Abstract
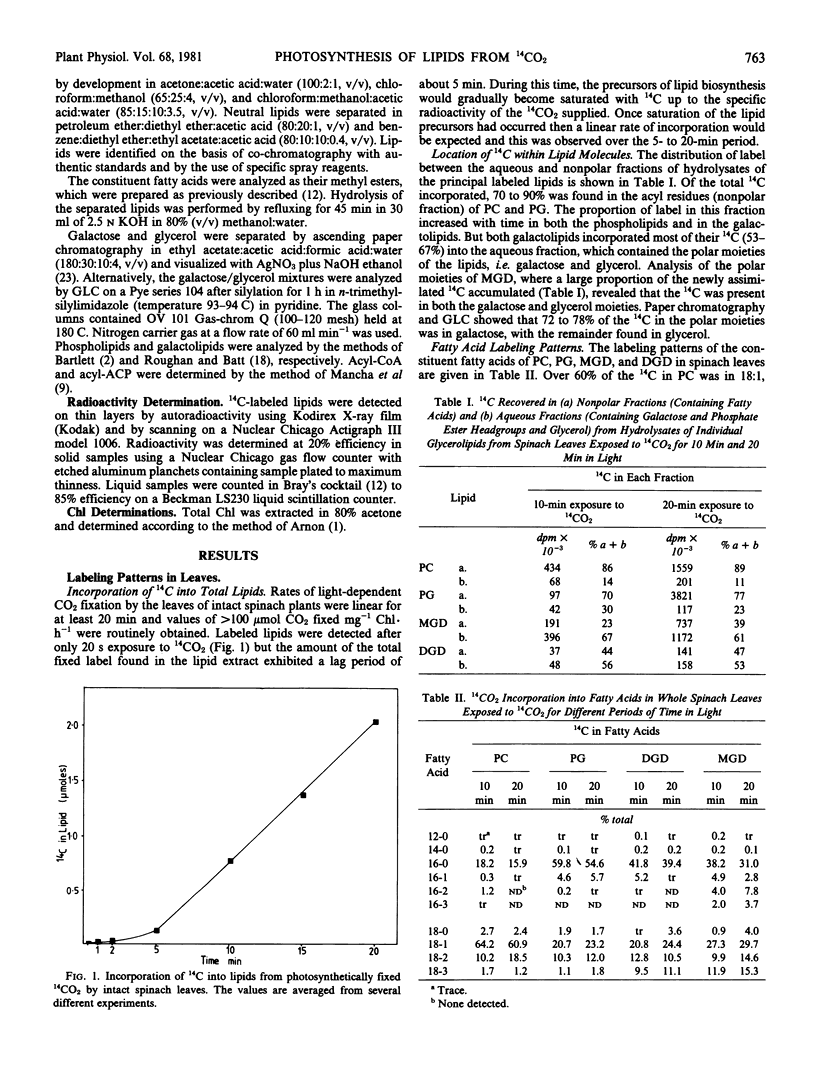
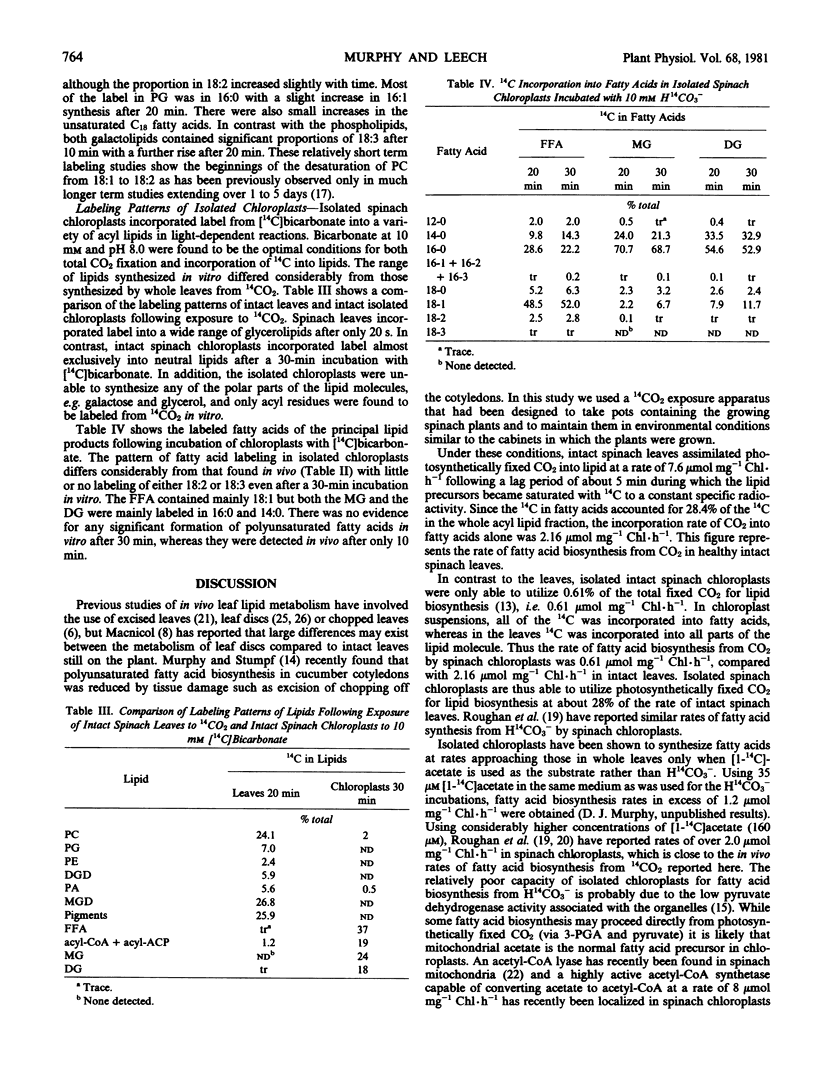
Young expanding spinach leaves exposed to 14CO2 under physiological conditions for up to 20 minutes assimilated CO2 into lipids at a mean rate of 7.6 micromoles per milligram chlorophyll per hour following a lag period of 5 minutes. Label entered into all parts of the lipid molecule and only 28% of the 14C fixed into lipids was found in the fatty acid moieties, i.e. fatty acids were synthesized from CO2in vivo at a mean rate of 2.1 micromoles per milligram chlorophyll per hour. Intact spinach chloroplasts isolated from these leaves incorporated H14CO3 into fatty acids at a maximal rate of 0.6 micromole per milligram chlorophyll per hour, but were unable to synthesize either the polar moieties of their lipids or polyunsaturated fatty acids. Since isolated chloroplasts will only synthesize fatty acids at rates similar to the one obtained with intact leaves in vivo if acetate is used as a precursor, it is suggested that acetate derived from leaf mitochondria is the physiological fatty acid precursor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. L. Fatty acid biosynthesis by avocado pear. Lipids. 1974 Nov;9(11):850–854. doi: 10.1007/BF02532608. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Jacobson B. S., Stumpf P. K. In vivo biosynthesis of -linolenic acid in plants. Biochem Biophys Res Commun. 1973 May 15;52(2):648–655. doi: 10.1016/0006-291x(73)90762-6. [DOI] [PubMed] [Google Scholar]
- Macnicol P. K. Rapid Metabolic Changes in the Wounding Response of Leaf Discs following Excision. Plant Physiol. 1976 Jan;57(1):80–84. doi: 10.1104/pp.57.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancha M., Stokes G. B., Stumpf P. K. Fat metabolism in higher plants. The determination of acyl-acyl carrier protein and acyl coenzyme A in a complex lipid mixture 1,2. Anal Biochem. 1975 Oct;68(2):600–608. doi: 10.1016/0003-2697(75)90655-7. [DOI] [PubMed] [Google Scholar]
- McKee J. W., Hawke J. C. The incorporation of [14C]acetate into the constituent fatty acids of monogalactosyldiglyceride by isolated spinach chloroplasts. Arch Biochem Biophys. 1979 Oct 1;197(1):322–332. doi: 10.1016/0003-9861(79)90252-2. [DOI] [PubMed] [Google Scholar]
- Murphy D. J., Leech R. M. Lipid biosynthesis from [14C]bicarbonate, [2(-14)C]pyruvate and [1(-14)C]acetate during photosynthesis by isolated spinach chloroplasts. FEBS Lett. 1977 May 15;77(2):164–168. doi: 10.1016/0014-5793(77)80226-3. [DOI] [PubMed] [Google Scholar]
- Murphy D. J., Stumpf P. K. Polyunsaturated Fatty Acid Biosynthesis in Cotyledons from Germinating and Developing Cucumis sativus L. Seedlings. Plant Physiol. 1980 Oct;66(4):660–665. doi: 10.1104/pp.66.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. Acetate is the preferred substrate for long-chain fatty acid synthesis in isolated spinach chloroplasts. Biochem J. 1979 Dec 15;184(3):565–569. doi: 10.1042/bj1840565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G. Phosphatidyl choline: Donor of 18-carbon unsaturated fatty acids for glycerolipid biosynthesis. Lipids. 1975 Oct;10(10):609–614. doi: 10.1007/BF02532725. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G. The kinetics of incorporation in vivo of (14C)acetate and (14C)carbon dioxide into the fatty acids of glycerolipids in developing leaves. Biochem J. 1975 Nov;152(2):217–228. doi: 10.1042/bj1520217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf P. K. Fatty acid synthesis by spinach chloroplasts. Methods Enzymol. 1972;24:394–397. doi: 10.1016/0076-6879(72)24085-x. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The synthesis of fatty acids in avocado mesocarp and cauliflower bud tissue. Biochem J. 1975 Feb;146(2):425–437. doi: 10.1042/bj1460425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. P., Watson G. R., Khan M. U., Leung S. Galactolipid Synthesis in Vicia faba Leaves: I. Galactose, Glycerol, and Fatty Acid Labeling after CO(2) Feeding. Plant Physiol. 1975 Jun;55(6):1038–1042. doi: 10.1104/pp.55.6.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. P., Watson G. R., Leung S. P. Galactolipid Synthesis in Vicia faba Leaves: II. Formation and Desaturation of Long Chain Fatty Acids in Phosphatidylcholine, Phosphatidylglycerol, and the Galactolipids. Plant Physiol. 1976 Feb;57(2):179–184. doi: 10.1104/pp.57.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]


