superoxide anion and other reactive oxygen intermediates.1 Hemizygous mutations in gp91phox cause the most common form, X-linked CGD (X-CGD), while autosomal CGD (A-CGD) is due to mutations in the other subunits. 2 CGD manifests clinically with recurrent infections and granulomatous complications. 3 Lower levels of residual ROS production by neutrophils are associated with earlier mortality. 2 Significantly elevated production of pro-inflammatory mediators by CGD myeloid cells (e.g., IL-8 4, LTB4 5), and decreased neutrophil apoptosis 6 are also thought to contribute to the excessive inflammation secondary or independent of infection that is often seen in CGD.
Beyond a role in immune defense, increased inflammation with associated increased reactive oxygen species generated by a family of NOX proteins, NOX2, NOX1 and NOX4, have been implicated in the pathogenesis of cardiovascular disease and atherosclerosis. 7 NADPH oxidases contribute to the differentiation and migration of vascular smooth muscle cells, endothelial cell response to nitric oxide, and are highly expressed in atherosclerotic plaque.8–12 Reduced NADPH oxidase activity may reduce vascular inflammation and thereby decrease susceptibility to atherosclerosis – a possibility that makes pharmacologic inhibition of NOX a potential target of therapy for cardiovascular diseases. 13
Mouse models of NOX deficiency have yielded conflicting results on atherosclerosis progression. 14–18 Pharmacologic inhibition of NOX in murine models has succeeded in reducing atherosclerosis progression. 19 Studies in gp91phox and p47phox - deficient human CGD subjects demonstrated significant differences in cardiovascular function. Enhanced arterial dilatation and vascular endothelial function following ischemia and reperfusion have been noted in CGD. 20, 21 Enhanced arterial dilatation was noted in male CGD patients and lower carotid intimal-medial thickness in X-CGD patients and female carriers compared to healthy subjects20,22, suggesting that even a 50% reduction in NOX2 function is sufficient for cardiovascular protective effects. To date, no studies have reported the effects of NOX2 deficiency in the coronary circulation. We investigated markers of inflammation and the prevalence of subclinical carotid and coronary atherosclerosis using noninvasive MRI and CT techniques in normal controls and patients with gp91phox or p47phox CGD.
METHODS
Patients
Patients over 18 years of age with a clinical diagnosis of CGD and either gp91phox or p47phox deficiency and healthy volunteers in the same age range were enrolled from 2010–2014 in an IRB approved protocol (10-I-0029) conducted at the NIH Clinical Center. All subjects provided documented informed consent. CGD diagnoses were confirmed by genetic sequencing and/or western blotting as well as quantitation of reactive oxygen species.2 Volunteers underwent history and physical examination to confirm that they were free of clinical cardiovascular disease or active systemic infection. Patients with fever, atrial fibrillation or contraindication to gadolinium or MR imaging were excluded. Patients with contraindication to iodinated contrast were eligible to undergo MR and non-contrast CT (calcium scoring). No CGD patients in this study had received a bone marrow transplant.
Acquisition and Analysis of Carotid MR Imaging
Carotid wall volume was determined to assess the extent of atherosclerotic disease.23 MR imaging was performed on a 3 T clinical scanner (Verio, Siemens) using four-channel carotid coils (Machnet). T1 pre & post contrast and T2 weighted fat-suppressed, ECG-gated black blood images were obtained using double inversion recovery fast spin echo sequences. Post-contrast T1 weighted images were obtained 5 minutes after an intravenous dose of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist, Bayer HealthCare). Scan resolution was 0.50 mm × 0.50 mm × 2.0 mm, with 5 consecutive slices and no gap obtained in each internal carotid artery (ICA) beginning at the carotid bifurcation.
ICA wall volume was quantified using QPlaque (version 1.0, Medis) by a blinded observer. The area within the external boundary of the vessel and the arterial lumen were semi-automatically contoured on post-contrast T1 images, using pre-contrast T1 and T2 weighted images to confirm vessel boundaries in slices with flow artifact. 24, 25 The corresponding volume was obtained by multiplying the area in each image by the slice thickness and summing the total number of slices obtained. Wall volume was calculated by subtracting lumen volume from total vessel volume.
Acquisition and Analysis of Cardiac CT Angiography
Coronary artery wall volume and calcium score were determined using CT angiography as measures of atherosclerotic disease.26 Pre- and post-contrast CT imaging was performed using a 320-detector row scanner with slice thickness 0.5 mm (Aquilion ONE, Toshiba Medical Systems). Calcium scoring was performed with prospective ECG gating with 350 msec gantry rotation, 120 kV tube voltage and 300 mA tube current and quantified by the Agatston method.27 CT angiography was performed after administration of intravenous iopamidol (Isovue 370, Bracco Diagnostics) using 120 kV tube voltage and tube current 350–580 mA dependent on BMI. Beta-blockers were administered if the resting heart rate was >60 beats per minute. Image analysis was performed on a Vitrea FX workstation (Version 6.1, Vital Images). The left main coronary artery and proximal coronary artery segments were segmented according to previously published definitions.28 The lumen and external vessel wall were semi-automatically contoured at 0.5 mm intervals. The area between the lumen and external wall was multiplied by slice thickness and summed over the total number of slices per segment to calculate coronary arterial wall volume (mm3). Coronary artery wall volume was indexed to segment length to account for variability in coronary anatomy. The resulting value is reported as the coronary plaque index (mm2). Inter-reader reproducibility for this method in a sub-sample of 10 consecutive participants was excellent (ICC=0.97).
Plasma Analytes
Glucose, lipids, and biomarker measurements were obtained after ≥ 12 hour fasting. Biomarker analysis was on plasma prepared from heparinized blood by 2 centrifugation steps at 500 g for 10 min. Plasma aliquots were stored in the vapor phase of liquid N2 freezer prior to analysis. IL-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12p70, IFN-γ, and TNF-α were measured with the TH1/TH2 Ultrasensitive 10-Plex (Meso Scale Discovery, Gaithersburg, MD) while IL-6, IL-17, GM-CSF, MIP-1α MIP-1β, RANTES, soluble TNF-RI, soluble TNF-RII, and soluble IL-6R were measured with customized multiplex cytokine immunoassays (Meso Scale Discovery) using a SECTOR™ Imager 6000 reader (Meso Scale Discovery). Standard curves were analyzed using nonlinear 4-parameter curve fitting and unknowns were calculated based on the best fit equation. For multiplex cytokine assays, an internal control was run on each plate to monitor inter-assay variability (mean CV% was 50.4%; range 16.3 – 111.4%). An aliquot of standard was spiked into a control plasma sample to determine the recovery of the specific cytokine standards in the sample matrix (mean recovery was 95.7%; range 78.2 – 119.8%). Commercial immunoassays were used for: oxidized LDL (Mercodia, Uppsala, Sweden), matrix metallopeptidase 9 (R&D Biosystems, Minneapolis, MN), lactoferrin (Oxis International, Portland, OR), α-defensins (Hycult Biotech, Plymouth Meeting, PA), and myeloperoxidase (Meso Scale Discovery). Plasma levels of total nitrate/nitrite (NOx−) and nitrite (NO2−) were determined using a Total Nitric Oxide and Nitrate/Nitrite kit (KGE001, R&D Systems, Minneapolis, MN).
Statistical Analysis
Continuous outcomes were summarized using means and standard deviations (SDs) or medians and interquartile ranges (IQRs) as appropriate, while counts and percentage values were reported for binary outcomes. Continuous outcomes of different subgroups were compared using t-tests with unequal variances. Binary outcomes were compared by chi-squared test. Spearman’s correlation coefficient was used to summarize the correlation between two continuous outcomes. Univariable and multivariable linear regression models were used to evaluate the unadjusted and adjusted effects of potential risk factors on carotid wall volume and coronary plaque burden. Two multivariable linear regression models were constructed: a base model adjusted for age and gender, and a second model including traditional clinical cardiovascular risk factors. Coefficients of determination R2 were reported to summarize the predictive power of a regression model. Carotid wall volume measures were logarithmically transformed (on a common log scale) before analysis to reduce skewness. Data analyses were carried out using STATA 10.1 (College Station, TX). All p values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of this study. Graphpad Prism version 6.0d was used for ANOVA with a Holm-Šídák post-test 29. P values less than 0.05 were considered statistically significant.
RESULTS
Patients
Twenty-two CGD patients with mutations in gp91phox, 19 patients with mutations in p47phox and 25 age- matched healthy controls were enrolled. Demographic and clinical characteristics of the study population are shown in Table 1. CGD patients were 32.5 ± 9.4 years old and 76% were male. CGD patients had significantly elevated cardiovascular risk factors compared with controls, including more prevalent hypertension, decreased HDL-c, as well as increased oxidized LDL. Amongst CGD patients, those with p47phox mutations had significantly greater residual superoxide production than those with gp91phox mutations (3.0 ± 0.7 vs. 1.7 ± 1.8 nM superoxide/1 × 106 neutrophils/60 min respectively, p<0.01), about 1.3 % and 0.7% of superoxide produced by neutrophils from normal volunteers (226.29 ± 3.01 nM superoxide/1 × 106 neutrophils/60 min).2
Table 1.
Clinical and biomarker characteristics of study population
| CGD (n=41) | Control (n=25) | |
|---|---|---|
| Clinical Characteristics | ||
| Age | 32.5±9.3 | 32.1±7.1 |
| Male, N (%)* | 31 (75.6%) | 12 (48.0%) |
| Hypertension* | 12 (29.3%) | 1(4.0%) |
| Diabetes | 3 (7.3%) | 0 (0%) |
| Smoking | 14 (34.2%) | 7 (28.0%) |
| Family CVD history* | 22 (56.4%) | 6 (24.0%) |
| Lipid Levels | ||
| Total Cholesterol (mg/dL) | 153.2±35.0 | 162.4±28.1 |
| LDL (mg/dL) | 92.7±33.9 | 84.7±21.7 |
| HDL (mg/dL) *** | 38.1±10.9 | 60.2±14.7 |
| Triglycerides (mg/dL) | 110.6±68.2 | 87.8±50.9 |
| Oxidized LDL (mg/dL) *** | 65.0±37.2 | 23.4±18.6 |
| Other Biomarkers | ||
| hsCRP (mg/L) *** | 8.9±10.1 | 2.4±3.8 |
| Nitrite (NO2−, μM) | 2.6±1.5 | 2.8±1.0 |
| Nitrate (NO3−, μM) | 24.7±16.8 | 22.8±17.6 |
| Neutrophil proteins | ||
| Lactoferrin (ng/ml) *** | 722.6±832.0 | 225.0±98.3 |
| MPO (ng/ml) ** | 473.0±810.7 | 118.4±76.3 |
| MMP-9 (ng/ml) *** | 375.7±420.4 | 134.5±63.9 |
| α-Defensins (ng/ml)* | 56.3±137.1 | 8.1±5.3 |
| Cytokines | ||
| IFN-γ (pg/ml) | 5.5±16.1 | 1.8±6.0 |
| TNF-α(pg/ml)** | 10.7±5.7 | 4.7±7.4 |
| GM-CSF (pg/ml)* | 3.1±6.8 | 0.7±0.7 |
| IL-1β(pg/ml) | 1.2±4.4 | 0.4±0.5 |
| IL-2 (pg/ml) | 0.8±0.8 | 1.2±2.5 |
| IL-4 (pg/ml) | 0.2±0.5 | 0.3±0.6 |
| IL-5 (pg/ml) | 1.8±6.1 | 2.3±7.1 |
| IL-6 (pg/ml)** | 10.3±11.0 | 4.4±5.5 |
| IL-10 (pg/ml) | 3.9±8.0 | 10.3±37.4 |
| IL-12p70 (pg/ml) | 10.0±56.7 | 31.9±146.9 |
| IL-13 (pg/ml) | 2.2±4.4 | 45.5±184.5 |
| IL-17 (pg/ml) | 12.6±19.1 | 7.6±7.8 |
| Chemokines | ||
| CCL3 (MIP-1α, pg/ml) | 38.8±42.2 | 27.7±13.7 |
| CCL4 (MIP-1β, pg/ml)** | 178.7±226.5 | 60.2±39.0 |
| CCL5 (RANTES, ng/ml) | 25.9±19.2 | 25.9±13. 3 |
| CXCL8 (IL-8, pg/ml)* | 19.6±37.6 | 5.5±6.6 |
| Soluble Selectins | ||
| sL-selectin (ng/ml) | 1,163.9±245.6 | 1,152.5±187.5 |
| sE-selectin (ng/ml)* | 39.8 ± 17.4 | 31.3±13.8 |
| Soluble Receptors | ||
| TNFR1 (pg/ml) | 3,376.0±2434.9 | 2,914.8±1,256.7 |
| TNFR2 (pg/ml) *** | 3,986.5±2,170.6 | 2,389.4±652.2 |
| IL-6R (pg/ml) | 18,223.8±4,780.8 | 18,695.1±5,162.2 |
Means ± SD.
denotes P ≤ 0.05,
denotes P ≤ 0.01, and
denotes P ≤ 0.001 for difference between CGD and control patients respectively.
Abbreviations: CGD, chronic granulomatous disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein; hsCRP, high sensitivity C-reactive protein; ESR, erythrocyte sedimentation rate; MPO, myeloperoxidase; IL, interleukin; TNF, tumor necrosis factor; GM-CSF, granulocyte macrophage colony stimulating factor; MIP, macrophage inflammatory protein; MMP, matrix metallopeptidase
Plasma markers of inflammation in CGD
CGD subjects had higher levels of classical acute phase reactants (hsCRP) and innate defense proteins (myeloperoxidase, lactoferrin, and α-defensins) suggesting increased neutrophil degranulation and systemic inflammation. Pro-inflammatory cytokines such as IL-6 and TNFα as well as the chemokines CXCL8 (IL-8) and CCL4 (MIP1β) were significantly elevated in CGD (Table 1).
Subclinical Atherosclerosis
Internal Carotid Artery
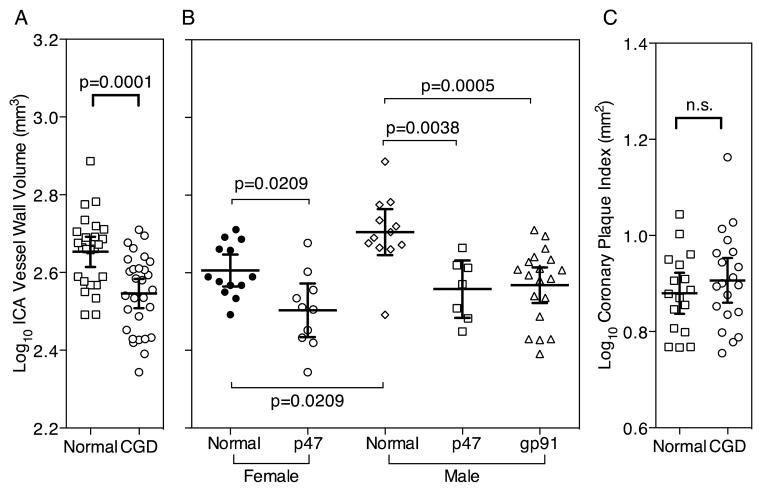
Patients with impaired NADPH oxidase function had 22% lower internal carotid arterial (ICA) wall volume than age-matched healthy individuals. The mean ICA wall volume in CGD patients was 361.3 ± 76.4 mm3, compared with 463.5 ± 104.7 mm3 in controls (p<0.001. To reduce skewness the data were log-transformed prior to the statistical analyses that follow. The mean ± standard deviation of the common log-transformed ICA wall volume in CGD patients was 2.55 ± 0.02 log10 mm3, compared with 2.66 ± 0.02 log10 mm3 in controls (p=0.0001, Table 2 & Figure 1A). No participant in either group had evidence of atherosclerotic plaque that resulted in significant carotid luminal stenosis.
Table 2.
Markers of subclinical atherosclerosis
| Control | CGD | p-value | |
|---|---|---|---|
| Internal carotid arterial wall volume (log10 mm3) | 2.66± 0.02 | 2.55 ± 0.02 | 0.0001 |
| Prevalent coronary arterial calcium (n, %) | 1 (6.3) | 6 (14.6) | 0.39 |
| Coronary plaque index (mm2) | 7.8 ± 1.5 | 8.3 ± 2.1 | 0.46 |
Figure 1.
Comparison of Subclinical Coronary and Carotid Atherosclerosis in Healthy Controls and CGD Patients. Bars denote mean and 95% CI of the mean and p-values are from t-tests (Panels A, C) and ANOVA with a Holm-Šídák post-test (Panel B).
There were significant univariate associations between ICA wall volume and CGD, HDL-c, IL-6, IL-10, IL-12p70 and IL-13. (Table 3) Only CGD (regression coefficient, −0.14; 95% CI, −0.21 – −0.08; p<0.001) male gender (regression coefficient, 0.06; 95% CI, 0.01 – 0.12; p=0.02) and hypertension (regression coefficient, 0.09; 95% CI, 0.02 – 0.15; p=0.01) remained as significant predictors of ICA wall volume after controlling for traditional cardiovascular risk factors in a multivariate linear regression model (R2 = 0.50).
Table 3.
Internal Carotid Arterial Wall Volume in Univariate and Multivariable Linear Regression Analyses
| Univariate Model | Multivariable base model (R2 = 0.38) | Multivariable model 2 (R2 = 0.50) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| coeff. | SE | p-value | coeff. | SE | p-value | coeff. | SE | p-value | |
|
|
|||||||||
| CGD | −0.11 | 0.03 | <0.001 | −0.13 | 0.02 | <0.001 | −0.14 | 0.03 | <0.001 |
| age | 0.001 | 0.002 | 0.56 | 0.002 | 0.001 | 0.23 | 0.001 | 0.001 | 0.70 |
| male gender | 0.05 | 0.03 | 0.09 | 0.08 | 0.02 | 0.002 | 0.06 | 0.03 | 0.02 |
| hypertension | 0.03 | 0.03 | 0.36 | 0.09 | 0.03 | 0.01 | |||
| smoking | −0.02 | 0.03 | 0.48 | −0.002 | 0.03 | 0.94 | |||
| family history | −0.01 | 0.03 | 0.81 | 0.04 | 0.03 | 0.09 | |||
| LDL | 0.08×10−3 | 0.48 ×10−3 | 0.87 | 0.05×10−3 | 0.4×10−3 | 0.90 | |||
| HDL | 2.0×10−3 | 0.8×10−3 | 0.02 | 1.0×10−3 | 1.0×10−3 | 0.31 | |||
| hsCRP | −0.0×10−3 | 1.5×10−3 | 0.55 | ||||||
| oxidized LDL | −0.6×10−3 | 0.4×10−3 | 0.11 | ||||||
| lactoferrin | −26×10−6 | 19×10−6 | 0.17 | ||||||
| MPO | −39×10−6 | 20×10−6 | 0.053 | ||||||
| MMP-9 | −32×10−6 | 39×10−6 | 0.40 | ||||||
| TNF-α | −0.2×10−6 | 0.1×10−6 | 0.08 | ||||||
| GM-CSF | 1.6×10−3 | 3.4×10−3 | 0.64 | ||||||
| IL-1β | −2.2×10−3 | 3.9×10−3 | 0.57 | ||||||
| IL-6 | 3.4×10−3 | 1.3×10−3 | 0.01 | ||||||
| IL-10 | 1.7×10−3 | 0.5×10−3 | 0.002 | ||||||
| IL-12p70 | 0.4×10−3 | 0.1×10−3 | 0.002 | ||||||
| IL-13 | 0.3×10−3 | 0.1×10−3 | 0.002 | ||||||
| IL-17 | 0.5×10−3 | 0.8×10−3 | 0.58 | ||||||
| CCL3 (MIP-1α) | −0.3×10−3 | 0.4×10−3 | 0.43 | ||||||
| CCL4 (MIP-1β) | 0.4×10−6 | 72×10−6 | 0.99 | ||||||
| CCL5 RANTES | −0.7×10−6 | 0.8×10−6 | 0.37 | ||||||
| CXCL8 (IL-8) | −0.3×10−3 | 0.4×10−3 | 0.50 | ||||||
| TNFR1 | 4×10−6 | 7×10−6 | 0.54 | ||||||
| TNFR2 | −8×10−6 | 8×10−6 | 0.29 | ||||||
| IL-6R | 2×10−6 | 3×10−6 | 0.43 | ||||||
Coeff is the estimated regression coefficient. SE is the variance of the estimated regression coefficient.
Coronary Plaque Index and Coronary Arterial Calcium (CAC)
Cardiac CT angiography and calcium scoring were performed in 21 CGD patients and 16 healthy volunteers. Analyzable results were obtained in 36 of 37 scans (97.3%). Four CGD patients and one volunteer had atherosclerotic plaque resulting in <50% luminal stenosis. One CGD patient and no volunteers had plaque resulting in >50% luminal stenosis. The coronary plaque index, including calcified and non-calcified plaque, was similar in CGD patients and controls (8.3 ± 2.1 and 7.8 ± 1.5 mm2 respectively, p=0.46).
Prevalent coronary calcification, defined as an Agatston score ≥ 1, was present in one healthy control and 6 CGD patients (6.3% and 14.6%, respectively, p=0.39). The median CAC in CGD patients with measurable calcification was 173 (IQR 9–366) and CAC for the control patient was 5.
Significant univariable associations with coronary plaque were present for age and hypertension but not CGD. (Table 4) A minimally adjusted multivariable linear regression model including CGD, age and gender found significant associations between age and gender – but not CGD – with coronary plaque. After correction for CGD and traditional cardiovascular risk factors in a multivariable linear regression model, only hypertension emerged as an independent predictor of coronary wall volume (coefficient of association=2.3; 95% CI, 0.5 – 4.1; p=0.02; overall r2 for model 0.52, p<0.01). ICA wall volume and coronary arterial wall volume were not significantly correlated (Spearman’s rho = 0.18, p=0.33).
Table 4.
Coronary Plaque Index in Univariable and Multivariable Linear Regression Analyses
| Univariate Model | Multivariate Model 1 (R2 = 0.28) | Multivariate Model 2 (R2 = 0.52) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| coeff. | SE | p-value | coeff. | SE | p-value | coeff. | SE | p-value | |
| CGD | 0.44 | 0.61 | 0.47 | 0.14 | 0.55 | 0.80 | −0.33 | 0.69 | 0.64 |
| age | 0.09 | 0.03 | 0.01 | 0.09 | 0.03 | 0.007 | 0.06 | 0.04 | 0.11 |
| male gender | 1.10 | 0.61 | 0.08 | 1.23 | 0.57 | 0.04 | 0.52 | 0.63 | 0.42 |
| hypertension | 3.2 | 0.69 | <0.001 | 2.29 | 0.89 | 0.02 | |||
| smoking | −0.15 | 0.68 | 0.83 | −0.21 | 0.59 | 0.72 | |||
| family history | −0.10 | 0.61 | 0.87 | −0.08 | 0.61 | 0.90 | |||
| LDL | −0.01 | 0.01 | 0.47 | −0.02 | 0.01 | 0.08 | |||
| HDL | −0.03 | 0.02 | 0.09 | −0.02 | 0.02 | 0.31 | |||
| hsCRP | 0.03 | 0.03 | 0.38 | ||||||
| oxidized LDL | 0.01 | 0.01 | 0.26 | ||||||
| MPO | −0.0006 | 0.0005 | 0.24 | ||||||
Coeff is the estimated regression coefficient. SE is the variance of the estimated regression coefficient.
Subgroup Analyses
Amongst patients with CGD, those with deficiency in gp91phox (X-linked CGD) were, as expected, entirely male while the p47phox patients were evenly divided between men and women (47% male). TNF-α levels were significantly lower in p47phox patients. We otherwise observed no significant differences in prevalent traditional cardiovascular risk factors, lipid subfraction levels, or markers of systemic inflammation by genotype. (Table 5)
Table 5.
Clinical and biomarker characteristics of CGD patients by genotype
| p47 (n=19) | gp91 (n=22) | |
|---|---|---|
| Clinical Characteristics | ||
| Age | 34.8±8.0 | 30.4±10.1 |
| Male, N (%)*** | 9 (47.4%) | 22 (100%) |
| Hypertension | 5 (26.3%) | 7 (31.8%) |
| Diabetes | 3 (15.8%) | 0 (0%) |
| Smoking | 8 (42.1%) | 6 (27.3%) |
| Family CVD history | 13 (68.4%) | 9 (45.0%) |
| Lipid Levels | ||
| Total Cholesterol (mg/dL) | 149.1 ± 24.4 | 156.8 ± 42.7 |
| LDL (mg/dL) | 85.9 ± 25.1 | 99.2 ± 40.1 |
| HDL (mg/dL) | 41.4 ±12.0 | 35.2 ±9.2 |
| Triglycerides (mg/dL) | 108.6 ± 57.1 | 112.3 ± 78.4 |
| Oxidized LDL (mg/dL) | 57.4 ± 36.8 | 71.6 ± 37.1 |
| Other Biomarkers | ||
| hsCRP (mg/L) | 7.5 ± 9.4 | 10.1 ± 10.8 |
| Nitrite (NO2−, μM) | 2.8±1.4 | 2.5±1.5 |
| Nitrate (NO3−, μM) | 21.3±12.7 | 27.5±19.6 |
| Neutrophil proteins | ||
| Lactoferrin (ng/ml) | 538.9 ± 708.7 | 881.3 ± 911.7 |
| MPO (ng/ml) | 442.1 ± 819.7 | 499.7 ± 821.2 |
| MMP-9 (ng/ml) | 248.2 ± 252.2 | 485.8± 504.7 |
| α-Defensins (ng/ml) | 63.3 ± 187. 9 | 50.2 ± 74.0 |
| Cytokines | ||
| IFN-γ (pg/ml) | 2.1 ± 2.0 | 8.4 ± 21.7 |
| TNF-α(pg/ml) | 8.6 ± 4.2* | 12.5 ± 6.2 |
| GM-CSF (pg/ml) | 1.4± 2.2 | 4.6 ± 8.9 |
| IL-1β(pg/ml) | 0.6 ±0.7 | 1.6 ± 5.9 |
| IL-2 (pg/ml) | 0.8 ±0.9 | 0.8 ±0.6 |
| IL-4 (pg/ml) | 0.3 ±0.6 | 0.1 ±0.3 |
| IL-5 (pg/ml) | 0.8 ±0.6 | 2.6 ±8.3 |
| IL-6 (pg/ml) | 11.1± 14.1 | 9.6 ± 7.8 |
| IL-10 (pg/ml) | 2.9 ± 1.8 | 4.8 ± 10.8 |
| IL-12p70 (pg/ml) | 1.2 ± 1.1 | 17.6 ± 77.4 |
| IL-13 (pg/ml) | 1.7 ± 3.5 | 2.5 ± 5.1 |
| IL-17 (pg/ml) | 11.0± 11.4 | 13.9 ± 24.1 |
| Chemokines | ||
| CCL3 (MIP-1α, pg/ml) | 39.2 ± 48.5 | 38.3 ± 37.2 |
| CCL4 (MIP-1β, pg/ml) | 128.9 ± 66.5 | 221.8 ± 299.5 |
| CCL5 (RANTES, ng/ml) | 23.7± 9.7 | 27.9 ± 24.8 |
| CXCL8 (IL-8, pg/ml) | 13.9 ± 24.6 | 24.6 ± 46.0 |
| Soluble Selectins | ||
| sL-selectin (ng/ml) | 1108.4 ± 218.5 | 1211.8 ± 262.2 |
| sE-selectin (ng/ml) | 38.5 ± 15.3 | 41.0± 19.3 |
| Soluble Receptors | ||
| TNFR1 (pg/ml) | 3057.0 ± 1373.8 | 3651.6 ± 3082.7 |
| TNFR2 (pg/ml) | 3514.7± 1204.8 | 4393.9 ± 2712.0 |
| IL-6R (pg/ml) | 17576.0 ± 3229.0 | 18783.2 ± 5821.6 |
Means ± SD.
denotes P ≤ 0.05,
denotes P ≤ 0.01, and
denotes P ≤ 0.001 for difference between CGD patients with deficiency p47phox and gp91phox deficiency.
As expected, in healthy control subjects the carotid artery wall volume was significantly lower in females compared with males (Fig 1B). CGD gp91phox deficient patients (all male) had significantly reduced carotid artery wall volume compared with age matched control males (Figure 1B). CGD p47phox deficient male and female patients also had significantly reduced carotid artery wall volume compared with age and sex matched controls (Figure 1B). There was no significant relationship between residual superoxide production and the internal carotid artery wall volume among all the CGD patients (not shown) nor was there a significant difference between common log-transformed internal carotid artery wall volume of p47phox deficient and gp91phox deficient patients (2.53 ± 0.02 vs. 2.57 ± 0.02 log10 mm3, p=0.19).
There was no difference between control subjects and CGD patients in coronary arterial plaque burden (p47phox, 8.4 ± 2.5 vs. gp91phox 8.1± 1.4 mm2, p=0.75) or the prevalence of coronary arterial calcium (p47phox 13.6% vs. gp91phox 15.8% p=0.85).
As intraconazole has been demonstrated to affect HDL levels 30 we conducted a sensitivity analysis excluding 16 patients on active itraconazole prophylaxis therapy. The remaining 20 CGD patients still had a significant reduction in carotid atherosclerosis compared to healthy controls (p=0.0012).
DISCUSSION
We investigated the prevalence of subclinical atherosclerosis in the carotid and coronary arteries of patients with CGD. Despite an adverse cardiovascular risk profile with significant elevations in multiple systemic markers of inflammation, the data demonstrate that CGD was associated with smaller carotid artery wall thickness, an established indicator of subclinical atherosclerosis, compared to healthy controls. This effect was independent of traditional cardiovascular risk factors. In contrast, CT angiography of coronary arteries did not show a significant difference between CGD and control subjects in the prevalence of coronary atherosclerosis.
CGD offers an opportunity to study the clinical consequences of reduced NOX2 activity on cardiovascular disease in humans. Violi and coauthors found a reduction in ultrasound carotid intimal-medial thickness in male patients with CGD 20 and in female carriers of X-linked CGD.22 While X-CGD carriers are generally healthy, many face an increased frequency of autoimmune disease 31 and, depending on the extent of lyonization in their myeloid cells, some face serious CGD-like infectious complications. 32 Nevertheless, a reduction in carotid intimal-medial thickness in both patients and carriers was associated with lower biomarkers of oxidative stress and increased brachial arterial flow mediated dilation. These investigators also reported decreased isoprostane formation and increased NO generation in X-CGD.20 Increased flow mediated dilation was reported in p47phox -deficient CGD patients but intimal-medial thickness did not differ from normal.33 These results provided the first evidence in vivo that NOX2 may play a role in arterial tone and hypertension, and possibly contribute to the pathogenesis of atherosclerotic disease.20, 22, 33 Although intimal-medial thickness is considered a useful surrogate marker for atherosclerosis, hypertension alone is a primary driver of intimal thickening, and can cause changes in carotid intimal-medial thickness that do not correspond histologically to atherosclerotic injury.34 It is plausible that the observed reduction in carotid intimal-medial thickness was driven by NOX2 related improvement in endothelial function in a process independent of atherosclerosis. Flow mediated dilation provides useful insights into arterial endothelial function, but has not been demonstrated to predict future cardiovascular events beyond traditional risk factors.35 The correlation between carotid and coronary atherosclerotic burden, while significant in some populations, has important limitations 36, and it remains possible that the observed reduction in carotid intimal-medial thickness may not translate into a protective effect in other vascular beds consistent with previous observations in a mouse model. 37 While associations between morphologic carotid atherosclerosis and coronary arterial disease 38, 39 incident cardiovascular events have been observed, the presence and strength of these correlations varies importantly by population. 36
The present study demonstrates an association between both p47phox and gp91phox CGD and lower carotid wall thickness using histologically validated high-resolution MRI techniques. 25, 40 Our analysis shows this difference in early vascular injury is independent of hypertension, suggesting that it may be mediated through other NOX2 effects as discussed below. Importantly, p47phox is not only a cytosolic regulator of NOX2 activity but can also regulate NOX1. 41 Deficiency in this protein may, therefore, alter multiple NADPH oxidases.
This is the first study, to our knowledge, to examine the prevalence of subclinical coronary atherosclerosis in CGD. In contrast to the findings in the carotid arteries, noninvasive quantification of wall thickening and coronary plaque burden showed no significant difference between NOX2-deficient patients and controls. Quantification of coronary arterial calcium, a finding representative of more advanced atherosclerotic lesions and a powerful independent predictor of cardiovascular events, 35, 42 showed no reduction and a trend towards a greater burden of calcified atherosclerosis in CGD patients. Physiologically, it is possible that NOX2-related mechanisms play a lesser role in coronary atherosclerosis than in other arterial beds. While there was a non-statistically significant trend towards greater coronary calcification in CGD patients, the total volume of calcified and noncalcified plaque, as measured by coronary plaque index, was nearly identical to controls. The ratio of calcified to non-calcified components of atherosclerotic plaque varies in different disease processes, 43–45 and patients with CGD may have more rapid progression of calcification than found in typical atherosclerosis. We cannot exclude the possibility that small sample size limited the power to detect a smaller magnitude difference in coronary plaque burden between groups. Given the increases in the lifespan of CGD patients since the advent of antimicrobial prophylaxis, further investigation of the aging CGD population will likely reveal whether ROS play a role in calcified coronary atherosclerosis. Our study of CGD patients also revealed significantly lower levels of HDL and a trend toward elevated triglycerides despite normal cholesterol. This is in contrast to the reported decrease in triglycerides in CGD mice. 14 Surprisingly, oxidized LDL was significantly higher in CGD suggesting not only that NOX2 is not primarily responsible for the production of oxidized LDL but possibly that NOX2 is involved in the catabolism of this lipid species. Importantly, CGD subjects are treated with prophylactic antibacterial and antifungal drugs including itraconazole, which was been shown to reduce LDL and increase HDL in immunocompetent men. 30 We did not detect any association between itraconazole prophylaxis or other antibiotic therapies our patients were receiving and atherosclerotic burden or lipid profiles.
Despite the absence of overt signs of infection including normal white cell counts, plasma from CGD subjects in this study contained significantly increased concentrations of clinically recognized cardiovascular risk factors such as hsCRP 46 and MPO 47 as well as other inflammatory biomarkers (e.g., TNFα, IL-6, GM-CSF). These elevations, which have not been reported previously in CGD, may be due to unrecognized infection or inflammation but may also relate to the direct regulatory role for reactive oxygen species in controlling inflammation. For example, ROS regulates the mRNA stability of IL8 4, the metabolism of leukotrienes resulting in accumulation of LTB4 5, IL-1β processing 48 and the apoptosis of CGD neutrophils 6. The role of cytokines as regulators of inflammation in cardiovascular disease has been reviewed 49 although specific pathophysiologic roles and clinical risk guidelines have yet to be established.
Interestingly, a common polymorphism in the IL-6 receptor that reduces function was associated in an 82-study meta-analysis with a decreased risk of coronary heart disease suggesting that signaling by IL-6, which was also elevated in CGD, may be pathogenic.50 In parallel with MPO, we also observed significant elevations in CGD patient plasma of other neutrophil-derived factors including gelatinase, lactoferrin and defensins. Further work will be required to determine whether or not the increases in these factors are due to mechanisms such as those controlling IL-8 (see above) or reflect neutrophil degranulation due to increased inflammation in CGD. The observation of reduced carotid but not coronary artery atherosclerosis in CGD patients raises questions about the role of NOX2 in the pathogenesis of atherosclerosis. Importantly, this finding also raises questions about whether or not NOX2 inhibitors may be beneficial in reducing all atherosclerosis or just that outside of the coronary circulation. Clearly, further high-resolution studies of possible links between deficiencies in or inhibition of different NOX proteins and atherogenesis in distinct anatomic vascular locations are indicated.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the participation of the CGD patients and the clinical support teams of the Laboratory of Host Defenses, the Laboratory of Clinical Infectious Diseases, and the Department of Radiology and Imaging Sciences.
Funding Sources: This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases and the National Institutes of Health Clinical Center.
Footnotes
Conflict of Interest Disclosures: The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors report no conflict of interest relevant to the content of this manuscript.
References
- 1.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med. 2000;343:1703–1714. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- 2.Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, Uzel G, DeRavin SS, Priel DA, Soule BP, Zarember KA, Malech HL, Holland SM, Gallin JI. Residual nadph oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363:2600–2610. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Lekstrom-Himes JA, Kuhns DB, Alvord WG, Gallin JI. Inhibition of human neutrophil il-8 production by hydrogen peroxide and dysregulation in chronic granulomatous disease. J Immunol. 2005;174:411–417. doi: 10.4049/jimmunol.174.1.411. [DOI] [PubMed] [Google Scholar]
- 5.Henderson WR, Klebanoff SJ. Leukotriene production and inactivation by normal, chronic granulomatous disease and myeloperoxidase-deficient neutrophils. J Biol Chem. 1983;258:13522–13527. [PubMed] [Google Scholar]
- 6.Kasahara Y, Iwai K, Yachie A, Ohta K, Konno A, Seki H, Miyawaki T, Taniguchi N. Involvement of reactive oxygen intermediates in spontaneous and CD95 (fas/apo-1)-mediated apoptosis of neutrophils. Blood. 1997;89:1748–1753. [PubMed] [Google Scholar]
- 7.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of nadph oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 9.Meng D, Lv DD, Fang J. Insulin-like growth factor-1 induces reactive oxygen species production and cell migration through nox4 and rac1 in vascular smooth muscle cells. Cardiovasc Res. 2008;80:299–308. doi: 10.1093/cvr/cvn173. [DOI] [PubMed] [Google Scholar]
- 10.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, Channon KM. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:333–339. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- 11.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, Channon KM. Endothelial nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: Studies in endothelial-targeted nox2 transgenic mice. Circ Res. 2007;100:1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 13.Cave A. Selective targeting of nadph oxidase for cardiovascular protection. Curr Op Pharmacol. 2009;9:208–213. doi: 10.1016/j.coph.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte nadph oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2000;20:1529–1535. doi: 10.1161/01.atv.20.6.1529. [DOI] [PubMed] [Google Scholar]
- 15.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in apoe−/− mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Keaney JF, Jr, Schulz E, Levison B, Shan L, Sakuma M, Zhang X, Shi C, Hazen SL, Simon DI. Decreased neointimal formation in nox2-deficient mice reveals a direct role for nadph oxidase in the response to arterial injury. Proc Natl Acad Sci U S A. 2004;101:13014–13019. doi: 10.1073/pnas.0405389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsich E, Segal BH, Pagano PJ, Rey FE, Paigen B, Deleonardis J, Hoyt RF, Holland SM, Finkel T. Vascular effects following homozygous disruption of p47(phox): An essential component of nadph oxidase. Circulation. 2000;101:1234–1236. doi: 10.1161/01.cir.101.11.1234. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Wang JJ, Yu Q, Chen K, Mahadev K, Zhang SX. Inhibition of reactive oxygen species by lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: Role of nadph oxidase 4. Diabetes. 2010;59:1528–1538. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinkade K, Streeter J, Miller FJ. Inhibition of nadph oxidase by apocynin attenuates progression of atherosclerosis. Int J Mol Sci. 2013;14:17017–17028. doi: 10.3390/ijms140817017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Violi F, Sanguigni V, Carnevale R, Plebani A, Rossi P, Finocchi A, Pignata C, De Mattia D, Martire B, Pietrogrande MC, Martino S, Gambineri E, Soresina AR, Pignatelli P, Martino F, Basili S, Loffredo L. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: Results of a multicenter study. Circulation. 2009;120:1616–1622. doi: 10.1161/CIRCULATIONAHA.109.877191. [DOI] [PubMed] [Google Scholar]
- 21.Loukogeorgakis SP, van den Berg MJ, Sofat R, Nitsch D, Charakida M, Haiyee B, de Groot E, MacAllister RJ, Kuijpers TW, Deanfield JE. Role of nadph oxidase in endothelial ischemia/reperfusion injury in humans. Circulation. 2010;121:2310–2316. doi: 10.1161/CIRCULATIONAHA.108.814731. [DOI] [PubMed] [Google Scholar]
- 22.Violi F, Pignatelli P, Pignata C, Plebani A, Rossi P, Sanguigni V, Carnevale R, Soresina A, Finocchi A, Cirillo E, Catasca E, Angelico F, Loffredo L. Reduced atherosclerotic burden in subjects with genetically determined low oxidative stress. Arterioscler Thromb Vasc Biol. 2013;33:406–412. doi: 10.1161/ATVBAHA.112.300438. [DOI] [PubMed] [Google Scholar]
- 23.Duivenvoorden R, de Groot E, Elsen BM, Lameris JS, van der Geest RJ, Stroes ES, Kastelein JJ, Nederveen AJ. In vivo quantification of carotid artery wall dimensions: 3.0-tesla MRI versus B-mode ultrasound imaging. Circ Cardiovasc Imaging. 2009;2:235–242. doi: 10.1161/CIRCIMAGING.108.788059. [DOI] [PubMed] [Google Scholar]
- 24.Sibley CT, Vavere AL, Gottlieb I, Cox C, Matheson M, Spooner A, Godoy G, Fernandes V, Wasserman BA, Bluemke DA, Lima JA. MRI-measured regression of carotid atherosclerosis induced by statins with and without niacin in a randomised controlled trial: The NIA plaque study. Heart. 2013;99:1675–1680. doi: 10.1136/heartjnl-2013-303926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasserman BA, Sharrett AR, Lai S, Gomes AS, Cushman M, Folsom AR, Bild DE, Kronmal RA, Sinha S, Bluemke DA. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution mri: The multi-ethnic study of atherosclerosis (MESA) Stroke. 2008;39:329–335. doi: 10.1161/STROKEAHA.107.498634. [DOI] [PubMed] [Google Scholar]
- 26.Inoue K, Motoyama S, Sarai M, Sato T, Harigaya H, Hara T, Sanda Y, Anno H, Kondo T, Wong ND, Narula J, Ozaki Y. Serial coronary ct angiography-verified changes in plaque characteristics as an end point: Evaluation of effect of statin intervention. JACC Cardiovasc Imaging. 2010;3:691–698. doi: 10.1016/j.jcmg.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 28.Miller JM, Dewey M, Vavere AL, Rochitte CE, Niinuma H, Arbab-Zadeh A, Paul N, Hoe J, de Roos A, Yoshioka K, Lemos PA, Bush DE, Lardo AC, Texter J, Brinker J, Cox C, Clouse ME, Lima JA. Coronary ct angiography using 64 detector rows: Methods and design of the multi-centre trial core-64. Eur Radiol. 2009;19:816–828. doi: 10.1007/s00330-008-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 30.Schneider B, Gerdsen R, Plat J, Dullens S, Bjorkhem I, Diczfalusy U, Neuvonen PJ, Bieber T, von Bergmann K, Lutjohann D. Effects of high-dose itraconazole treatment on lipoproteins in men. Int J Clin Pharmacol Ther. 2007;45:377–384. doi: 10.5414/cpp45377. [DOI] [PubMed] [Google Scholar]
- 31.Cale CM, Morton L, Goldblatt D. Cutaneous and other lupus-like symptoms in carriers of x-linked chronic granulomatous disease: Incidence and autoimmune serology. Clin Exp Immunol. 2007;148:79–84. doi: 10.1111/j.1365-2249.2007.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen-Wolff A, Soldan W, Heyne K, Bickhardt J, Gahr M, Roesler J. Increased susceptibility of a carrier of x-linked chronic granulomatous disease (CGD) to aspergillus fumigatus infection associated with age-related skewing of lyonization. Ann Hematol. 2001;80:113–115. doi: 10.1007/s002770000230. [DOI] [PubMed] [Google Scholar]
- 33.Loffredo L, Carnevale R, Sanguigni V, Plebani A, Rossi P, Pignata C, De Mattia D, Finocchi A, Martire B, Pietrogrande MC, Martino S, Gambineri E, Giardino G, Soresina AR, Martino F, Pignatelli P, Violi F. Does NADPH oxidase deficiency cause artery dilatation in humans? Antioxid Redox Signal. 2013;18:1491–1496. doi: 10.1089/ars.2012.4987. [DOI] [PubMed] [Google Scholar]
- 34.Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: A point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30:177–181. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
- 35.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams MR, Nakagomi A, Keech A, Robinson J, McCredie R, Bailey BP, Freedman SB, Celermajer DS. Carotid intima-media thickness is only weakly correlated with the extent and severity of coronary artery disease. Circulation. 1995;92:2127–2134. doi: 10.1161/01.cir.92.8.2127. [DOI] [PubMed] [Google Scholar]
- 37.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in apoe(−/−) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB., Sr Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polak JF, Tracy R, Harrington A, Zavodni AE, O’Leary DH. Carotid artery plaque and progression of coronary artery calcium: The multi-ethnic study of atherosclerosis. J Am Soc Echocardiogr. 2013;26:548–555. doi: 10.1016/j.echo.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, Takaya N, Polissar NL, Yuan C. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: Comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–3444. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 41.Lambeth JD, Kawahara T, Diebold B. Regulation of nox and duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 43.Dollar AL, Kragel AH, Fernicola DJ, Waclawiw MA, Roberts WC. Composition of atherosclerotic plaques in coronary arteries in women less than 40 years of age with fatal coronary artery disease and implications for plaque reversibility. Am J Cardiol. 1991;67:1223–1227. doi: 10.1016/0002-9149(91)90931-a. [DOI] [PubMed] [Google Scholar]
- 44.Kragel AH, Reddy SG, Wittes JT, Roberts WC. Morphometric analysis of the composition of coronary arterial plaques in isolated unstable angina pectoris with pain at rest. Am J Cardiol. 1990;66:562–567. doi: 10.1016/0002-9149(90)90482-g. [DOI] [PubMed] [Google Scholar]
- 45.Schmermund A, Schwartz RS, Adamzik M, Sangiorgi G, Pfeifer EA, Rumberger JA, Burke AP, Farb A, Virmani R. Coronary atherosclerosis in unheralded sudden coronary death under age 50: Histo-pathologic comparison with ‘healthy’ subjects dying out of hospital. Atherosclerosis. 2001;155:499–508. doi: 10.1016/s0021-9150(00)00598-0. [DOI] [PubMed] [Google Scholar]
- 46.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of c-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 47.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 48.van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, van der Meer JW, Netea MG. Reactive oxygen species-independent activation of the IL-1β inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci U S A. 2010;107:3030–3033. doi: 10.1073/pnas.0914795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearson TA, Bazzarre TL, Daniels SR, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Hong Y, Mensah GA, Sallis JF, Jr, Smith S, Jr, Stone NJ, Taubert KA American Heart Association Expert Panel on P, Prevention S. American heart association guide for improving cardiovascular health at the community level: A statement for public health practitioners, healthcare providers, and health policy makers from the american heart association expert panel on population and prevention science. Circulation. 2003;107:645–651. doi: 10.1161/01.cir.0000054482.38437.13. [DOI] [PubMed] [Google Scholar]
- 50.Collaboration IRGCERF. Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjaerg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundstrom J, Wassertheil-Smoller S, Mellstrom D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, Davey-Smith G, de Bakker PI, O’Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten A, Ljunggren O, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Holm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O, Danesh J. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.