Abstract
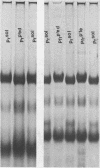
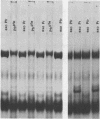
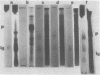
Phytochrome that has been photoinduced to pellet by irradiation of intact oat (cv. Garry) shoots and recovered from a pellet obtained by centrifugation of crude extracts exhibits modified behavior when compared to soluble phytochrome isolated from shoots that had never been irradiated. This modified behavior includes retarded mobility during sodium dodecyl sulfate polyacrylamide gel electrophoresis (Boeshore ML, LH Pratt 1980 Plant Physiol 66: 500-504). The electrophoretic mobility of several different kinds of phytochrome preparations were examined to study how this modification might arise.
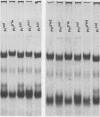
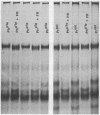
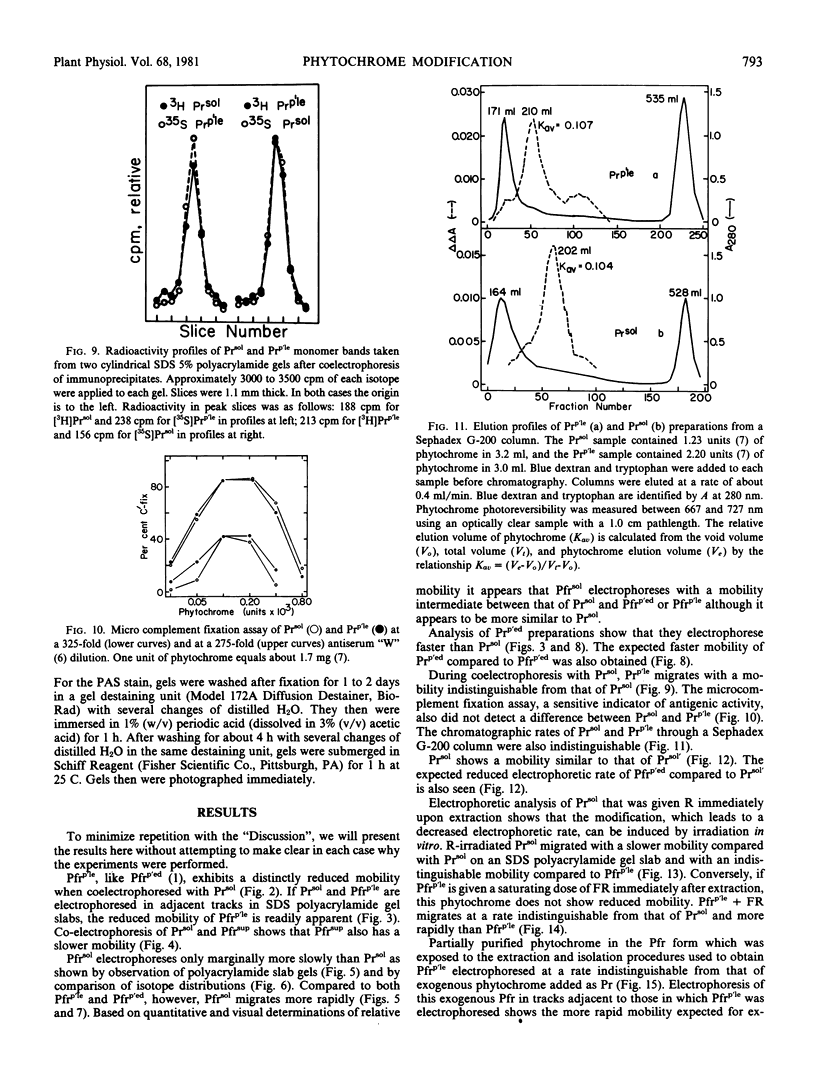
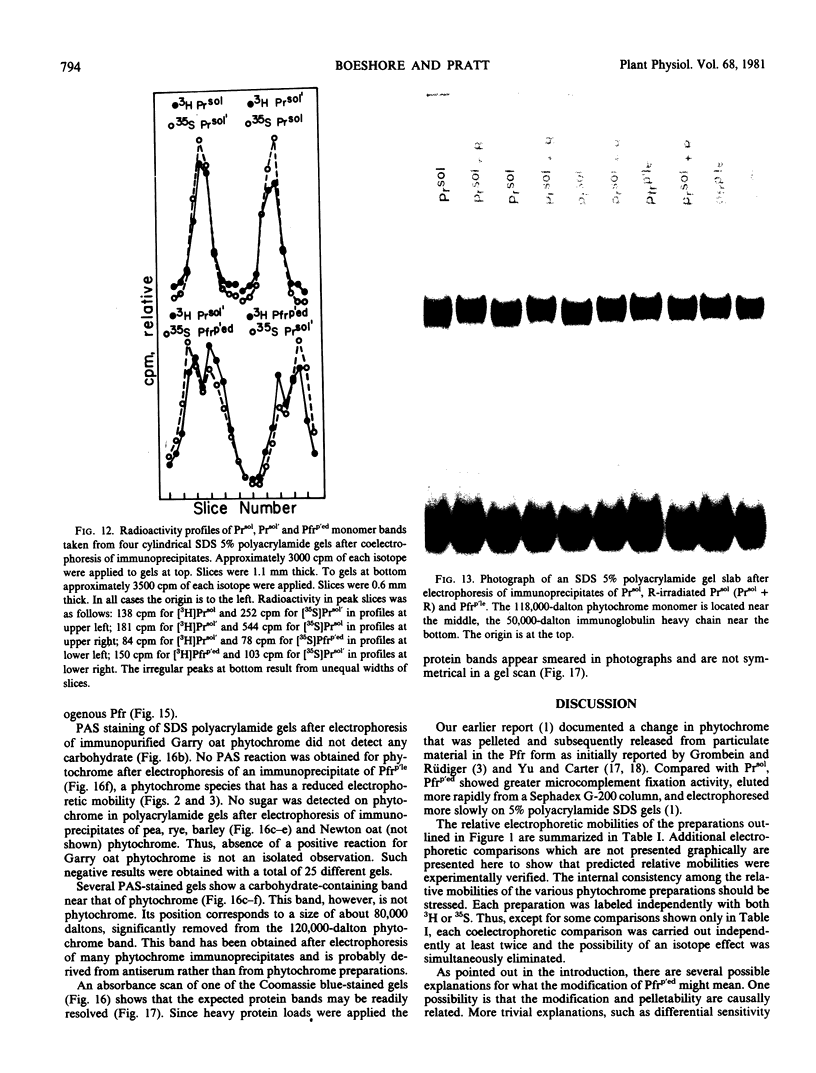
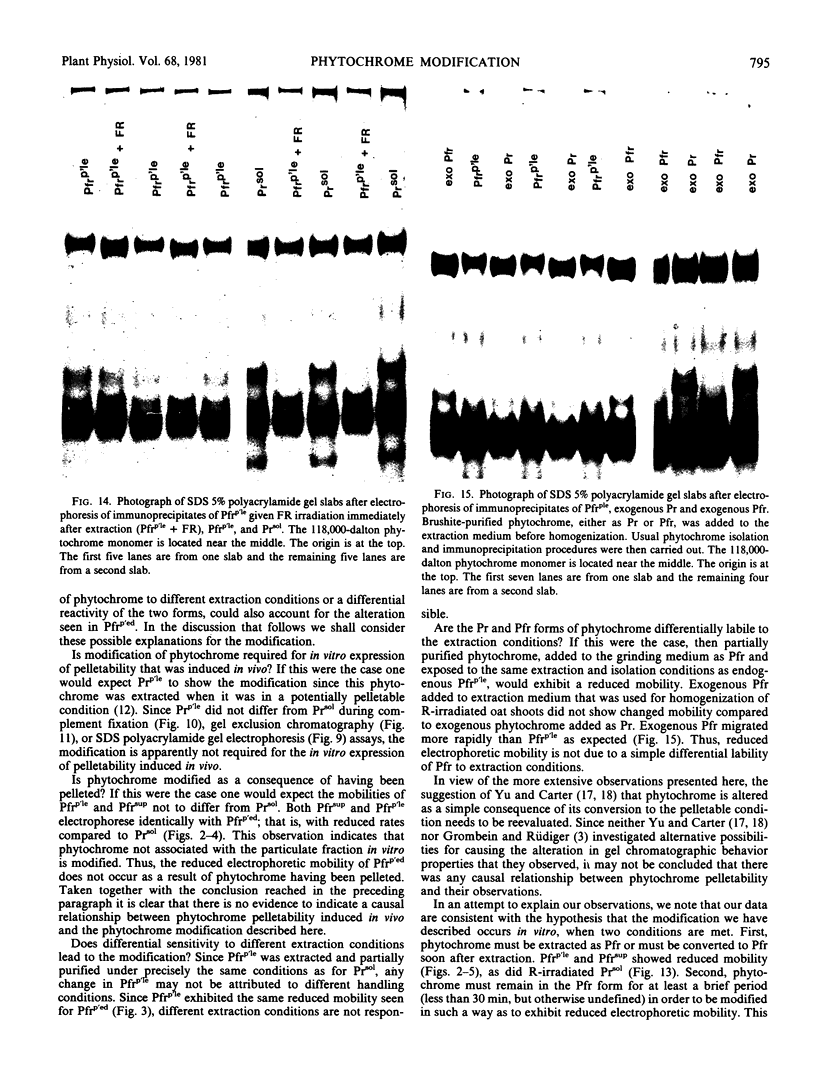
Phytochrome that was extracted in the pelletable condition from red-, far-red-irradiated tissue, but without added divalent cation so that it did not pellet, did not exhibit an altered electrophoretic mobility. Hence, this modification of phytochrome is not required for the expression in vitro of pelletability induced in vivo. Phytochrome that was extracted in the pelletable condition and in the far-red-absorbing form, but without added divalent cation so that it did not pellet, and phytochrome in the far-red-absorbing form that remained in the supernatant after collection of pellets containing pelleted phytochrome both electrophoresed with reduced mobility. Thus, this modification does not arise as a consequence of phytochrome having been pelleted. Differential sensitivity of phytochrome to different handling conditions also is not the cause of this modification since the far-red-absorbing form of phytochrome, which was extracted in the pelletable condition but by the same protocol used to extract soluble phytochrome, also exhibited reduced mobility. Furthermore, the reduced electrophoretic rate is not due to a simple differential lability of the far-red-absorbing form of phytochrome to extraction conditions, since partially purified soluble phytochrome that was exposed in the far-red-absorbing form to the isolation and extraction conditions used for preparation of soluble phytochrome did not exhibit the alteration.
The data are instead consistent with the more complex interpretation that phytochrome is modified in vitro if two conditions are met: (a) that phytochrome is extracted in the far-red-absorbing form or is converted to the far-red-absorbing form in the crude extract soon after extraction and (b), that phytochrome remains in the far-red-absorbing form in the crude extract for at least a brief period.
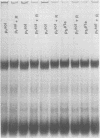
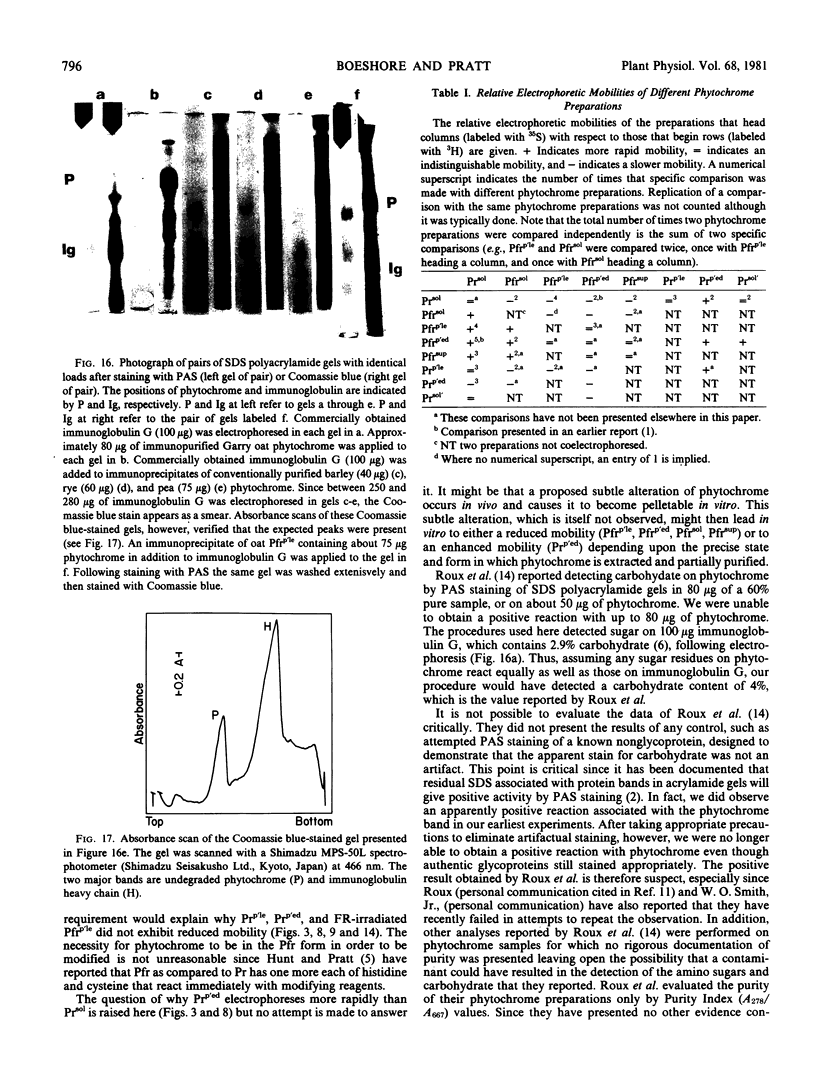
The possibility that the phytochrome modification studied here might have arisen because of a change in carbohydrate content was tested by periodic acid Schiff staining of sodium dodecyl sulfate polyacrylamide gels. No carbohydrate was detected in any of the phytochrome preparations that were examined. This inability to detect carbohydrate is in direct contrast to the report of Roux et al. (1975 Physiol Plant 35: 85-90).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeshore M. L., Pratt L. H. Phytochrome Modification and Light-enhanced, In Vivo-induced Phytochrome Pelletability. Plant Physiol. 1980 Sep;66(3):500–504. doi: 10.1104/pp.66.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Grombein S., Rüdiger W. On the molecular weight of phytochrome: a new high molecular phytochrome species in oat seedlings. Hoppe Seylers Z Physiol Chem. 1976 Jul;357(7):1015–1018. [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Physicochemical differences between the red- and the far-red-absorbing forms of phytochrome. Biochemistry. 1981 Feb 17;20(4):941–945. doi: 10.1021/bi00507a046. [DOI] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Phytochrome immunoaffinity purification. Plant Physiol. 1979 Aug;64(2):332–336. doi: 10.1104/pp.64.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H. Comparative immunochemistry of phytochrome. Plant Physiol. 1973 Jan;51(1):203–209. doi: 10.1104/pp.51.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Marmé D. Red Light-enhanced Phytochrome Pelletability: Re-examination and Further Characterization. Plant Physiol. 1976 Nov;58(5):686–692. doi: 10.1104/pp.58.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail P. H., Briggs W. R. Irradiation-enhanced Phytochrome Pelletability: Requirement for Phosphorylative Energy in Vivo. Plant Physiol. 1978 Nov;62(5):773–778. doi: 10.1104/pp.62.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]