Abstract
Recently, there has been increased interest in ultra-rapid freezing with mammalian spermatozoa, especially for vitrification in the absence of cryoprotectants. Sperm cryopreservation in non-human primates has been successful, but the use of frozen–thawed sperm in standard artificial insemination (AI) remains difficult, and removal of permeable cryoprotectant may offer opportunities for increased AI success. The present study intended to explore the possibility of freezing rhesus monkey sperm in the absence of permeable cryoprotectants. Specifically, we evaluated various factors such as presence or absence of egg yolk, the percentage of egg yolk in the extenders, and the effect of cooling and thawing rate on the success of freezing without permeable cryoprotectants. Findings revealed that freezing with TEST in the absence of egg yolk offers little protection (<15% post-thaw motility). Egg yolk of 40% or more in TEST resulted in decreased motility, while egg yolk in the range of 20–30% yielded the most motile sperm. Cooling at a slow rate (29 °C/min) reduced post-thaw motility significantly for samples frozen with TEST-yolk alone, but had no effect for controls in the presence of glycerol. Similarly, slow thawing in room temperature air is detrimental for freezing without permeable cryoprotectant (<2% motility). In addition to motility, the ability of sperm to capacitate based on an increase in intracellular calcium levels upon activation with cAMP and caffeine suggested no difference between fresh and frozen–thawed motile sperm, regardless of treatment. In summary, the present study demonstrates that ejaculated and epididymal sperm from rhesus monkeys can be cryopreserved with TEST-yolk (20%) in the absence of permeable cryoprotectant when samples were loaded in a standard 0.25-mL straw, cooled rapidly in liquid nitrogen vapor at 220 °C/min, and thawed rapidly in a 37 °C water bath. This study also represents the first success of freezing without permeable cryoprotectant in non-human primates.
Keywords: Freezing, Semen, Egg yolk, Epididymal sperm, Macaca mulatta, Non-human primate
Introduction
The discovery of glycerol as a cryoprotectant by Polge et al. [42] has led to its widespread use in sperm cryopreservation of species ranging from fish to mammals [5,35,45]. With the addition of glycerol, sperm were usually frozen at a slow rate in a magnitude of tens of degrees per min (e.g., 20–40 °C/min). Ultra-rapid freezing such as vitrification has been considered inappropriate in sperm freezing due to the high concentrations of cryoprotectant needed [21]. In addition, possibly because of the commercial availability of controlled rate freezers, which in general have a maximum cooling rate of 50 °C/min, slow cooling has been the focus of the majority of sperm freezing studies. Along with its popularity as the main cryoprotectant for mammalian spermatozoa, glycerol has also been found to adversely affect fertility in chickens, turkeys, boars, bulls, and stallions [2,30,41]. In humans, glycerol in combination with a TES, Tris, and egg yolk based medium has long been used routinely for clinical practices [45]. However, a series of recent studies has begun to explore freezing human sperm without cryoprotectants through the method of vitrification, and success has been reported [19,22–24,40].
In non-human primates, sperm cryopreservation has been studied most extensively in cynomolgus and rhesus macaques [34]. Despite that, propagation using frozen–thawed sperm in standard artificial insemination (AI) has not been successful, and live births were only reported through either intrauterine insemination [46,49] or intracytoplasmic sperm injection (ICSI) [54]. The unique tortuous cervical canal of the rhesus macaque has been suspected to be the greatest barrier for the successful application of standard AI [10]. However, it is possible that glycerol may also have a negative effect on the fertility of sperm cells. This study explored the possibility of freezing without glycerol by the use of a rapid freezing method. Excluding permeable cryoprotectants may also be beneficial for in vitro fertilization (IVF) or ICSI with frozen–thawed sperm.
In the present study, freezing in the absence of permeable cryoprotectants was evaluated for the first time for ejaculated and epididymal sperm of rhesus macaques. Specifically, this study (1) tested the percent egg yolk that offers the most effective protection for samples frozen without permeable cryoprotectant; (2) compared freezing with or without egg yolk or permeable cryoprotectant; (3) evaluated the effect of cooling rate on freezing success without permeable cryoprotectant; and (4) determined the effect of thawing methods on freezing outcome without permeable cryoprotectant.
Materials and methods
Semen collection and processing
Rhesus monkeys (Macaca mulatta) were housed at the California National Primate Research Center (CNPRC) and maintained according to Institutional Animal Care and Use Committee protocols at the University of California. Experiments were conducted in accordance with the National Research Council publication “Guide for Care and Use of Laboratory Animals” (copyright 1996, National Academy of Sciences). Both ejaculated and epididymal sperm samples were used in this study. Ejaculated semen samples were collected from nine adult males (Table 1) that were individually caged at the CNPRC with lights on from 06:00 to 18:00 h at 25–27 °C. The males were trained to chair restraint and semen was collected by direct penile stimulation with a Grass 6 stimulator equipped with EKG pad electrodes (30–50 V, 20 ms duration, 18 pulses/s) [49]. Ejaculates were collected in the morning every Tuesday and Thursday between October and November 2007, and one ejaculate was collected from each male every week. A total of 25 ejaculates were used in this study (1–4 ejaculates per male) and the mean volume of ejaculates ranged from 20 to 1200 μL (Table 1). Samples were allowed to liquefy for 30 min before processing. Epididymides were obtained from five males that were scheduled to necropsy for other research projects at the CNPRC, and sperm were extracted from the cauda epididymis as described previously by [11].
Table 1.
Basic characteristics of ejaculated semen of the nine males used in this study.
| Male number | Age (yr) | Weight (kg) | No. of ejaculate | Volume per ejaculate (μL) | Sperm density (×108/mL) | Motility before and after washing (%)
|
|
|---|---|---|---|---|---|---|---|
| Before | After | ||||||
| 1 | 18 | 13.4 | 1 | 1200 | 9.2 | 90 | 80 |
| 2 | 17 | 11.5 | 1 | 650 | 2.4 | 70 | 80 |
| 3 | 16 | 13.7 | 3 | 497 ± 145 | 5.7 ± 2.1 | 85 ± 5 | 85 ± 9 |
| 4 | 15 | 13.8 | 2 | 500, 800 | 18.2, 3.4 | 95, 90 | 99, 90 |
| 5 | 13 | 12.2 | 2 | 400, 20 | 22.0, 30.6 | 90, 70 | 90, 90 |
| 6 | 12 | 11.1 | 4 | 338 ± 442 | 41.4 ± 21.1 | 71 ± 21 | 85 ± 10 |
| 7 | 11 | 12.1 | 4 | 675 ± 119 | 7.3 ± 4.2 | 73 ± 15 | 93 ± 3 |
| 8 | 10 | 12.2 | 4 | 370 ± 184 | 11.2 ± 2.9 | 73 ± 10 | 88 ± 5 |
| 9 | 6 | 13.7 | 4 | 105 ± 42 | 27.0 ± 8.1 | 45 ± 17 | 90 ± 0 |
Values presented were mean ± SD. For males with two ejaculates, numbers separated by a comma indicate values for the first and second ejaculate, respectively.
A dilution of 1:20 (v/v) of semen to modified Tyrode’s medium supplemented with bovine serum albumin (TL-BSA, 290 mOsm/kg) [50] was used for sperm motility estimation of fresh semen, and a second dilution of 1:20 (v/v) of sperm to distilled water was used for hemacytometer counts (Hausser Scientific, Horsham, PA). The mean sperm density ranged from 2.4 to 41.4 × 108/mL (Table 1). Sperm suspensions were washed twice with TL-BSA at 300g for 10 min, and resuspended to 1 × 108 cells/mL of total motile sperm (sperm density × initial motility) with TEST solution (43.25 g TES, 10.265 g Tris, 10 g glucose in 1 L distilled water, pH 7.4, 350 mOsm/kg, modified from [49]) or TEST containing varying amounts of egg yolk depending on the design of each experiment. Unless specified otherwise, TEST-yolk generally refers to TEST with 20% egg yolk (v/v) in the present study. Suspended semen samples were then subjected to various treatments (detailed below). All chemicals used for preparation of solutions were of reagent grade (Sigma Chemical Corporation, St. Louis, Missouri).
Freezing and thawing procedure
Fifty microliter aliquants of sperm suspensions were drawn into 0.25-mL French straws (IMV International, Minneapolis) manually with a 1 cc syringe and were heat-sealed (MP-4 Impulse Sealer, Midwest Pacific). Straws were placed into a 600-mL glass beaker containing 500 mL of room temperature distilled water, and equilibrated at 4 °C in a refrigerator for 2 h before initiation of the freezing process. Freezing followed the methods described previously [10]. In brief, a Styrofoam box (inside dimensions: 33 × 24 × 23 cm) was filled with a depth of 4 cm liquid nitrogen and a 1 or 5 cm thick Styrofoam ‘boat’ was floated on top of it for 10 min, then straws were placed on top of the ‘boat’, and equilibrated for 10 min before being plunged into liquid nitrogen. The cooling rate was measured using a data logger thermometer (Type T thermocouple, Omega, Stamford, CT) with the wire inserted into a 0.25-mL straw filled with TEST-20% yolk-3% glycerol, and a minimum of five measurements were recorded. The average cooling rate from −10 °C to −70 °C was ~220 °C/min for the 1 cm boat and ~29 °C/min for the 5 cm boat. For post-thaw motility estimation, except for the thawing method experiments, three straws per treatment were thawed in a 37 °C water bath for 30 s (ISOTEMP 102, Fisher Scientific, Pittsburg, PA). Samples were evaluated after a minimum of 8 days storage in liquid nitrogen.
Sperm motility and capacitation estimation
A 10-μl drop of pre-freeze or post-thaw semen, covered with a 22 mm square coverglass, was visualized with 20 × positive-phase objective and a condenser setting of 100 (pseudo-dark field) on an Olympus BH-series phase-contrast microscope (Scientific Instrument Co, Sunnyvale, CA). An air curtain incubator (Sage Instruments, Model 279, Orion Research Inc., Cambridge, MA) maintained the microscope stage at 37 °C. The initial motility was in the range of 45%–93% before washing, and 80%–99% after washing (Table 1). Post-thaw motility was estimated without any dilution or washing immediately after thawing or after incubation at 37 °C in 5% CO2 in air for 1 h. Evaluation at 1 h post-thaw simulated the time required for insemination and fertilization in real practice. Forward progression was estimated with an adjusted motility index (AMI) as described previously [11]. In brief, the percent forward progression was subjectively estimated with a five point scale, and this was integrated with the percent motility into one number with the formula as follows: AMI = (Scale value/4) × percent motility. Samples were presented in random order each time so that the operator did not know their identity. The ability of sperm to capacitate after various treatments was measured based on an increase in intracellular calcium levels upon activation with cAMP and caffeine (detailed below).
Effect of egg yolk on freezing without permeable cryoprotectant
Ejaculated semen from six males was used in this experiment. Each ejaculate was divided into five samples for the following treatments: freeze without permeable cryoprotectant in TEST with 20%, 30%, 40%, and 50% egg yolk, and controls with 3% glycerol in TEST-yolk. Samples were frozen at 220 °C/min in liquid nitrogen vapor as described above or by immersing straws directly into liquid nitrogen. Post-thaw motility and forward progression were estimated on thawing and after incubation at 37 °C in 5% CO2 in air for 1 h.
Effect of freezing with and without permeable cryoprotectant
There were two trials in this experiment; for the first trial, ejaculates collected from nine males were used to compare the effect of the following treatments: 3% glycerol in TEST-yolk, 2% ethylene glycol in TEST-yolk, TEST with 20% egg yolk, TEST with 30% egg yolk, and TEST alone without egg yolk. For the second trial, sperm collected from epididymides of five males were used for the same treatments. Samples were frozen at 220 °C/min, and post-thaw motility and forward progression were estimated on thawing and after incubation at 37 °C in 5% CO2 in air for 1 h.
Effect of cooling rate on freezing without permeable cryoprotectant
Ejaculated sperm from six males were suspended in 3% glycerol in TEST-yolk (control), and TEST with 20% and 30% egg yolk without glycerol. Samples were cooled at 220 and 29 °C/min, and post-thaw motility and forward progression were estimated on thawing and after incubation at 37 °C in 5% CO2 in air for 1 h.
Effect of thawing on freezing without permeable cryoprotectant
Ejaculated sperm from five males were suspended in 3% glycerol in TEST-yolk (control), and TEST with 20% egg yolk without glycerol. Samples were cooled at 220 °C/min and thawed at three different conditions: in a 37 °C water bath for 30 s, at 23 °C room temperature air for 10 min, and in a 70 °C water bath for 5 s. Post-thaw motility and forward progression were estimated on thawing and after incubation at 37 °C in 5% CO2 in air for 1 h.
Effect of cryopreservation on intracellular calcium response upon activation
Sperm cryopreserved with 3% glycerol, 20% egg yolk, and TEST without egg yolk were washed once with TL-BSA at 300g for 10 min after thawing and resuspended to ~30 × 106 sperm/mL. Fresh sperm were washed twice and resuspended as above. Samples were incubated with 5 μM of the cell permeant calcium indicator dye, Fluo-4-AM ester (Invitrogen, Carlsbad, CA) in TL-BSA (final DMSO concentration of 0.5%), for 40 min at 37 °C and were washed twice by centrifugation at 300g for 5 min with TL-BSA to remove excess dye. Samples were resuspended in TL-H-PVA (modified Tyrodes with 10 mM HEPES and 0.1 mg/mL PVA based on [6]) containing 1% methyl cellulose alone or with the addition of cAMP/caffeine (1 mM each) and incubated another 20 min at 37 °C to allow de-esterification of the dye. Samples were prepared for live cell imaging by placing 10 μl drops on a glass bottom dish (0.17 mm; Electron Microscopy Sciences, Hatfield, PA) and covering the drops with warm mineral oil.
Images were taken with a Delta Vision system (Applied Precision, Issaquah, WA), a cooled charge-coupled device (CCD) camera (CH350; Roper Scientific/Princeton Instruments Inc., Trenton, NJ), and an Olympus IX70 microscope using a 60 × /1.2 water lens (UPlanApo), 1.5 × auxiliary lens and softWoRx 3.22 software suite. Images were scaled to the same minimum/maximum values, and pseudo-color was applied using Olympus MicroSuite Biological Suite FIVE, Build 1089, by applying a color look-up key that assigned specific RGB values to defined intensity ranges (representing 20 intensity units each). The resulting images varied from black (lowest intensity, 0–20 units) to blue, green, yellow, orange, red and white (highest intensity, 241–255). Images were processed in Adobe Photoshop CS by cropping and rotating for visual consistency.
Data analysis
Data were analyzed using repeated measures ANOVA (SAS 9.1). When a significant difference (P < 0.05) was observed among treatments, Tukey’s studentized range test was used for post-test comparisons. Percent motility was arcsine-square root transformed and means of three straws per treatment were used for analysis. Values presented are means ± SD.
Results
Effect of egg yolk on freezing without permeable cryoprotectant
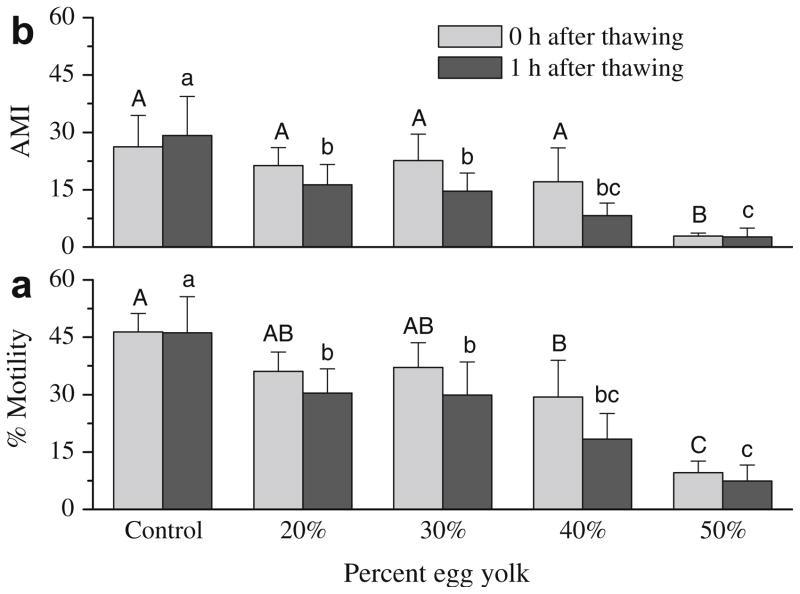
Directly plunging straws into liquid nitrogen resulted in no motility for all samples (data not shown). For samples cooled in liquid nitrogen vapor, motility at 0 h after thawing was not different (P > 0.05) between samples frozen with 20% (36.1 ± 5.0%) and 30% (37.1 ± 6.5%) egg yolk and controls with 3% glycerol (46.4 ± 4.8%) (Fig. 1a). However, samples frozen with 20% (30.4 ± 6.3%) and 30% (29.9 ± 8.6%) egg yolk had lower (P < 0.05) motility than controls with glycerol (46.2 ± 9.4%) after 1 h incubation at 37 °C. Similar trends were observed for AMI (Fig. 1b). Samples frozen with 40% and 50% egg yolk without glycerol had significantly lower (P < 0.05) motility and AMI than those of 20% egg yolk or controls with glycerol, especially at 1 h after thawing.
Fig. 1.
Post-thaw motility (a) and adjusted motility index (AMI) (b) of rhesus monkey ejaculated sperm samples frozen in 3% glycerol–TEST-yolk (control), and in TEST with 20%, 30%, 40%, and 50% egg yolk without glycerol. Bars sharing the same letter indicate no significant difference.
Effect of freezing with and without permeable cryoprotectant
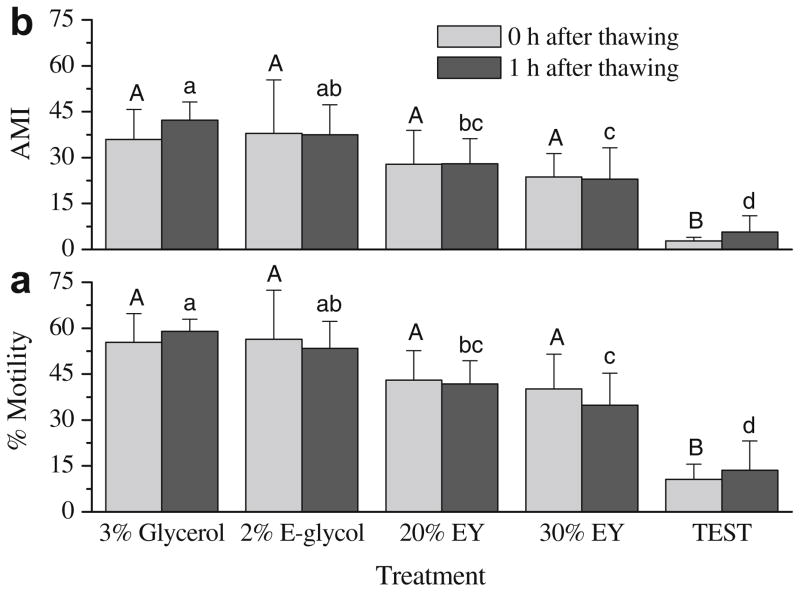
For ejaculated sperm samples (Fig. 2), similar to the previous experiment, motility at 0 h after thawing was not different (P > 0.05) between samples frozen in 20% egg yolk (43.0 ± 9.7%) and in 3% glycerol (55.4 ± 9.3%) or 2% ethylene glycol (56.4 ± 16.0) (Fig. 2a). After 1 h incubation at 37 °C, motility (41.8 ± 7.5%) and AMI (28.0 ± 8.2) of samples frozen with 20% egg yolk were significantly lower (P < 0.05) than that of 3% glycerol (59.0 ± 3.9% for motility; 42.2 ± 6.0 for AMI). The lowest motility (10.6 ± 5.0%) and AMI (2.8 ± 1.2) were observed in samples frozen with TEST alone (without egg yolk) at 0 h after thawing.
Fig. 2.
Post-thaw motility (a) and adjusted motility index (AMI) (b) of rhesus monkey ejaculated sperm samples frozen with 3% glycerol, 2% ethylene glycol (E-glycol), and without permeable cryoprotectants–TEST with 20% (20% EY), 30% egg yolk (30% EY), and TEST alone. Bars sharing the same letter indicate no significant difference.
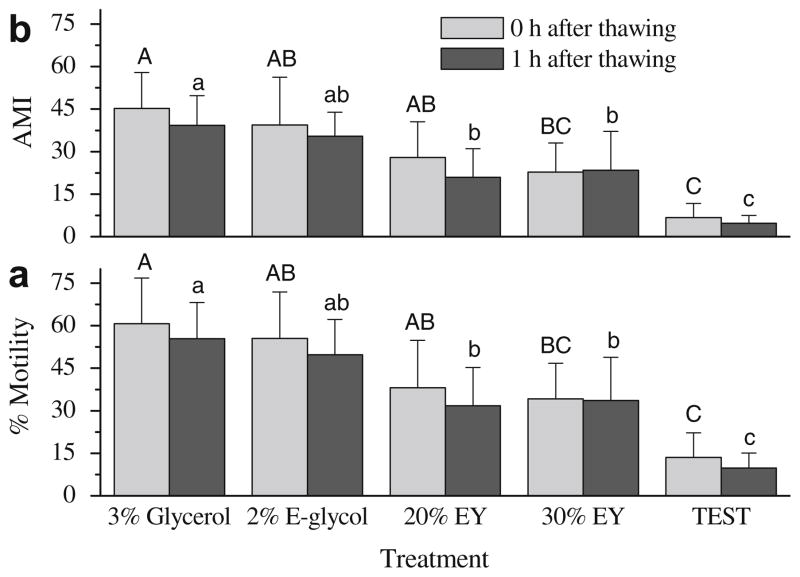
The same trend was observed for epididymal sperm (Fig. 3). Samples frozen in 20% egg yolk (38.1 ± 16.7%) had similar motility at 0 h after thawing compared to that in 3% glycerol (60.7 ± 16.1%) or 2% ethylene glycol (55.5 ± 16.4%). At 1 h after thawing, motility (31.8 ± 13.4%) and AMI (20.9 ± 10.1) of samples with 20% egg yolk were significantly lower than those of samples with 3% glycerol (55.4 ± 12.8% for motility; 39.3 ± 10.4 for AMI). The lowest motility (9.8 ± 5.3%) and AMI (4.7 ± 2.8) were observed in samples frozen with TEST alone at 1 h after thawing.
Fig. 3.
Post-thaw motility (a) and adjusted motility index (AMI) (b) of rhesus monkey epididymal sperm samples frozen with 3% glycerol, 2% ethylene glycol (E-glycol), and without permeable cryoprotectants–TEST with 20% (20% EY), 30% egg yolk (30% EY), and TEST alone. Bars sharing the same letter indicate no significant difference.
Effect of cooling rate on freezing without permeable cryoprotectant
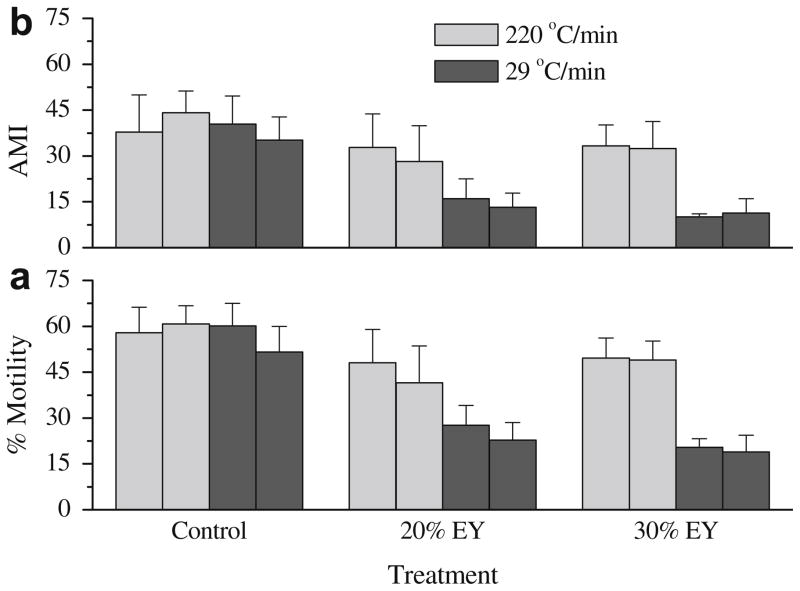
For freezing trials in 20% and 30% egg yolk, samples cooled at 220 °C/min had significantly higher (P < 0.05) motility and AMI at 0 h and 1 h after thawing than those cooled at 29 °C/min (Fig. 4). However, for freezing trials with 3% glycerol, there were no differences (P > 0.05) in motility and AMI between samples cooled at 220 and 29 °C/min (Fig. 4).
Fig. 4.
Post-thaw motility (a) and adjusted motility index (AMI) (b) of rhesus monkey ejaculated sperm samples frozen with 3% glycerol (control), and without glycerol in TEST with 20% (20% EY) and 30% egg yolk (30% EY). Samples were cooled at 220 and 29 °C/min. Post-thaw motility and AMI were estimated after incubation at 37 °C in 5% CO2 in air for 0 h (left bars of the same color) and 1 h (right bars of the same color).
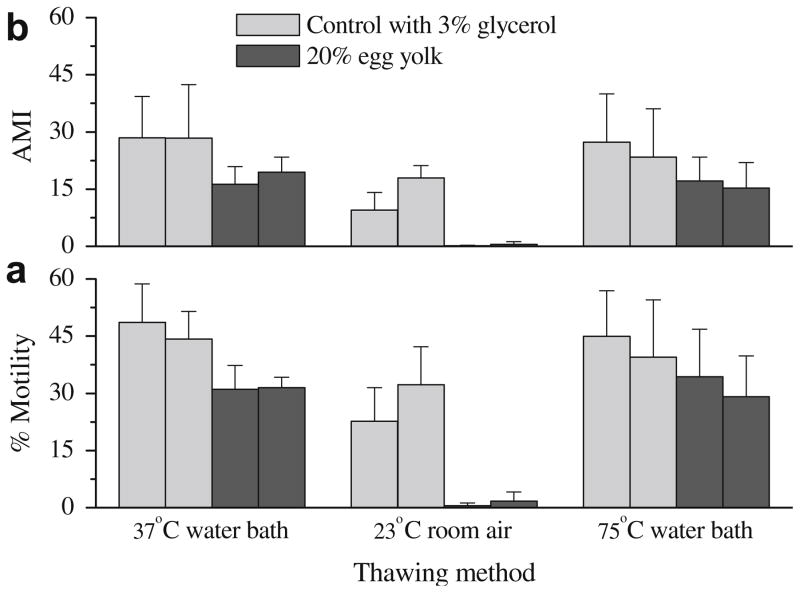
Effect of thawing on freezing without permeable cryoprotectant
For freezing trials in 20% egg yolk (Fig. 5), there were no differences (P > 0.05) in motility and AMI at 0 and 1 h after thawing for samples thawed in a 37 °C water bath for 30 s and in a 70 °C water bath for 5 s, however, both were significantly higher (P < 0.05) than those thawed at 23 °C room temperature air (<5% for motility, and <1 for AMI). Similar trends were observed for controls with 3% glycerol, but thawing at 23 °C room temperature air still yielded 32.3 ± 9.9% motility and 18.0 ± 3.3 AMI when estimated after 1 h incubation at 37 °C.
Fig. 5.
Post-thaw motility (a) and adjusted motility index (AMI) (b) of rhesus monkey ejaculated sperm samples frozen with 3% glycerol and without glycerol in TEST with 20% egg yolk, and thawed with three different methods: in a 37 °C water bath for 30 s, at 23 °C room temperature air for 10 min, and in a 70 °C water bath for 5 s. Samples were cooled at 220 °C/min. Post-thaw motility and AMI were estimated after incubation at 37 °C in 5% CO2 in air for 0 h (left bars of the same color) and 1 h (right bars of the same color).
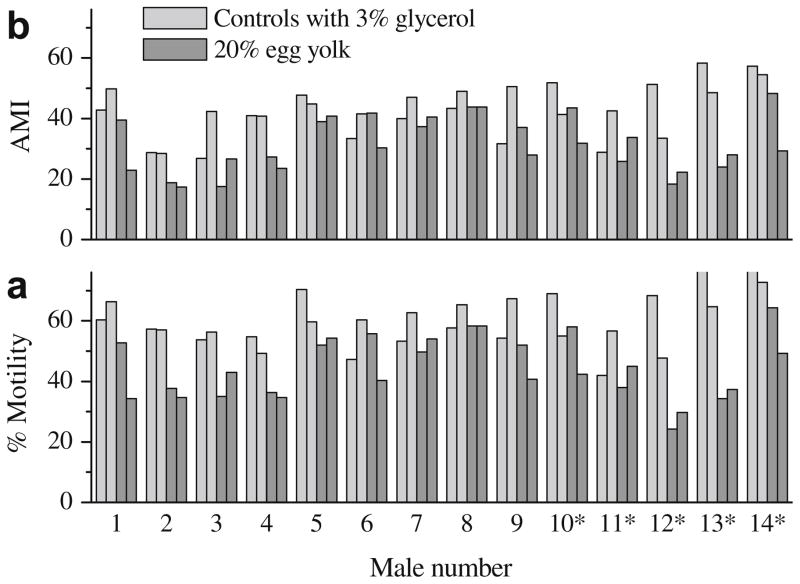
Male variations
The highest post-thaw motility and AMI for each individual male (n = 14) from the above experimental trials were compared (Fig. 6). When normalized with the post-thaw motility of controls with 3% glycerol, the recovery rate (mean ± SD) for freezing with 20% egg yolk was 79 ± 22% at 0 h and 71 ± 12% at 1 h after thawing. The recovery for forward progressive motility estimated by AMI was 82 ± 25% at 0 h and 68 ± 14% at 1 h after thawing. For some males, similar protection was derived from egg yolk alone or control with glycerol (e.g., male 8), while others showed inferior motility immediately after thawing, but better motility after 1 h incubation (e.g., male 12).
Fig. 6.
The highest post-thaw motility (a) and adjusted motility index (AMI) (b) of rhesus monkey sperm obtained from 14 individual males (* indicate epididymal sperm sample). Samples were suspended in TEST with 20% egg yolk without glycerol and 3% glycerol (control), and cooled at 220 °C/min. Post-thaw motility and AMI were estimated after incubation at 37 °C in 5% CO2 in air for 0 h (left bars of the same color) and 1 h (right bars of the same color).
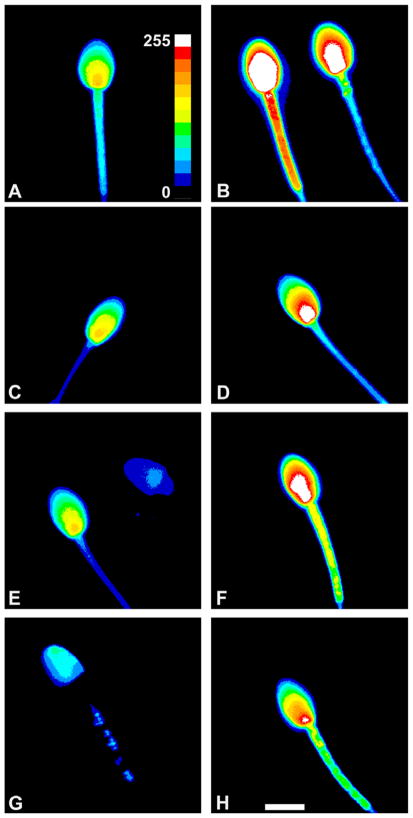
Effect of cryopreservation on intracellular calcium response upon activation
Sperm capitation is an important step towards successful fertilization, and calcium plays an important role in this process. To determine if sperm cryopreserved by the methods used in this study were capable of exhibiting an increase in intracellular calcium upon activation, sperm frozen in either 3% glycerol, 20% egg yolk or TEST without egg yolk were thawed, loaded with Fluo-4 and imaged (Fig. 7). Fresh sperm were also included for comparison (Fig. 7A and B). The basal level of intracellular calcium detected in non-activated samples was low, and varied from almost non-detectable (see sperm head in the right top corner of E) to a higher calcium level (as in A, C and sperm on the left in E). The majority of thawed motile sperm that had been frozen in either 3% glycerol (D) or 20% egg yolk (F) exhibited an increase in calcium upon activation compared to the non-activated (Fluo-4 only) samples. Sperm from these treatments not only responded similarly to each other but also to fresh, activated sperm (B). The calcium level detected in activated samples varied from a moderate increase (Fig. 7H) to a robust increase (Fig. 7B) based on the increased level of fluorescence observed. Based on the pseudo-colored images, calcium increased the most in the post-acrosomal or basal region of the sperm head, and in some sperm this extended to the apical sperm head and into the midpiece. Approximately, 90% of the sperm that had been cryopreserved in TEST alone (G, H) were found to be non-motile. Regardless of treatment, non-motile sperm typically exhibited a specific calcium pattern with discrete spots of calcium in the midpiece and no detectable calcium in the basal sperm head (G). However, the small portion of motile sperm thawed after freezing in TEST alone showed an increase in their calcium level (H) when activated with cAMP and caffeine.
Fig. 7.
Fresh and cryopreserved sperm exhibit similar intracellular calcium increases after activation. Fresh sperm (A, B) and sperm that were cryopreserved in either 3% glycerol (C, D), TEST with 20% egg yolk (E, F) or TEST alone (G, H) and thawed were incubated with the calcium indicator dye Fluo-4 (A, C, E and G) or with Fluo-4 containing 1 mM cAMP and caffeine (B, D, F, H). Live sperm samples were imaged using a Delta Vision system with a 60× water objective lens with 1.5× auxillary magnification, and were pseudo-colored as described in the methods section. The inset in (A) shows the color look-up key used to colorize the images, and the scale bar in (H) is 4 μm.
Discussion
Similar to studies with human sperm, glycerol in combination with TEST-yolk is by far the most widely used and successful cryoprotectant for sperm of non-human primates. The present study demonstrated for the first time that ejaculated and epididymal sperm from rhesus macaque could be successfully cryopreserved in TEST-yolk extender in the absence of permeable cryoprotectants such as glycerol or ethylene glycol. Although the post-thaw motility of samples frozen with egg yolk alone was not as good as controls with glycerol, the recovery rates were approximately 70–80% of the controls, which is maintained even after samples were incubated for 1 h after thawing. Cynomolgus macaques (M. fascicularis) are similar to rhesus in many aspects including sperm biology; however, early studies with pellet freezing on dry ice of sperm from cynomolgus have indicated the necessity of glycerol as there was no motility obtained after freezing when glycerol was omitted from the egg yolk (20%)-lactose (11%) extender [32]. This discrepancy may rely on different methods employed between these two studies. As illustrated in this study, the feasibility of freezing rhesus macaque sperm without glycerol depends on the presence and amount of egg yolk in the extender, rapid cooling rate (220 °C/min), fast thawing, and possibly the extended pre-cooling and equilibration period (~2 h) as it minimizes the effects of temperature shock [10].
Despite the failure in cynomolgus monkey sperm [32], earlier studies of pellet freezing on dry ice have shown success with bovine sperm suspended in 20% egg yolk without glycerol [4,15,37,38]. However, these researchers suggested that the sugar component of their extender offered the protection, not the egg yolk. Research done by Pace and Graham [41] revealed similar results on bovine sperm freezing in the absence of glycerol, but indicated that egg yolk was the main component that offered the protection. This was confirmed by the present study, in which TEST (containing glucose) alone only yielded minimal post-thaw motility, while TEST plus glycerol offered some protection, but not as much as TEST-yolk (data not shown). Similar to this effect of TEST alone, the present study has also shown that excessive egg yolk offers little protection, suggesting that the protective role of egg yolk is dose dependent. Egg yolk has long been recognized for protecting sperm cells from cold shock [41]. However, the discovery of glycerol as a protective agent in 1949 has essentially diverted researchers from the study of the protective role of egg yolk. Recently, the interest on the protective role of egg yolk has been resumed and several mechanisms involving its function for sperm protection have been proposed [1,3,36]. However, these hypotheses have not been tested and verified, and its protection mechanism remains elusive. Findings in the current study highlight the importance of revealing such mechanisms.
Permeable cryoprotectants are believed to help lower the freezing point of the solution, minimize osmotic shock by replacing the water inside the cell, and reduce formation of lethal intracellular ice [9,44]. Meanwhile, they also cause damage to cells due to their chemical toxicity and osmotic stress during addition before cooling and removal after thawing [8,16]. Prior to the discovery of glycerol as a protective agent, success was reported for frog sperm with vitrification in the absence of cryoprotectant [31]. Despite that, sperm vitrification has seldom been found to be practical due to the lack of an appropriate container, and the belief that vitrification could only be achieved with high concentrations of cryoprotectant that were lethal to most mammalian sperm [21]. Recent progress in embryo freezing and thawing techniques (e.g., [25,27]) has inspired studies of freezing low numbers of sperm in patients with severe oligozoospermia [47], which involved cooling samples very rapidly with or, preferably without permeable cryoprotectant [19]. In the latter study, moderate success was achieved when samples were frozen rapidly in a small drop (10 μl) with a sucrose solution in the absence of permeable cryoprotectants. Supported with the results presented in this study, it is reasonable to suggest that rapid cooling is one of the key factors for freezing success without permeable cryoprotectants. However, earlier studies have shown that mouse spermatozoa can be frozen successfully at a relatively slow cooling rate of 20–40 °C/min in skimmed milk and raffinose [26,39,55]. Most likely, it is the overall combination of sperm properties, extenders, cooling, and thawing that determines the final outcome of freezing without permeable cryoprotectants.
All previous studies involving rapid freezing without permeable cryoprotectants employed a small sample volume through the use of specially designed carriers such as cryoloops, micro-drops, or pellets. In contrast, the present study used a standard 0.25-mL straw loaded with 50 or 200 μl sperm suspension. If the success in all previous studies was mainly attributed to the very small sample size, then success obtained in this study with regular 0.25-mL straw could suggest that rhesus monkey sperm may have high membrane elasticity in the presence of egg yolk, and could better cope with the stress induced by the freezing and thawing process. In the future, comparisons of freezing with the exact same methods among monkeys, humans, and other species would allow us to better understand the differences in tolerance to cryopreservation procedures. Moreover, the present study also shows that rhesus monkey sperm suspended in 0.25-mL straws resulted in 0% motility when plunging directly into liquid nitrogen regardless of treatments. This was in agreement with findings on human sperm where vitrification in standard 0.25-mL straws yielded almost 100% non-motile sperm [40], perhaps because the thawing rate for specimens plunged directly into liquid nitrogen was not rapid enough to prevent ice crystals from forming [47]. This idea is supported by a study in which human sperm vitrified with the copper loop method resulted in no survival if not warmed rapidly enough [24].
The present study also indicated male variation in response to freezing without permeable cryoprotectants (Fig. 6); some males demonstrated equally good post-thaw motility as the control treatment with glycerol, while others had only ~50% of the controls. Males with good post-thaw motility after freezing without glycerol may have a better chance to succeed in standard AI procedures because of the lack of influence from the cryoprotectant. Our next step is to test this hypothesis. Because environmental influences might adversely affect the genetic quality of the oocyte [20], the absence of glycerol in frozen–thawed sperm may also be beneficial for other assisted reproduction techniques such as IVF or ICSI, especially since ICSI is widely used for human clinical IVF practices. In addition, it is possible to further improve this method through procedure modifications such as pre-selection of only highly motile sperm [12,13], optimizing equilibration methods, or finding ways to increase the cooling or thawing rates. Subsequently, it may allow more males with moderate cryodamage susceptibility to be cryopreserved equally successfully with extenders in the absence and presence of permeable cryoprotectant. Future studies will be needed to explore these possibilities.
In addition to motility, another measure of sperm function is the ability to undergo capacitation, a complex series of maturation events and a prerequisite to fertilization in mammalian species [52,53]. Calcium is thought to be one of the key regulators of the signaling pathways important to this process [17,52]. Increased intracellular calcium in bovine sperm has been detected after stimulation with NH4Cl [33] and is important for achieving hyperactivated motility [18,48], a process associated with capacitation. Macaque sperm require an incubation period with caffeine and cAMP in order to capacitate in vitro [7,51]. In the present study, sperm frozen with egg yolk in the absence and presence of glycerol displayed similar increased calcium levels after thawing and activation, which was also comparable to fresh (non-frozen), activated sperm. To our knowledge, this study for the first time detected an increase in intracellular calcium in monkey sperm after activation with cAMP and caffeine compared to non-activated sperm. This suggests that calcium may also be a regulator of the events of capacitation in monkey sperm.
Due to the expense of AI trials in monkeys, AI success is not an endpoint in this study; however, our results were convincing as motility and forward progression could last for more than 1 h without significant loss after thawing. In addition, frozen–thawed sperm demonstrated similar patterns of calcium response upon activation as non-frozen fresh samples. Motility has been found to be the most sensitive indicator of sperm quality in non-human primates. For example, post-thaw motility has been found to be more sensitive than the sperm penetration assay in the lowland Gorilla [43], is positively correlated with membrane integrity in cynomolgus macaques [14,49], and is the most convincing parameter for sperm function analysis when compared with sperm acrosome integrity (evaluated with FITC-PNA) [29]. More recently, analysis of chromosome damage in rhesus macaques suggested no differences between fresh and frozen–thawed sperm when motile sperm were selected for ICSI [28]. In the present study, the motile sperm of frozen–thawed samples (regardless of treatment) and fresh controls displayed similar increased intracellular calcium level when activated with cAMP and caffeine. All these findings further confirmed that motility is a simple and reliable measure of sperm quality after thawing in monkey sperm.
In summary, the present study demonstrates that ejaculated and epididymal sperm from rhesus monkeys can be cryopreserved with TEST-yolk (20%) in the absence of permeable cryoprotectant when samples were loaded in a standard 0.25-mL straw, cooled rapidly in liquid nitrogen vapor at 220 °C/min, and thawed rapidly in a 37 °C water bath. This study also represents the first success of freezing without permeable cryoprotectant in non-human primates.
Acknowledgments
We thank S.E. Rodenburg and D.L. Hill for assistance with data collection and critical review.
Footnotes
This work was supported by NIH Grants RR00169 and RR13439.
References
- 1.Amirat L, Tainturier D, Jeanneau L, Thorin C, Gerard O, Courtens JL, Anton M. Bull semen in vitro fertility after cryopreservation using egg yolk LDL: a comparison with Optidyl, a commercial egg yolk extender. Theriogenology. 2004;61:895–907. doi: 10.1016/s0093-691x(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 2.Bedford SJ, Jasko DJ, Graham JK, Amann RP, Squires EL, Pickett BW. Effect of seminal extenders containing egg yolk and glycerol on motion characteristics and fertility of stallion spermatozoa. Theriogenology. 1995;43:955–967. doi: 10.1016/0093-691x(95)00045-a. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron A, Manjunath P. New insights towards understanding the mechanisms of sperm protection by egg yolk and milk. Mol Reprod Dev. 2006;73:1338–1344. doi: 10.1002/mrd.20565. [DOI] [PubMed] [Google Scholar]
- 4.Berndston WE, Foote RH. The freezability of spermatozoa after minimal pre-freezing exposure to glycerol or lactose. Cryobiology. 1972;9:57–60. doi: 10.1016/0011-2240(72)90010-7. [DOI] [PubMed] [Google Scholar]
- 5.Blaxter T. Sperm storage and cross-fertilization of spring and autumn spawning herring. Nature. 1953;172:1189–1190. [Google Scholar]
- 6.Boatman DE. In vitro growth of non-human primate pre-and peri-implantation embryos. In: Bavister BD, editor. The Mammalian Preimplantation Embryo. Plenum Press; New York: 1987. pp. 273–308. [Google Scholar]
- 7.Boatman DE, Bavister BD. Stimulation of rhesus monkey sperm capacitation by cyclic nucleotide mediators. J Reprod Fertil. 1984;84:357–366. doi: 10.1530/jrf.0.0710357. [DOI] [PubMed] [Google Scholar]
- 8.Critser JK, Huse Benda AR, Aaker DV, Arneson BW, Ball GD. Cryopreservation of human spermatozoa III. The effect of cryoprotectants on motility. Fertil Steril. 1988;50:314–320. [PubMed] [Google Scholar]
- 9.Doebbler GF. Cryoprotective compounds – review and discussion of structure and function. Cryobiology. 1966;3:2–11. doi: 10.1016/s0011-2240(66)80144-x. [DOI] [PubMed] [Google Scholar]
- 10.Dong Q, Rodenburg SE, Huang C, VandeVoort CA. Effect of pre-freezing conditions on semen cryopreservation of rhesus monkey. Theriogenology. 2008;70:61–69. doi: 10.1016/j.theriogenology.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong Q, Rodenburg SE, Huang C, VandeVoort CA. Cryopreservation of rhesus monkey (Macaca mulatta) epididymal spermatozoa before and after refrigerated storage. J Androl. 2008;29:283–292. doi: 10.2164/jandrol.107.003921. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly E, McClure N, Lewis EM. Cryopreservation of human semen and prepared sperm: effect on motility parameters and DNA integrity. Fertil Steril. 2001;76:892–900. doi: 10.1016/s0015-0282(01)02834-5. [DOI] [PubMed] [Google Scholar]
- 13.Esteves SC, Sharma RK, Thomas AJ, Agarwal A. Improvement in motion characteristics and acrosome status in cryopreserved human spermatozoa by swim-up processing before freezing. Hum Reprod. 2000;15:2173–2179. doi: 10.1093/humrep/15.10.2173. [DOI] [PubMed] [Google Scholar]
- 14.Feradis AH, Pawitri D, Suatha IK, Amin MR, Yusuf TL, Sajuthi D, Budiarsa IN, Hayes ES. Cryopreservation of epididymal spermatozoa collected by needle biopsy from cynomolgus monkeys (Macaca fascicularis) J Med Primatol. 2001;30:100–106. doi: 10.1034/j.1600-0684.2001.300205.x. [DOI] [PubMed] [Google Scholar]
- 15.Gibson CD, Graham EF. The relationship between fertility and post-freeze motility of bull spermatozoa (by pellet freezing) without glycerol. J Reprod Fertil. 1969;20:155–157. doi: 10.1530/jrf.0.0200155. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore JA, Liu J, Gao DY, Critser JK. Determination of optimal cryoprotectants and procedures for their addition and removal from human spermatozoa. Hum Reprod. 1997;12:112–118. doi: 10.1093/humrep/12.1.112. [DOI] [PubMed] [Google Scholar]
- 17.Handrow RR, First NL, Parrish JJ. Calcium requirement and increased association with bovine sperm during capacitation by heparin. J Exp Zool. 1989;252:174–182. doi: 10.1002/jez.1402520209. [DOI] [PubMed] [Google Scholar]
- 18.Ho HC, Suarez SS. An inositol 1,4,5-triphosphate receptor-gated intracellular Ca(2+) store is involved in regulating sperm hyperactivated motility. Biol Reprod. 2001;65:1606–1615. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- 19.Hossain AM, Osuamkpe CO. Sole use of sucrose in human sperm cryopreservation. Arch Androl. 2007;53:99–103. doi: 10.1080/01485010701225675. [DOI] [PubMed] [Google Scholar]
- 20.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Isachenko E, Isachenko V, Katkov II, Dessole S, Nawroth F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: from past practical difficulties to present success. Reprod BioMed Online. 2003;6:191–200. doi: 10.1016/s1472-6483(10)61710-5. [DOI] [PubMed] [Google Scholar]
- 22.Isachenko E, Isachenko V, Katkov II, Rahimi G, Schondorf T, Mallmann P, Dessole S, Nawroth F. DNA integrity and motility of human spermatozoa after standard slow freezing versus cryoprotectant-free vitrification. Hum Reprod. 2004;19:932–939. doi: 10.1093/humrep/deh194. [DOI] [PubMed] [Google Scholar]
- 23.Isachenko V, Isachenko E, Katkov II, Montag M, Dessole S, Nawroth F, van der Ven H. Cryoprotectant-free cryopreservation of human spermatozoa by vitrification and freezing in vapor: effect on motility, DNA integrity, and fertilization ability. Biol Reprod. 2004;71:1167–1173. doi: 10.1095/biolreprod.104.028811. [DOI] [PubMed] [Google Scholar]
- 24.Isachenko V, Isachenko E, Montag M, Zaeva V, Krivokharchenko I, Nawroth F, Dessole S, Katkov II, van der Ven H. Clean technique for cryoprotectant-free vitrification of human spermatozoa. Reprod BioMed Online. 2005;10:350–354. doi: 10.1016/s1472-6483(10)61795-6. [DOI] [PubMed] [Google Scholar]
- 25.Kattera S, Chen C. A modified embryo cryopreservation method increases post-thaw survival with a concomitant increase in implantation. Fertil Steril. 2005;84:1498–1504. doi: 10.1016/j.fertnstert.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 26.Koshimoto C, Gamliel E, Mazur P. Effect of osmolality and oxygen tension on the survival of mouse sperm frozen to various temperatures in various concentrations of glycerol and raffinose. Cryobiology. 2000;41:204–231. doi: 10.1006/cryo.2000.2281. [DOI] [PubMed] [Google Scholar]
- 27.Lane M, Schoolcraft WB, Gardner DK. Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. Fertil Steril. 1999;73:1073–1078. doi: 10.1016/s0015-0282(99)00418-5. [DOI] [PubMed] [Google Scholar]
- 28.Li MW, Meyers S, Tollner TL, Overstreet JW. Damage to chromosomes and DNA of rhesus monkey sperm following cryopreservation. J Androl. 2007;28:493–501. doi: 10.2164/jandrol.106.000869. [DOI] [PubMed] [Google Scholar]
- 29.Li YH, Cai KJ, Kovacs A, Ji WZ. Effects of various extenders and permeating cryoprotectants on cryopreservation of cynomolgus monkey (Macaca fascicularis) spermatozoa. J Androl. 2005;26:387–395. doi: 10.2164/jandrol.04147. [DOI] [PubMed] [Google Scholar]
- 30.Long JA, Kulkarni G. An effective method for improving the fertility of glycerol-exposed poultry semen. Poultry Sci. 2004;83:1594–1601. doi: 10.1093/ps/83.9.1594. [DOI] [PubMed] [Google Scholar]
- 31.Luyet BJ, Hodapp A. Revival of frog spermatozoa vitrified in liquid air. Proc Meet Soc Exp Biol. 1938;39:433–434. [Google Scholar]
- 32.Mahone JP, Dukelow WR. Semen preservation in Macaca fascicularis. Lab Anim Sci. 1978;28:556–561. [PubMed] [Google Scholar]
- 33.Marquez B, Suarez SS. Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+ influx. Biol Reprod. 2007;76:660–665. doi: 10.1095/biolreprod.106.055038. [DOI] [PubMed] [Google Scholar]
- 34.Morrell JM, Hodges JK. Cryopreservation of non-human primate sperm: priorities for future research. Anim Reprod Sci. 1998;53:43–63. doi: 10.1016/s0378-4320(98)00126-2. [DOI] [PubMed] [Google Scholar]
- 35.Mounib MS. Cryogenic preservation of fish and mammalian spermatozoa. J Reprod Fertil. 1978;53:13–18. doi: 10.1530/jrf.0.0530013. [DOI] [PubMed] [Google Scholar]
- 36.Moussa M, Martinet V, Trimeche A, Tainturier D, Anton M. Low density lipoproteins extracted from hen egg yolk by an easy method: cryoprotective effect on frozen–thawed bull semen. Theriogenology. 2002;57:1695–1706. doi: 10.1016/s0093-691x(02)00682-9. [DOI] [PubMed] [Google Scholar]
- 37.Nagase H, Niwa T. Deep freezing bull semen in concentrated pellet form. I. Factors affecting survival of spermatozoa. V Int. Congr. Anim. Reprod. Artif. Insem; Trento, Italy. 1964. pp. 410–415. [Google Scholar]
- 38.Nagase H, Yamashita S, Irie S. Protective effect of sugars against freezing injury of bull spermatozoa. VI Int. Congr. Anim. Reprod. Artif. Insem; Paris, France. 1968. pp. 1111–1113. [Google Scholar]
- 39.Nakagata N, Takeshima T. High fertilizing ability of mouse spermatozoa diluted slowly after cryopreservation. Theriogenology. 1992;37:1263–1291. [Google Scholar]
- 40.Nawroth F, Isachenko V, Dessole S, Rahimi G, Farina M, Vargiu N, Mallmann P, Dattena M, Capobianco G, Peters D, Orth I, Isachenko E. Vitrification of human spermatozoa without cryoprotectants. CryoLetters. 2002;23:93–102. [PubMed] [Google Scholar]
- 41.Pace MM, Graham EF. Components in egg yolk which protect bovine sperm during freezing. J Anim Sci. 1974;39:1144–1149. doi: 10.2527/jas1974.3961144x. [DOI] [PubMed] [Google Scholar]
- 42.Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164:666. doi: 10.1038/164666a0. [DOI] [PubMed] [Google Scholar]
- 43.Pope CE, Dresser BL, Chin NW, Liu JH, Loskutoff NM, Behnke EJ, Brown C, McRae MA, Sinoway CE, Campbell MK, Cameron KN, Owens OM, Johnson CA, Evans RR, Cedars MI. Birth of a western lowland gorilla (Gorilla gorilla) following in vitro fertilization and embryo transfer. Amer J Primatol. 1997;41:247–260. doi: 10.1002/(SICI)1098-2345(1997)41:3<247::AID-AJP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 44.Rowe AW. Biochemical aspects of cryoprotective agents in freezing and thawing. Cryobiology. 1966;3:12–18. doi: 10.1016/s0011-2240(66)80145-1. [DOI] [PubMed] [Google Scholar]
- 45.Royere D, Barthelemy C, Hamamah S, Lansac J. Cryopreservation of spermatozoa: a 1996 review. Hum Reprod Update. 1996;2:553–559. doi: 10.1093/humupd/2.6.553. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez Partida LG, Maginnis G, Dominko T, Martinovich C, McVay B, Fanton J, Schatten G. Live rhesus offspring by artificial insemination using fresh sperm and cryopreserved sperm. Biol Reprod. 2000;63:1092–1097. doi: 10.1095/biolreprod63.4.1092. [DOI] [PubMed] [Google Scholar]
- 47.Schuster T, Keller LM, Dunn RL, Ohl DA, Smith GD. Ultra-rapid freezing of very low numbers of sperm using cryoloops. Hum Reprod. 2003;18:788–795. doi: 10.1093/humrep/deg162. [DOI] [PubMed] [Google Scholar]
- 48.Suarez SS, Dai X. Intracellular calcium reaches different levels of elevation in hyperactivated and acrosome-reacted hamster sperm. Mol Reprod Dev. 1995;42:325–333. doi: 10.1002/mrd.1080420310. [DOI] [PubMed] [Google Scholar]
- 49.Tollner TL, VandeVoort CA, Overstreet JW, Drobnis EZ. Cryopreservation of spermatozoa from cynomolgus monkeys (Macaca fascicularis) J Reprod Fertil. 1990;90:347–352. doi: 10.1530/jrf.0.0900347. [DOI] [PubMed] [Google Scholar]
- 50.VandeVoort CA. High quality sperm for nonhuman primate ART: production and assessment. Reprod Biol Endocrinol. 2004;16:33. doi: 10.1186/1477-7827-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.VandeVoort CA, Tollner TL, Overstreet JW. Separate effects of caffeine and dbcAMP on macaque sperm motility and interaction with the zona pellucida. Mol Reprod Dev. 1994;37:299–304. doi: 10.1002/mrd.1080370309. [DOI] [PubMed] [Google Scholar]
- 52.Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- 53.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 189–317. [Google Scholar]
- 54.Yeoman RR, Mitalipov S, Gerami-Naini B, Nusser KD, Wolf DP. Low temperature storage of rhesus monkey spermatozoa and fertility evaluation by intracytoplasmic injection. Theriogenology. 2005;63:2356–2371. doi: 10.1016/j.theriogenology.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 55.Yokoyama M, Akiba H, Katsuki M, Nomura T. Production of normal young following transfer of mouse embryos obtained by in vitro fertilization using cryopreserved spermatozoa. Jikken Dobutsu. 1990;39:125–128. doi: 10.1538/expanim1978.39.1_125. [DOI] [PubMed] [Google Scholar]