Abstract
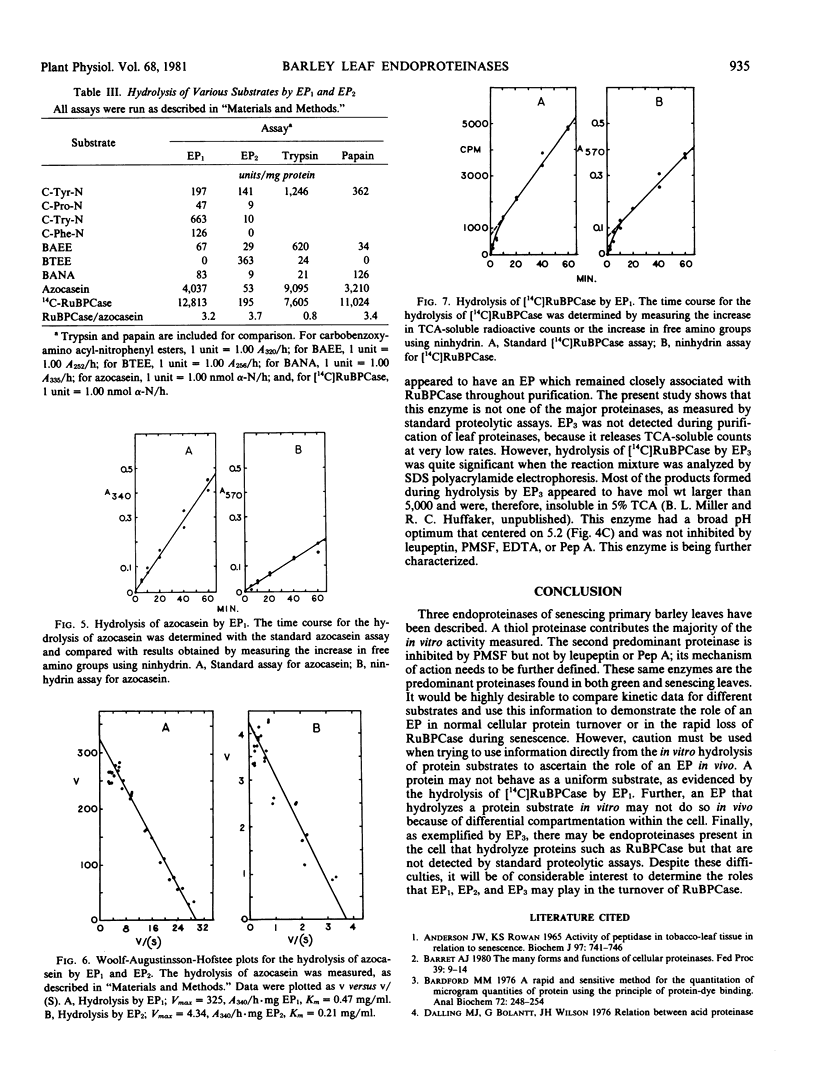
Two major endoproteinases were purified from senescing primary barley leaves. The major enzyme (EP1) appeared to be a thiol proteinase and accounted for about 85% of the total proteolytic activity measured in vitro. This proteinase was purified 5,800-fold and had a molecular weight of 28,300. It was highly unstable in the absence of dithiothreitol or at a pH greater than 7.5. Leupeptin, at a concentration of 10 micromolar, inhibited this enzyme 100%. A second proteinase (EP2) was purified approximately 50-fold and had a molecular weight of 67,000. It was inhibited 20% by 1 millimolar dithiothreitol and 50% by 1 millimolar phenylmethyl sulfonylfluoride. EP2 contributed about 15% of the total proteolytic activity measured in vitro. Both proteinases hydrolyzed a variety of artificial and protein substrates, and both had pH optima of 5.5 to 5.7 when either azocasein or [14C]ribulose-1,5-bisphosphate carboxylase ([14C]RuBPCase) was the substrate. The thiol endoproteinase hydrolyzed azocasein linearly but hydrolyzed [14C]RuBPCase biphasically. A third endoproteinase (EP3), not detected by standard proteolytic assays, was observed when [14C]RuBPCase was the substrate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Rowan K. S. Activity of peptidase in tobacco-leaf tissue in relation to senescence. Biochem J. 1965 Dec;97(3):741–746. doi: 10.1042/bj0970741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. The many forms and functions of cellular proteinases. Fed Proc. 1980 Jan;39(1):9–14. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Drivdahl R. H., Thimann K. V. Proteases of Senescing Oat Leaves: II. Reaction to Substrates and Inhibitors. Plant Physiol. 1978 Apr;61(4):501–505. doi: 10.1104/pp.61.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Feller U. K., Soong T. S., Hageman R. H. Leaf Proteolytic Activities and Senescence during Grain Development of Field-grown Corn (Zea mays L.). Plant Physiol. 1977 Feb;59(2):290–294. doi: 10.1104/pp.59.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp R., De Filippis L. F. Plastid Protease Activity and Prolamellar Body Transformation during Greening. Plant Physiol. 1980 Apr;65(4):663–668. doi: 10.1104/pp.65.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. A simplified purification and some properties of ribulose 1,5-diphosphate carboxylase from barley. Plant Physiol. 1970 Aug;46(2):204–207. doi: 10.1104/pp.46.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968 Dec 10;243(23):6281–6283. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Pike C. S., Briggs W. R. Partial Purification and Characterization of a Phytochrome-degrading Neutral Protease from Etiolated Oat Shoots. Plant Physiol. 1972 Apr;49(4):521–530. doi: 10.1104/pp.49.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragster L. V., Chrispeels M. J. Azocoll-digesting Proteinases in Soybean Leaves: Characteristics and Changes during Leaf Maturation and Senescence. Plant Physiol. 1979 Nov;64(5):857–862. doi: 10.1104/pp.64.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K., Ryan C. Radial diffusion as a sensitive method for screening endopeptidase activity in plant extracts. Anal Biochem. 1977 Jan;77(1):1–9. doi: 10.1016/0003-2697(77)90283-4. [DOI] [PubMed] [Google Scholar]
- Vavreinová S., Turková J. SH-proteinase from bean Phaseolus vulgaris var. Perlicka. Biochim Biophys Acta. 1975 Oct 22;403(2):506–513. doi: 10.1016/0005-2744(75)90078-9. [DOI] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]