Abstract
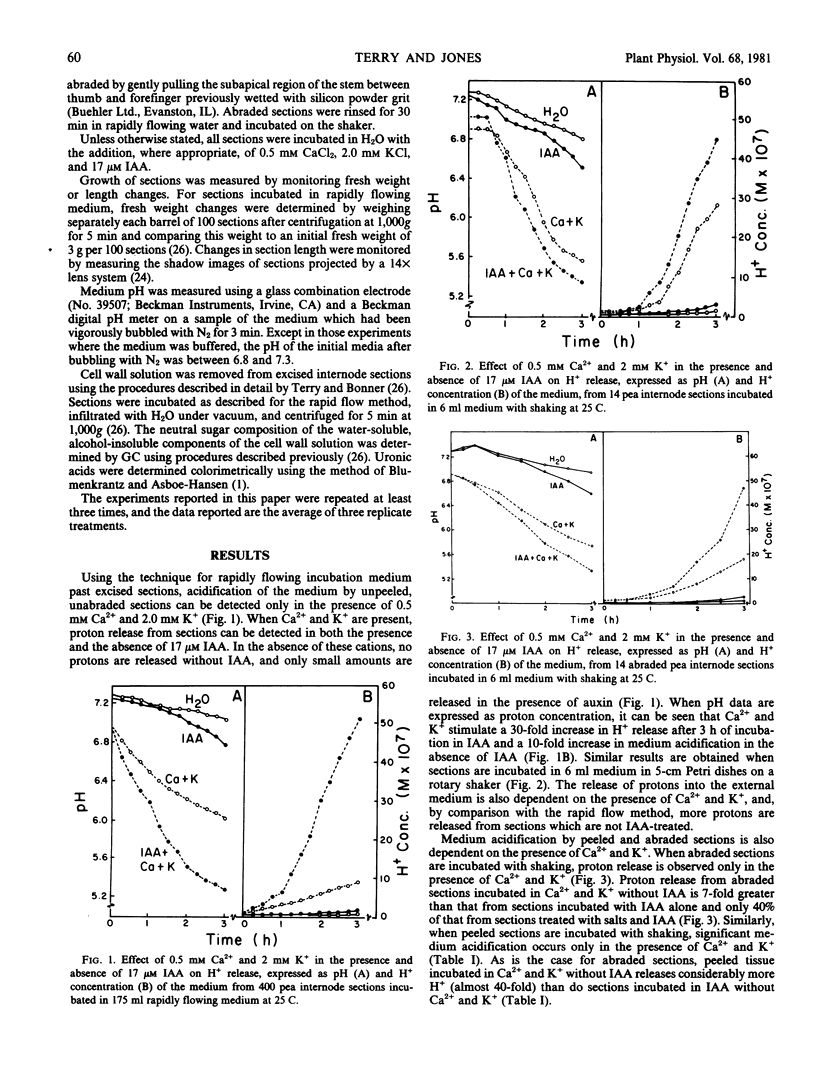
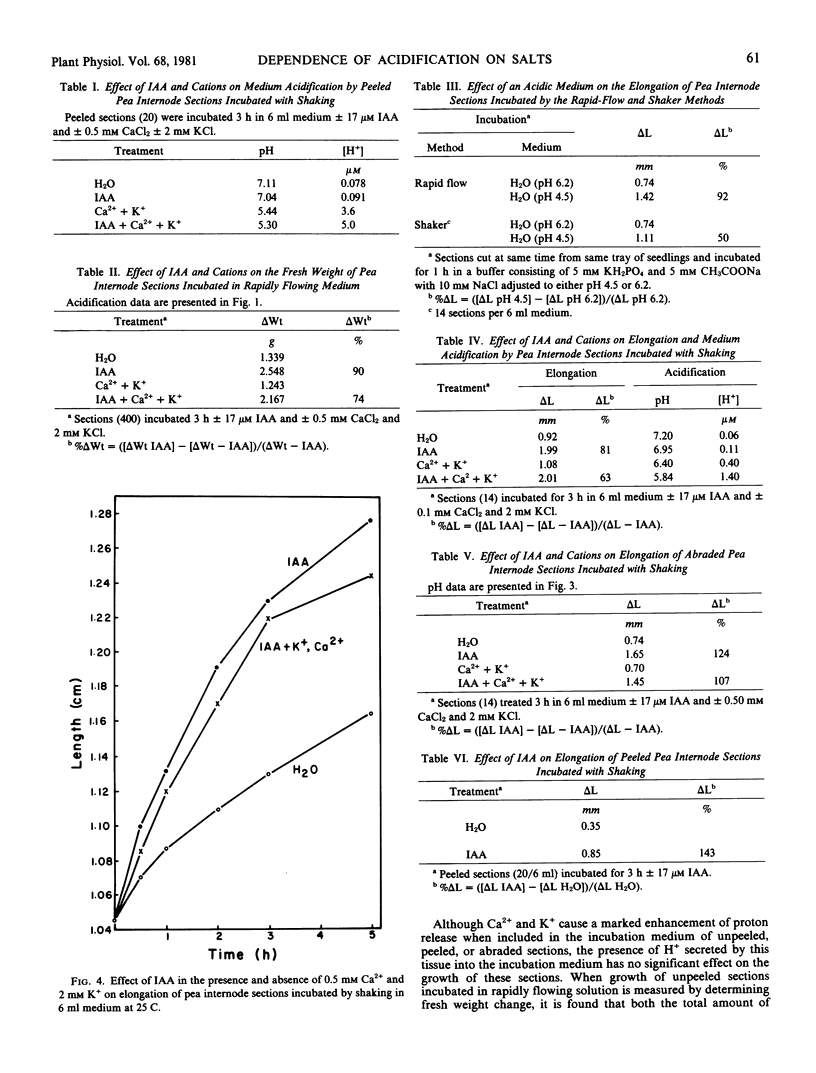
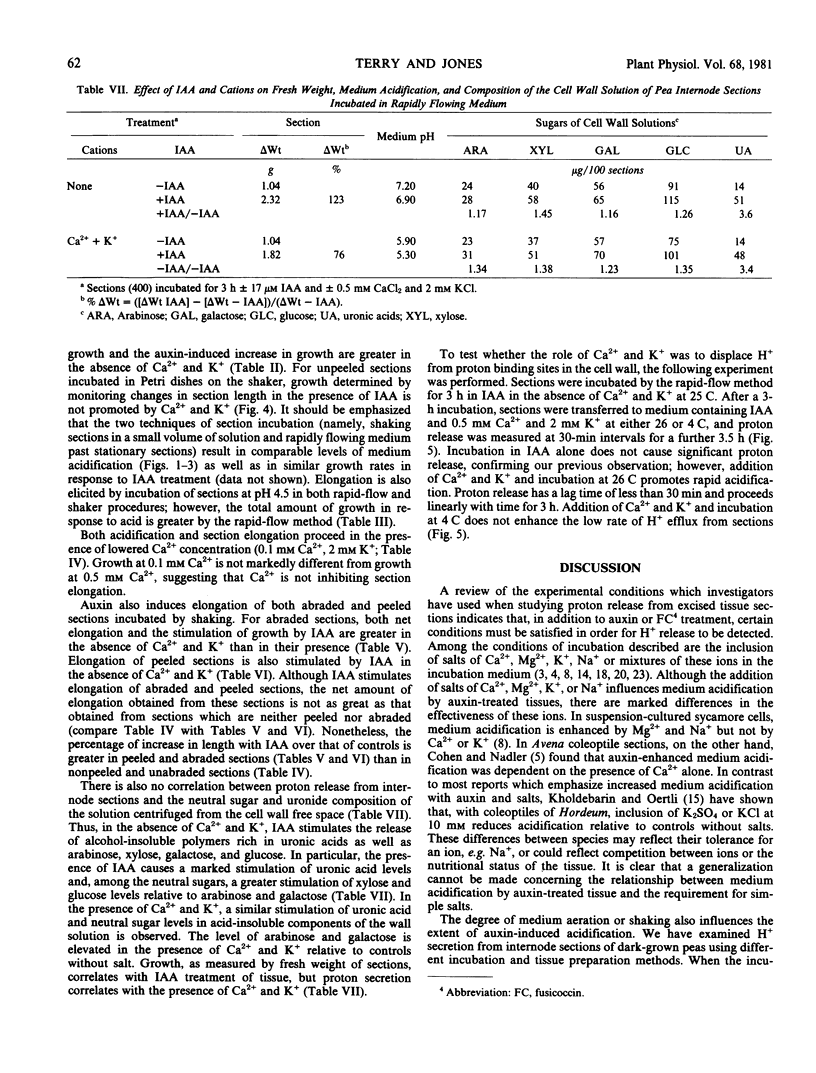
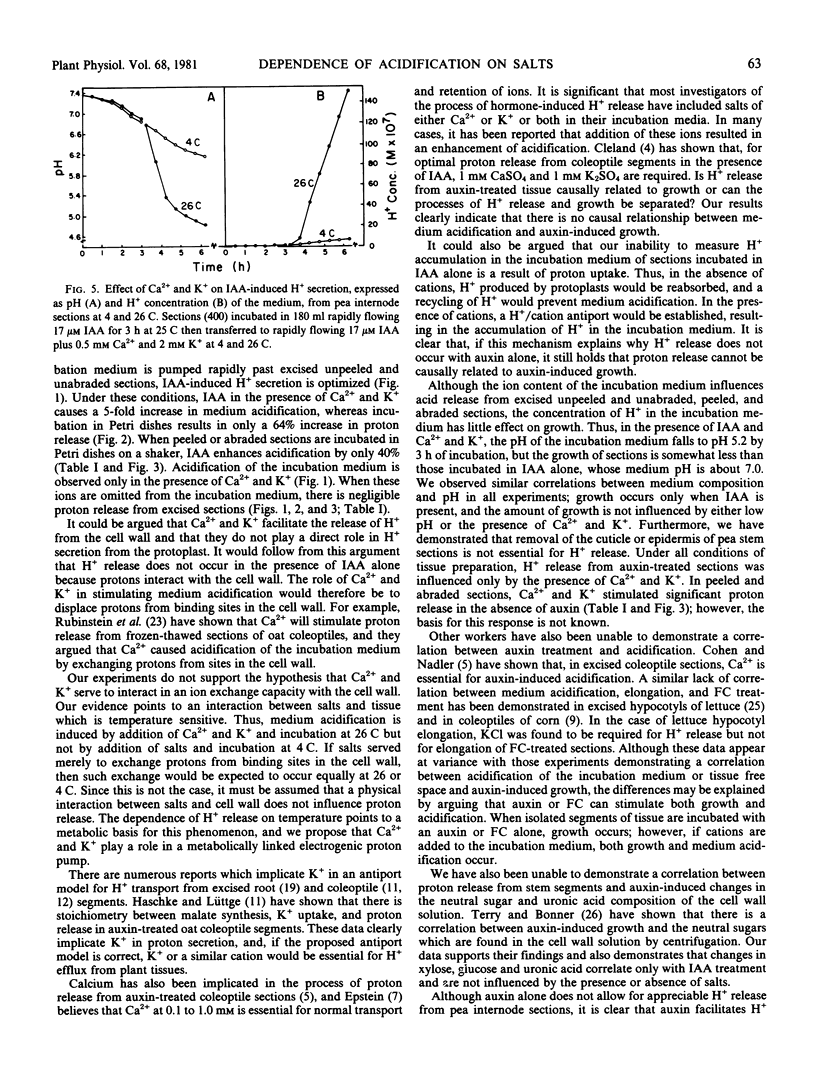
The capacity of excised internode sections of pea to grow and secrete protons in response to indoleacetic acid (IAA) and Ca2+ and K+ treatments was examined. By incubating unpeeled and unabraded sections in rapidly flowing solutions, it was shown that acidification of the external medium in the presence or absence of IAA is dependent on the presence of Ca2+ and K+. Similar results were obtained when unpeeled and unabraded sections were incubated in dishes with shaking. When peeled or abraded sections were incubated with shaking in IAA, H+ release was also dependent on the presence of Ca2+ and K+. The release of H+ from sections incubated in Ca2+ and K+ is not caused by displacement of H+ from binding sites in the cell wall. Rather, the release of protons from sections is temperature dependent, and it is concluded that this is a metabolically linked process. Although Ca2+ and K+ are essential for the release of H+ from isolated stem sections of peas, these cations do not influence elongation. Despite the large increase in proton release induced by Ca2+ and K+ either in the presence or absence of auxin, growth in the presence of these ions was never greater than it was in their absence. Furthermore, cations do not affect the neutral sugar or uronic acid composition of the solution which can be centrifuged from isolated sections. As is the case for growth, an increase in the neutral sugar and uronide composition of the cell wall solution is dependent only on IAA. It is concluded that IAA-induced growth of pea stem sections is independent of the secretion of protons.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Cleland R. E. Kinetics of Hormone-induced H Excretion. Plant Physiol. 1976 Aug;58(2):210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Auxin-induced hydrogen ion excretion from Avena coleoptiles. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3092–3093. doi: 10.1073/pnas.70.11.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D., Nadler K. D. Calcium Requirement for Indoleacetic Acid-induced Acidification by Avena Coleoptiles. Plant Physiol. 1976 Mar;57(3):347–350. doi: 10.1104/pp.57.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. L., Albersheim P. Characterization of a H Efflux from Suspension-cultured Plant Cells. Plant Physiol. 1974 Mar;53(3):464–468. doi: 10.1104/pp.53.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschke H. P., Lüttge U. Stoichiometric Correlation of Malate Accumulation with Auxin-dependent K-H Exchange and Growth in Avena Coleoptile Segments. Plant Physiol. 1975 Nov;56(5):696–698. doi: 10.1104/pp.56.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Promotion of Xyloglucan Metabolism by Acid pH. Plant Physiol. 1975 Sep;56(3):373–376. doi: 10.1104/pp.56.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentze J., Raymond B., Cohen J. D., Rayle D. L. Auxin-induced H Secretion in Helianthus and Its Implications. Plant Physiol. 1977 Oct;60(4):509–512. doi: 10.1104/pp.60.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish D. J., Davies P. J. On the Relationship between Extracellular pH and the Growth of Excised Pea Stem Segments. Plant Physiol. 1977 Apr;59(4):574–578. doi: 10.1104/pp.59.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G. Active H Efflux from Cells of Low-salt Barley Roots during Salt Accumulation. Plant Physiol. 1970 Jun;45(6):787–790. doi: 10.1104/pp.45.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Enhancement of wall loosening and elongation by Acid solutions. Plant Physiol. 1970 Aug;46(2):250–253. doi: 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk W. K., Jones R. L. Gibberellin response in lettuce hypocotyl sections. Plant Physiol. 1975 Aug;56(2):267–272. doi: 10.1104/pp.56.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry M. E., Bonner B. A. An Examination of Centrifugation as a Method of Extracting an Extracellular Solution from Peas, and Its Use for the Study of Indoleacetic Acid-induced Growth. Plant Physiol. 1980 Aug;66(2):321–325. doi: 10.1104/pp.66.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Findley J. S., Burke J. J., Blizzard W. E. Auxin Has No Effect on Modification of External pH by Soybean Hypocotyl Cells. Plant Physiol. 1977 May;59(5):1000–1003. doi: 10.1104/pp.59.5.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Lu T. Y., Williams C. A. Comparison of Auxin-induced and Acid-induced Elongation in Soybean Hypocotyl. Plant Physiol. 1977 May;59(5):1004–1007. doi: 10.1104/pp.59.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A., Williams C. A., Brinkmann K. A. Additional evidence for separable responses to auxin in soybean hypocotyl. Plant Physiol. 1976 May;57(5):817–819. doi: 10.1104/pp.57.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]


