Abstract
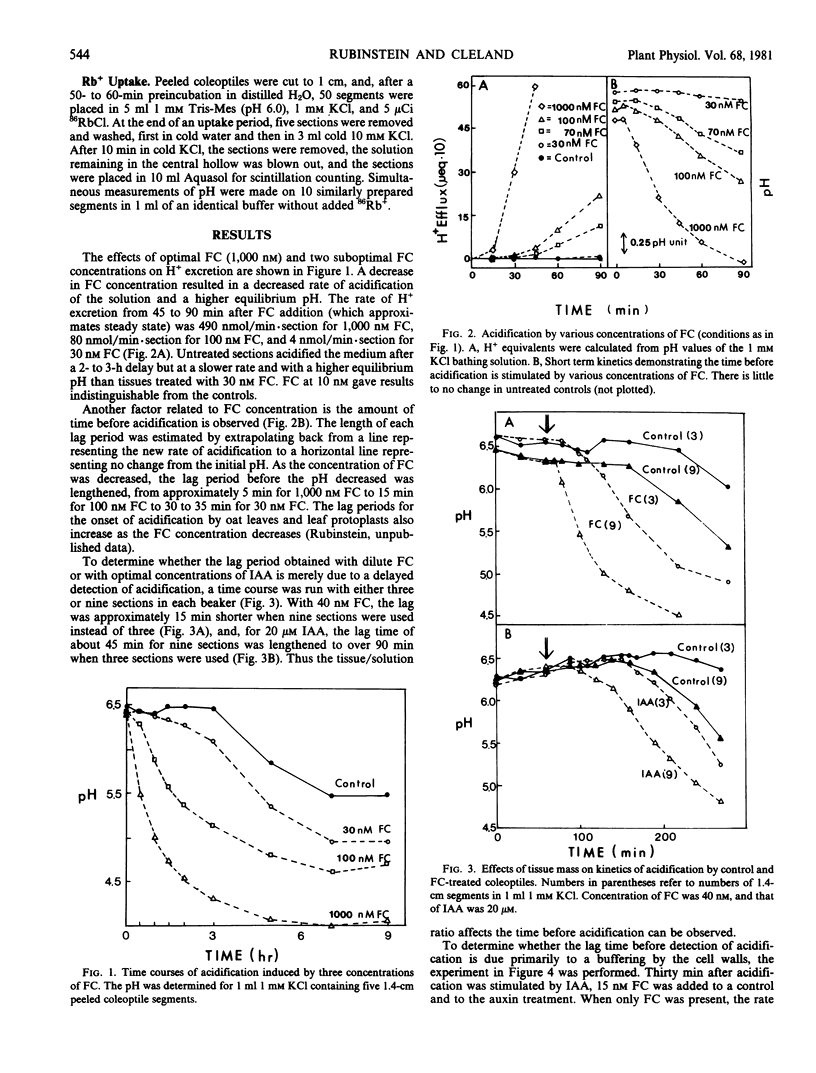
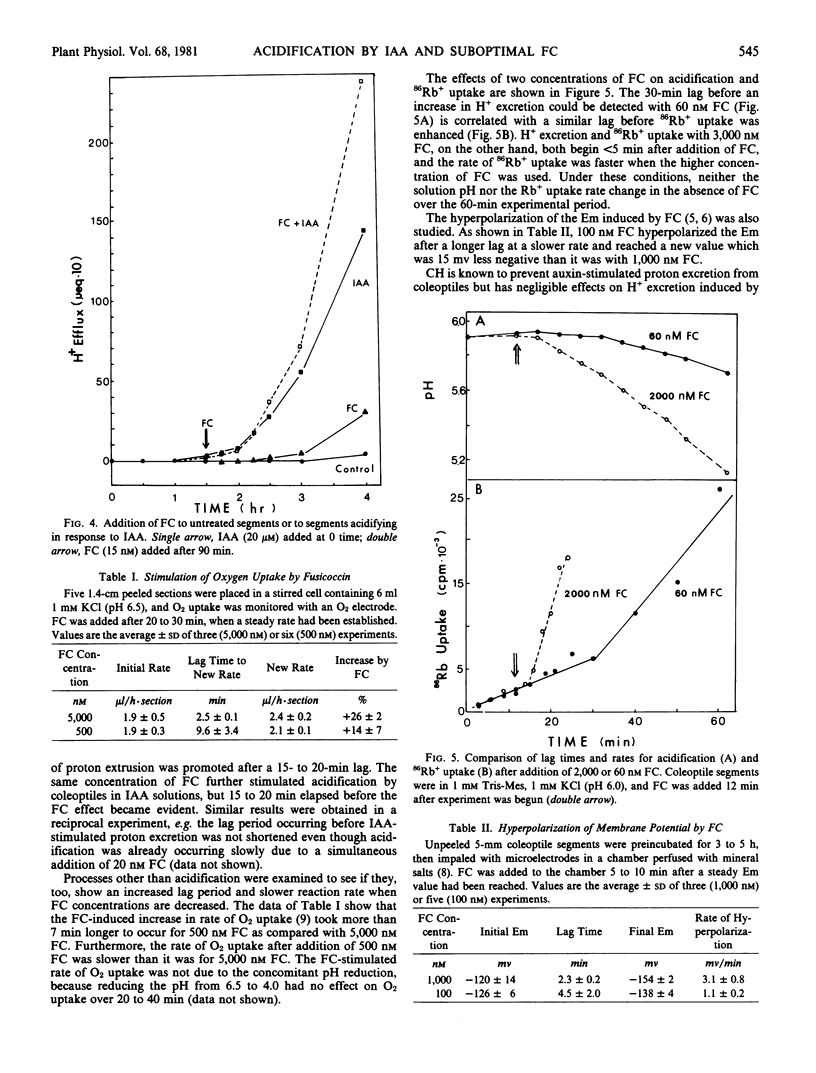
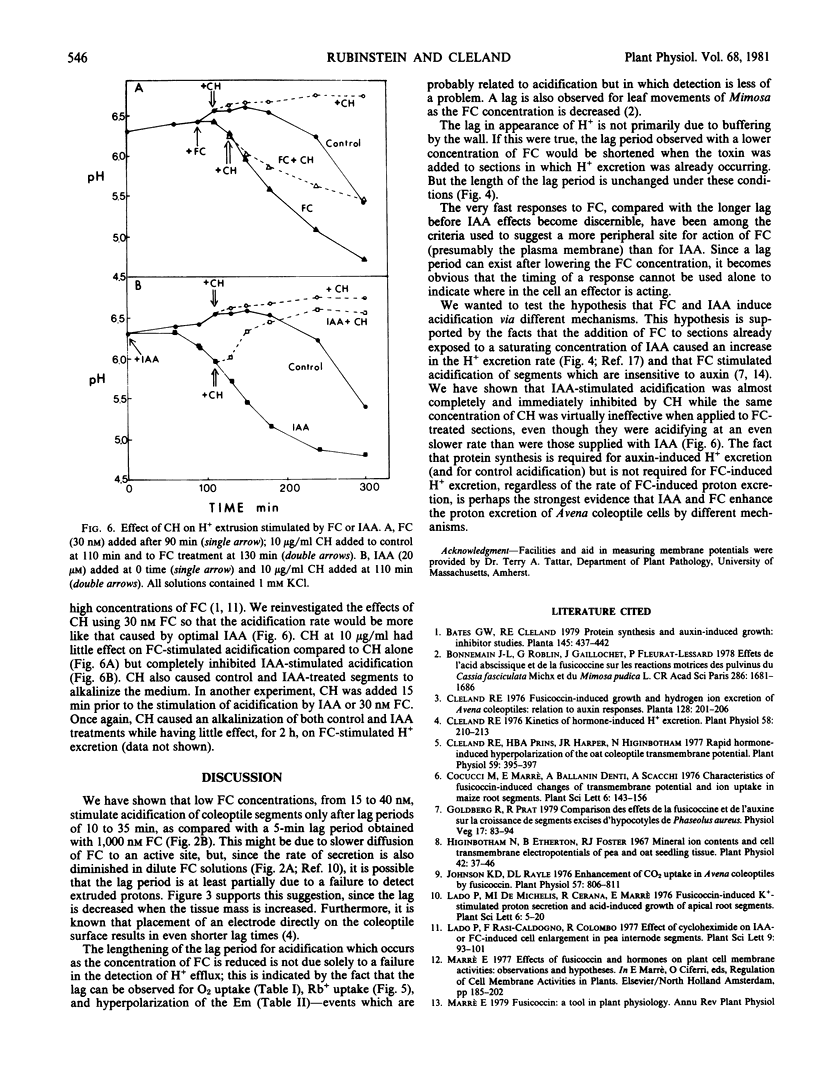
Proton excretion induced by optimal concentrations of indoleacetic acid (IAA) and fusicoccin (FC) differs not only in maximum rate of acidification but also in the lag before onset of H+ excretion and in sensitivity to cycloheximide. Because these differences might simply be a consequence of the difference in rate of proton excretion, FC and IAA have now been compared using oat coleoptiles (cv. Victory) under conditions where the rates of acidification are more similar, i.e. suboptimal FC versus optimal IAA. As the concentration of FC is reduced, the rate of H+ excretion decreases, the final equilibrium pH increases, and the lag before detectable acidification increases up to 7-fold. This enhanced lag period is not primarily a consequence of wall buffering, inasmuch as it persists when a low concentration of FC is added to sections which were already excreting H+ in response to IAA. An extended lag also occurs, upon reduction of FC levels, in the hyperpolarization of the membrane potential, before enhancement of O2 uptake and before the increased rate of Rb+ uptake. The presence or absence of a lag is not a distinguishing feature between FC and IAA actions on H+ excretion and cannot be used to discriminate between their sites of action. In contrast, the insensitivity of FC-induced H+ excretion to cycloheximide, as compared with the nearly complete inhibition of this auxin effect by cycloheximide, persists even at dilute concentrations of FC. This seems to be a basic difference in H+ excretion by IAA and FC.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland R. E. Kinetics of Hormone-induced H Excretion. Plant Physiol. 1976 Aug;58(2):210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E., Prins H. B., Harper J. R., Higinbotham N. Rapid Hormone-induced Hyperpolarization of the Oat Coleoptile Transmembrane Potential. Plant Physiol. 1977 Mar;59(3):395–397. doi: 10.1104/pp.59.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Mineral ion contents and cell transmembrane electropotentials of pea and oat seedling tissue. Plant Physiol. 1967 Jan;42(1):37–46. doi: 10.1104/pp.42.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Rayle D. L. Enhancement of CO(2) Uptake in Avena Coleoptiles by Fusicoccin. Plant Physiol. 1976 May;57(5):806–811. doi: 10.1104/pp.57.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]


