Abstract
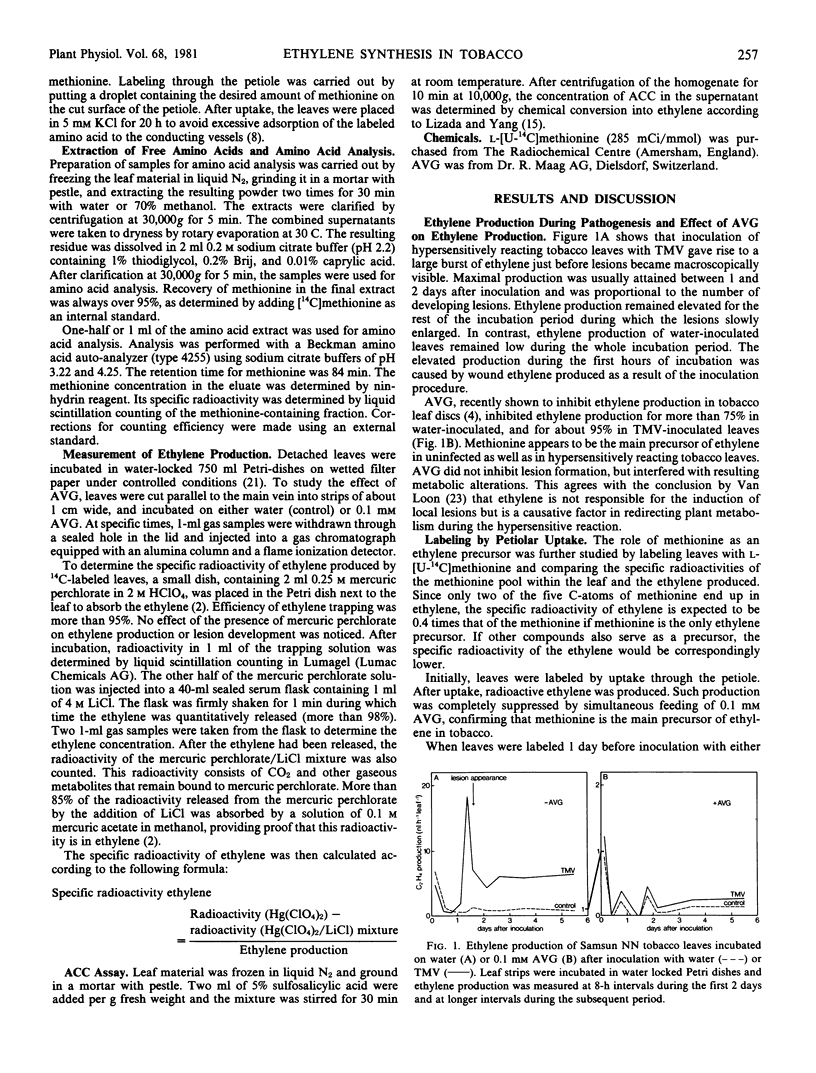
The hypersensitive reaction of Samsun NN tobacco leaves to tobacco mosaic virus (TMV) was accompanied by a large increase in ethylene production, just before necrotic local lesions became visible. Normal and virus-induced ethylene production were both largely inhibited by 0.1 millimolar aminoethoxyvinylglycine indicating that methionine is a main ethylene precursor.
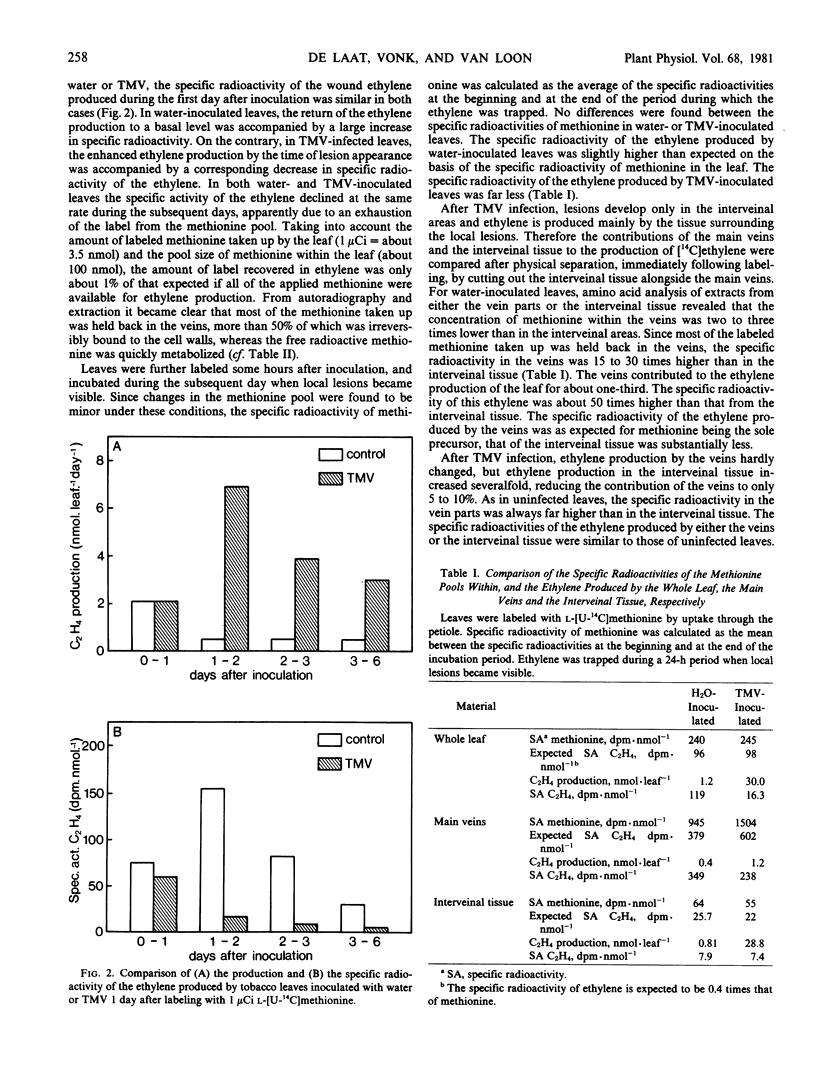
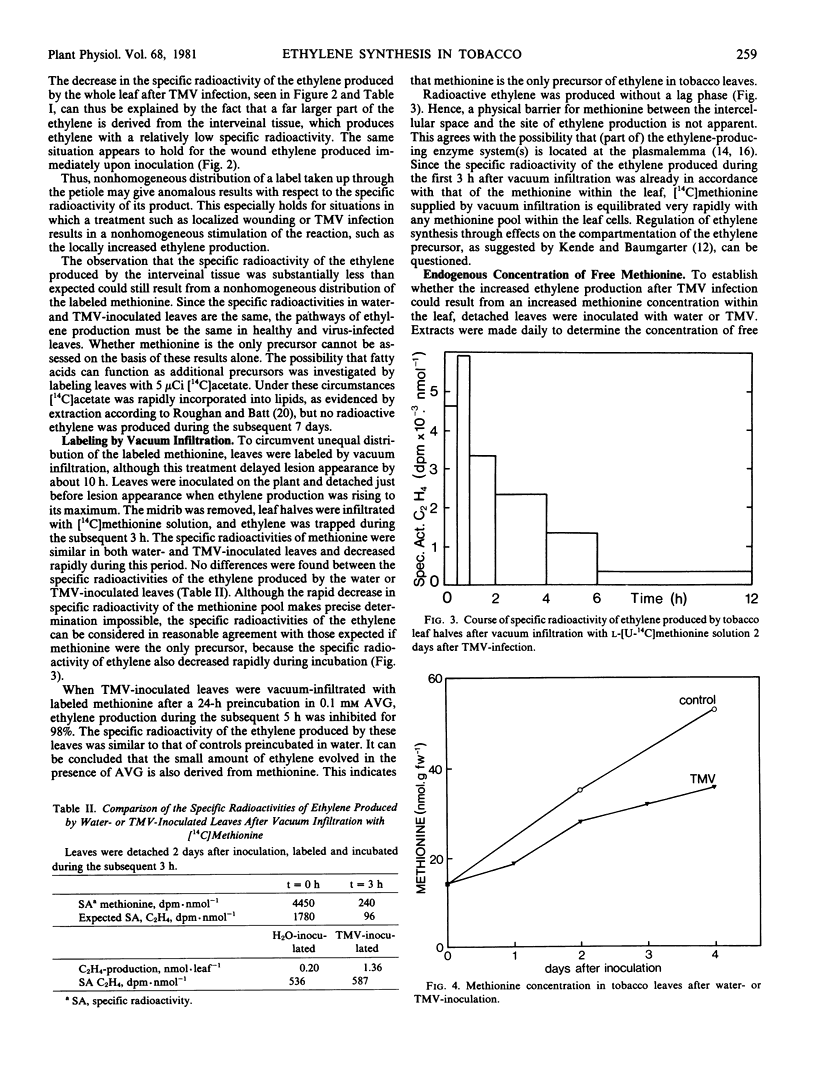
The contribution of methionine to ethylene production was estimated by labeling leaves with l-[U-14C]methionine and comparing the specific activities of methionine within and ethylene produced by the leaf. When taken up through the petiole, methionine was largely retained in the veins, leading to production of ethylene with a far higher specific activity in the veins than in the interveinal tissue. After TMV infection, ethylene production increased only in the interveinal tissue, resulting in a decrease in specific activity of the ethylene produced. In the interveinal tissue, the specific radioactivity of the ethylene was lower than expected if methionine were the only precursor. After labeling by vacuum infiltration, the specific activities of the ethylene produced by water- and TMV-inoculated leaves were both identical and in accordance with the specific radioactivity of methionine. Inasmuch as the content of 1-aminocyclopropane-1-carboxylic acid was increased severalfold two days after TMV infection, methionine can be considered to be the only ethylene precursor in healthy and in TMV-infected tobacco leaves.
The increase in ethylene production after TMV-infection was not accompanied by an increased concentration of free methionine within the leaf. Compartmentation of methionine does not appear to be a regulating factor since labeled methionine supplied to the leaf by vacuum infiltration is equilibrated very rapidly with any methionine pool within the leaf cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L. Biochemical Pathway of Stress-induced Ethylene. Plant Physiol. 1972 Oct;50(4):496–498. doi: 10.1104/pp.50.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N., Lieberman M. Ethylene as a regulator of senescence in tobacco leaf discs. Plant Physiol. 1979 Nov;64(5):801–804. doi: 10.1104/pp.64.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Methionine metabolism and ethylene biosynthesis in senescent flower tissue of morning-glory. Plant Physiol. 1976 Apr;57(4):528–537. doi: 10.1104/pp.57.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Lieberman M. Localization of the Ethylene-synthesizing System in Apple Tissue. Plant Physiol. 1977 Nov;60(5):794–799. doi: 10.1104/pp.60.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagaki Y., Hirai T., Stahmann M. A. Ethylene production by detached leaves infected with tobacco mosaic virus. Virology. 1970 Jan;40(1):1–9. doi: 10.1016/0042-6822(70)90372-7. [DOI] [PubMed] [Google Scholar]
- Pritchard D. W., Ross A. F. The relationship of ethylene to formation of tobacco mosaic virus lesions in hypersensitive responding tobacco leaves with and without induced resistance. Virology. 1975 Apr;64(2):295–307. doi: 10.1016/0042-6822(75)90106-3. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Batt R. D. Quantitative analysis of sulfolipid (sulfoquinovosyl diglyceride) and galactolipids (monogalactosyl and digalactosyl diglycerides) in plant tissues. Anal Biochem. 1968 Jan;22(1):74–88. doi: 10.1016/0003-2697(68)90261-3. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. Regulation of Auxin-induced Ethylene Production in Mung Bean Hypocotyls: Role of 1-Aminocyclopropane-1-Carboxylic Acid. Plant Physiol. 1979 Mar;63(3):589–590. doi: 10.1104/pp.63.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon L. C. Induction by 2-chloroethylophosphonic acid of viral-like lesions, associated proteins, and systemic resistance in tobacco. Virology. 1977 Jul 15;80(2):417–420. doi: 10.1016/s0042-6822(77)80016-0. [DOI] [PubMed] [Google Scholar]


