Abstract
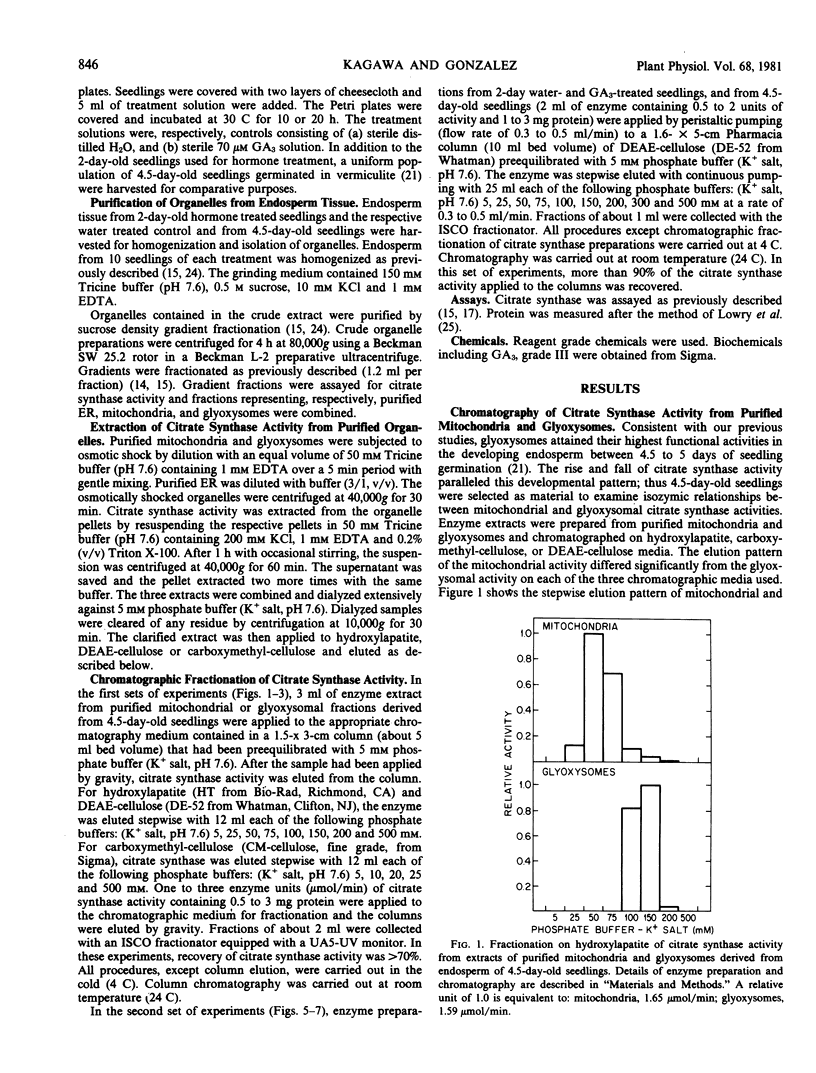
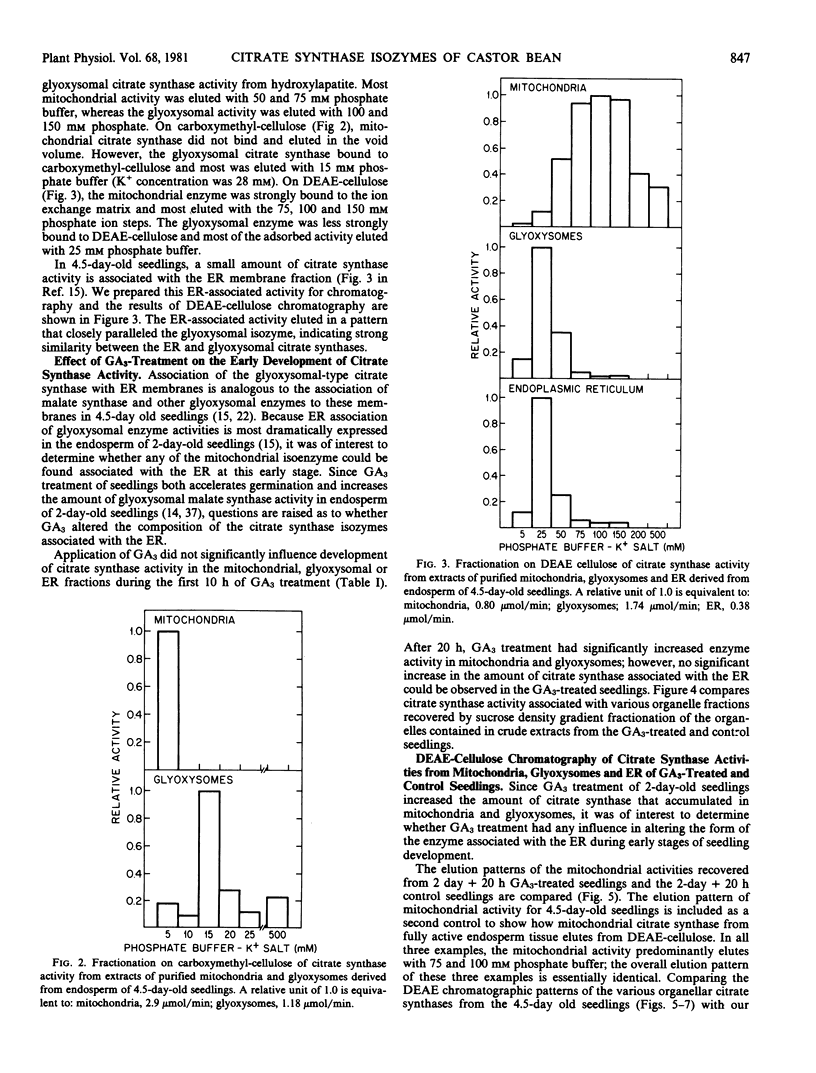
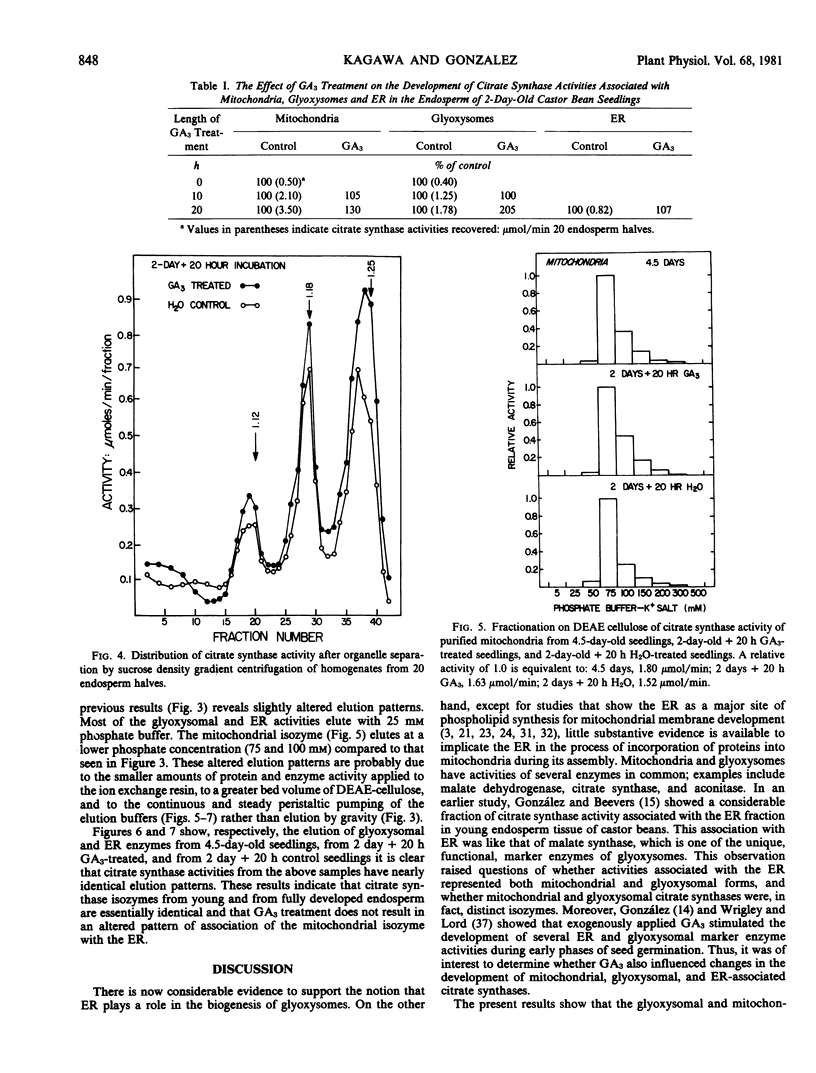
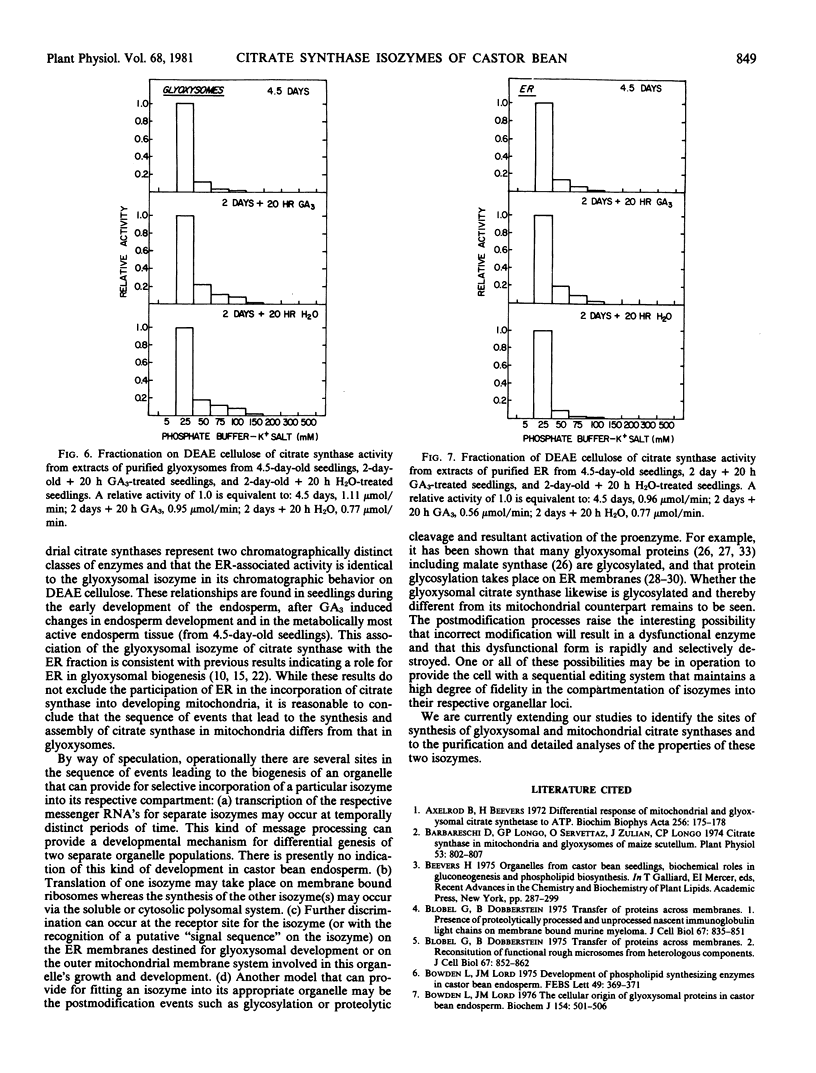
Chromatographic analysis of organelle-associated citrate synthase activity revealed distinct mitochondrial and glyoxysomal forms of the enzyme. The chromatographic elution patterns on hydroxylapatite, carboxymethylcellulose and DEAE-cellulose of citrate synthase from the endosperm of 4.5-day-old castor bean seedlings revealed significant differences for mitochondrial and glyoxysomal activities of the enzyme. The endoplasmic reticulum-associated citrate synthase activity eluted from DEAE-cellulose in a pattern that was identical to that of the glyoxysomal activity. The same kinds of organelle specific isozyme elution patterns were observed with young, developing seedlings. Gibberellic acid-treatment of young seedlings increased total recoverable citrate synthase activity from endosperm tissue but did not modify the organelle specific isozyme relationships.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod B., Beevers H. Differential response of mitochondrial and glyoxysomal citrate synthase to ATP. Biochim Biophys Acta. 1972 Feb 28;256(2):175–178. doi: 10.1016/0005-2728(72)90050-3. [DOI] [PubMed] [Google Scholar]
- Barbareschi D., Longo G. P., Servettaz O., Zulian T., Longo C. P. Citrate synthetase in mitochondria and glyoxysomes of maize scutellum. Plant Physiol. 1974 Jun;53(6):802–807. doi: 10.1104/pp.53.6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. Development of phospholipid synthesizing enzymes in castor bean endosperm. FEBS Lett. 1975 Jan 1;49(3):369–371. doi: 10.1016/0014-5793(75)80787-3. [DOI] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. Purification and comparative properties of microsomal and glyoxysomal malate synthase from castor bean endosperm. Plant Physiol. 1978 Feb;61(2):259–265. doi: 10.1104/pp.61.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. Similarities in the polypeptide composition of glyoxysomal and endoplasmic-reticulum membranes from castor-bean endosperm. Biochem J. 1976 Feb 15;154(2):491–499. doi: 10.1042/bj1540491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. The cellular origin of glyoxysomal proteins in germinating castor-bean endosperm. Biochem J. 1976 Feb 15;154(2):501–506. doi: 10.1042/bj1540501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M. Glyoxysomes in megagamethophyte of germinating ponderosa pine seeds. Plant Physiol. 1970 Sep;46(3):475–482. doi: 10.1104/pp.46.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E., Beevers H. Role of the endoplasmic reticulum in glyoxysome formation in castor bean endosperm. Plant Physiol. 1976 Mar;57(3):406–409. doi: 10.1104/pp.57.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González E. Effect of gibberellin a(3) on the endoplasmic reticulum and on the formation of glyoxysomes in the endosperm of germinating castor bean. Plant Physiol. 1978 Sep;62(3):449–453. doi: 10.1104/pp.62.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruban Z., Rechcigl M., Jr Microbodies and related particles. Morphology, biochemistry, and physiology. Int Rev Cytol. 1969;(Suppl):1–296. [PubMed] [Google Scholar]
- Huang A. H., Bowman P. D., Beevers H. Immunological and biochemical studies on isozymes of malate dehydrogenase and citrate synthetase in castor bean glyoxysomes. Plant Physiol. 1974 Sep;54(3):364–367. doi: 10.1104/pp.54.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Beevers H. The development of microbodies (glyoxysomes and leaf peroxisomes) in cotyledons of germinating watermelon seedlings. Plant Physiol. 1975 Feb;55(2):258–264. doi: 10.1104/pp.55.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. The origin and turnover of organelle membranes in castor bean endosperm. Plant Physiol. 1973 Jan;51(1):61–65. doi: 10.1104/pp.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Bowden L. Evidence that glyoxysomal malate synthase is segregated by the endoplasmic reticulum. Plant Physiol. 1978 Feb;61(2):266–270. doi: 10.1104/pp.61.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Beevers H. Intracellular distribution of enzymes of the cytidine diphosphate choline pathway in castor bean endosperm. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2429–2432. doi: 10.1073/pnas.69.9.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. S., Lord J. M., Kagawa T., Beevers H. Enzymes of phospholipid metabolism in the endoplasmic reticulum of castor bean endosperm. Plant Physiol. 1973 Jul;52(1):50–53. doi: 10.1104/pp.52.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. S. Phosphatidylglycerol synthesis in castor bean endosperm: kinetics, requirements, and intracellular localization. Plant Physiol. 1974 Aug;54(2):164–168. doi: 10.1104/pp.54.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]


