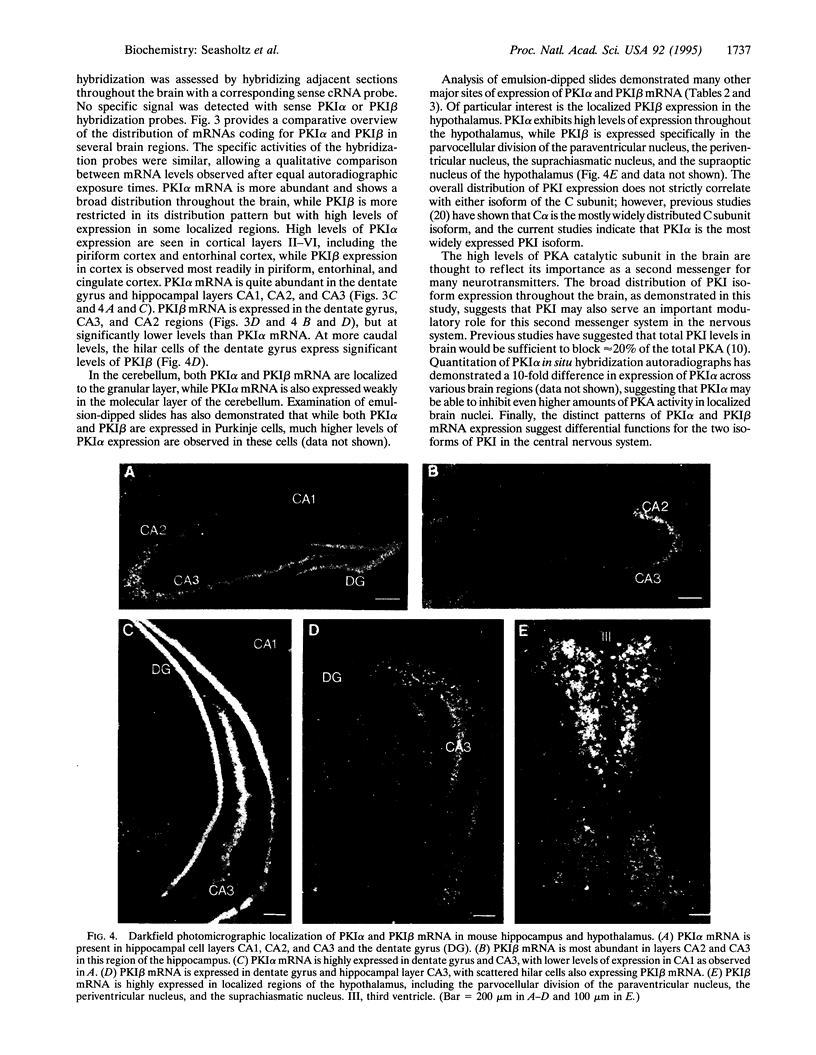
Abstract
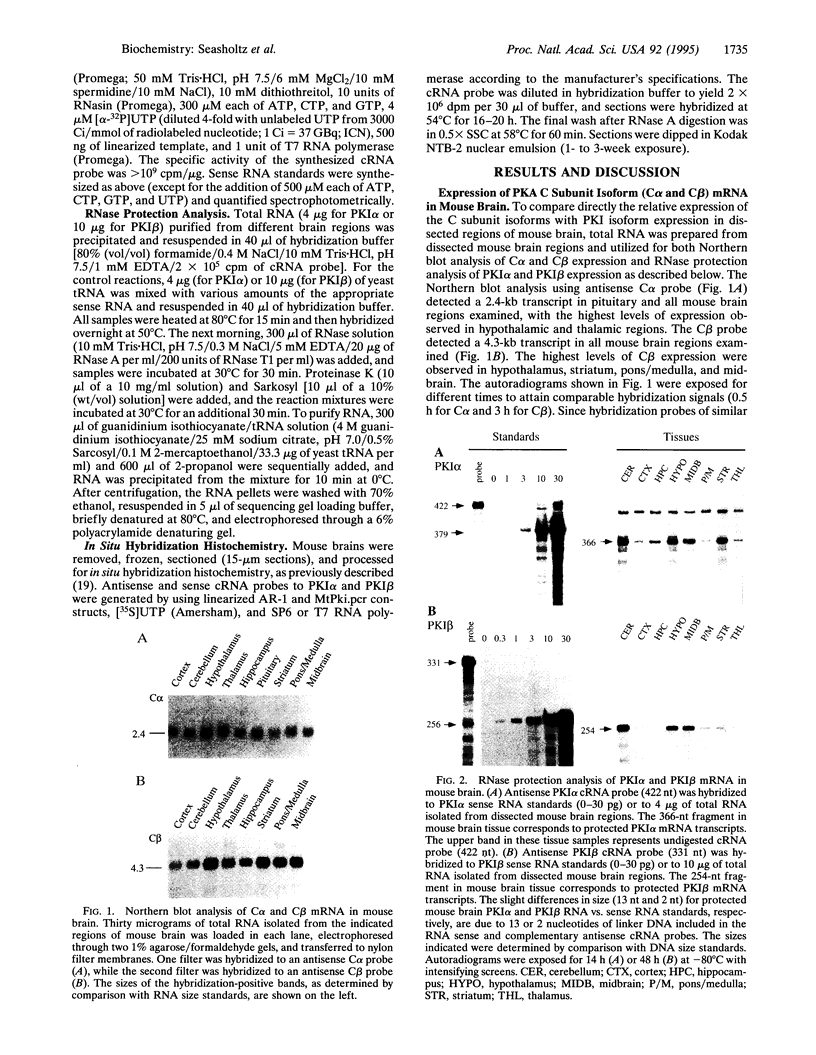
Many neurotransmitters are known to regulate neuronal cell function by means of activation of cAMP-dependent protein kinase (PKA) and phosphorylation of neuronal substrate proteins, including transcription factors and ion channels. Here, we have characterized the gene expression of two isoforms of a protein kinase inhibitor (PKI) specific for PKA in mouse brain by RNase protection and in situ hybridization histochemistry. The studies demonstrate that the PKI alpha isoform is abundant in many regions of the adult mouse brain but particularly in cerebellum, hypothalamus, hippocampus, and cortex. In contrast, PKI beta is present at much lower levels in most brain regions but is found in significant amounts in the cerebellum, as well as in distinct nuclei within the pons, medulla, and hypothalamus. These results are consistent with a regulatory role of endogenous PKI in PKA-mediated signal transduction in brain and suggest differential functions for the two isoforms of PKI within the central nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey C. H., Kandel E. R. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Cadd G., McKnight G. S. Distinct patterns of cAMP-dependent protein kinase gene expression in mouse brain. Neuron. 1989 Jul;3(1):71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis R. L. Mushroom bodies and Drosophila learning. Neuron. 1993 Jul;11(1):1–14. doi: 10.1016/0896-6273(93)90266-t. [DOI] [PubMed] [Google Scholar]
- Day R., Schafer M. K., Collard M. W., Watson S. J., Akil H. Atypical prodynorphin gene expression in corticosteroid-producing cells of the rat adrenal gland. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1320–1324. doi: 10.1073/pnas.88.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantozzi D. A., Harootunian A. T., Wen W., Taylor S. S., Feramisco J. R., Tsien R. Y., Meinkoth J. L. Thermostable inhibitor of cAMP-dependent protein kinase enhances the rate of export of the kinase catalytic subunit from the nucleus. J Biol Chem. 1994 Jan 28;269(4):2676–2686. [PubMed] [Google Scholar]
- Hawkins R. D., Kandel E. R., Siegelbaum S. A. Learning to modulate transmitter release: themes and variations in synaptic plasticity. Annu Rev Neurosci. 1993;16:625–665. doi: 10.1146/annurev.ne.16.030193.003205. [DOI] [PubMed] [Google Scholar]
- Lee K. A. Transcriptional regulation by cAMP. Curr Opin Cell Biol. 1991 Dec;3(6):953–959. doi: 10.1016/0955-0674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- McKnight G. S. Cyclic AMP second messenger systems. Curr Opin Cell Biol. 1991 Apr;3(2):213–217. doi: 10.1016/0955-0674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Olsen S. R., Uhler M. D. Isolation and characterization of cDNA clones for an inhibitor protein of cAMP-dependent protein kinase. J Biol Chem. 1991 Jun 15;266(17):11158–11162. [PubMed] [Google Scholar]
- Scarpetta M. A., Uhler M. D. Evidence for two additional isoforms of the endogenous protein kinase inhibitor of cAMP-dependent protein kinase in mouse. J Biol Chem. 1993 May 25;268(15):10927–10931. [PubMed] [Google Scholar]
- Scott J. D. Cyclic nucleotide-dependent protein kinases. Pharmacol Ther. 1991;50(1):123–145. doi: 10.1016/0163-7258(91)90075-w. [DOI] [PubMed] [Google Scholar]
- Swope S. L., Moss S. J., Blackstone C. D., Huganir R. L. Phosphorylation of ligand-gated ion channels: a possible mode of synaptic plasticity. FASEB J. 1992 May;6(8):2514–2523. [PubMed] [Google Scholar]
- Uhler M. D., McKnight G. S. Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1987 Nov 5;262(31):15202–15207. [PubMed] [Google Scholar]
- Van Patten S. M., Howard P., Walsh D. A., Maurer R. A. The alpha- and beta-isoforms of the inhibitor protein of the 3',5'-cyclic adenosine monophosphate-dependent protein kinase: characteristics and tissue- and developmental-specific expression. Mol Endocrinol. 1992 Dec;6(12):2114–2122. doi: 10.1210/mend.6.12.1491692. [DOI] [PubMed] [Google Scholar]
- Van Patten S. M., Ng D. C., Th'ng J. P., Angelos K. L., Smith A. J., Walsh D. A. Molecular cloning of a rat testis form of the inhibitor protein of cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5383–5387. doi: 10.1073/pnas.88.12.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas S. I., Greengard P. Protein phosphorylation and neuronal function. Pharmacol Rev. 1991 Sep;43(3):299–349. [PubMed] [Google Scholar]
- Walsh D. A., Glass D. B., Mitchell R. D. Substrate diversity of the cAMP-dependent protein kinase: regulation based upon multiple binding interactions. Curr Opin Cell Biol. 1992 Apr;4(2):241–251. doi: 10.1016/0955-0674(92)90039-f. [DOI] [PubMed] [Google Scholar]