Abstract
Medicago truncatula is a model legume forage crop native to the arid and semi-arid environments of the Mediterranean. Given its drought-adapted nature, it is an ideal candidate to study the molecular and biochemical mechanisms conferring drought resistance in plants. Medicago plants were subjected to a progressive drought stress over 14 d of water withholding followed by rewatering under controlled environmental conditions. Based on physiological measurements of plant water status and changes in morphology, plants experienced mild, moderate and severe water stress before rehydration. Transcriptome analysis of roots and shoots from control, mildly, moderately and severely stressed, and rewatered plants, identified many thousands of genes that were altered in expression in response to drought. Many genes with expression tightly coupled to the plant water potential (i.e. drought intensity) were identified suggesting an involvement in Medicago drought adaptation responses. Metabolite profiling of drought-stressed plants revealed the presence of 135 polar and 165 non-polar compounds in roots and shoots. Combining Medicago metabolomic data with transcriptomic data yielded insight into the regulation of metabolic pathways operating under drought stress. Among the metabolites detected in drought-stressed Medicago plants, myo-inositol and proline had striking regulatory profiles indicating involvement in Medicago drought tolerance.
Global transcriptional and metabolic responses to drought and rewatering were investigated in Medicago truncatula, a naturally drought-adapted model legume species. Integration of metabolomic and transcriptomic data yielded insights into the regulation of metabolic pathways underlying drought-stress adaptation. Many genes and metabolites with expression tightly coupled to drought intensity were identified, suggesting active involvement in Medicago drought resistance. These could prove useful targets for future translational approaches to improve closely related crop plants such as common bean, soybean and pea.
Keywords: legumes, drought stress, resistance, tolerance, microarray, gene profiling, transcriptomics, metabolomics, proline, ABA
Introduction
Periodic drought is the primary limitation on plant growth and yield of crops in agricultural systems (Boyer 1982). As human populations grow and urban demand for water increases, agriculture faces the dual challenge of increasing food and feed production with static or dwindling supplies of water. Since plants colonized land over 400 million years ago, they have evolved numerous strategies to escape, avoid and/or tolerate periodic drought (Levitt 1972). For millennia, farmers have maximized crop yields by synchronizing planting and plant development with the rainy season. Nevertheless, breeding and biotechnological advances have focused mainly on increasing yield under optimal conditions and not on maintaining yield under drought conditions (Marris 2008). Efforts to improve crop performance under environmental stresses have been somewhat stymied by a lack of understanding of the fundamental mechanisms of stress tolerance (Yamaguchi & Blumwald 2005; Tuberosa & Salvi 2006). In the case of drought resistance, this can be attributed to the complexity of the traits and genes involved (Bray 1993; Pennisi 2008; Salekdeh et al. 2009). In recent years, some light has been shed on the physiological, biochemical and genetic basis of plant-drought adaptation, which has the potential to accelerate the breeding process (Stockinger et al. 1997; Liu et al. 1998; Kasuga et al. 1999; Bruce et al. 2002; Pnueli et al. 2002; Capell et al. 2004; Hazen et al. 2005; Wang et al. 2005; Umezawa et al. 2006; Valliyodan & Nguyen 2006; Xiong et al. 2006; Nelson et al. 2007; Seki et al. 2007; Shinozaki & Yamaguchi-Shinozaki 2007; Cuellar-Ortiz et al. 2008; Yu et al. 2008; Julia et al. 2009; Kohzuma et al. 2009). Plants adapt to short-term drought at the physiological level by limiting transpiration water loss by closing stomata and leaf rolling (Mansfield & Davies 1981; Brodribb & Holbrook 2003; Lawlor & Tezara 2009). Longer-term adaptations to drought involve developmental changes, such as expanding the root system at the expense of shoot growth to maximize soil water capture (Sharp & Davies 1989; Turner 1979; Sharp et al. 2004; Benjamin & Nielsen 2006). Ultimately, physiological and developmental responses to drought are underpinned by reprogramming of gene expression and metabolism (Ozturk et al. 2002; Zhu 2002; Oono et al. 2003; Rabbani et al. 2003; Boominathan et al. 2004; Reddy et al. 2004; Yamaguchi-Shinozaki & Shinozaki 2006; Talame et al. 2007).
Technological innovations over the past decade have made it possible to measure changes in gene expression (transcript levels) and metabolite levels on genome- and metabolome-wide scales (Udvardi et al. 2007; Benedito et al. 2008; Urano et al. 2010). This enables an unprecedented overview of the global molecular changes occurring under drought stress. There are many published reports on transcriptomic variation induced by drought treatments in a variety of plant species. However, interpreting such transcriptomic variation has not been a straightforward task (Deyholos 2010; Sanchez 2013). The major challenge is identifying the significant genes/gene networks driving plant adaptation to water stress among the thousands of genes that are differentially expressed. A plethora of different treatments have been used as a proxy for drought. These range from the use of osmotic agents in agar plates (i.e. Polyethylene glycol, mannitol), air drying on filter paper of agar-grown plants, to withholding watering from soil-grown plants. Each treatment will result in very different time scales for water deficit development (which seldom is physiologically assessed). This inevitably results in very different transcriptomic responses, with only a very small fraction of common variation, as shown in Arabidopsis thaliana (Bray 1997) and wheat (Talame et al. 2007). The choice of the germplasm must also be considered when interpreting the transcriptomic changes occurring in response to drought. For instance, the majority of the studies on transcriptome response to drought stress have been made using A. thaliana, which is a drought-sensitive plant that does not tolerate low water potentials (Deyholos 2010; Des Marais et al. 2012). Relevant transcriptome changes observed under drought stress in Arabidopsis will be more related to drought-avoidance processes (Des Marais et al. 2012) than to more severe drought tolerance.
The Leguminosae are second only to the Gramineae in importance as a source of food for humans, feed for livestock and raw materials for industry (Graham & Vance 2003). Legumes are the lynch pin of sustainable agriculture because they carry out symbiotic nitrogen fixation, which injects between 40 and 60 million tons of nitrogen per annum into agricultural systems (Smil 1999). Food legumes of global importance include soybean (Glycine max), bean (Phaseolus vulgaris), pea (Pisum sativum) and many others. Alfalfa (Medicago sativa L.) is the most important forage legume species and one of the most valuable crops in the USA. Unfortunately, it is not ideal for genetics or genomics research because of its large, tetraploid genome and out-crossing nature. However, its close relative, M. truncatula is self-fertile and has a relatively small diploid genome, which makes it useful for both genetics and genomics studies. For these and other reasons, M. truncatula has been developed as a model species for the legume family (Barker et al. 1990; Cook 1999; Young et al. 2005). In addition to a complete genome sequence for the genotype A17 (Young et al. 2011) and of hundreds of other accessions potentiated by the Medicago hapmap project (http://www.medicagohapmap.org), resources for transcriptomics, proteomics, metabolomics and for forward- and reverse-genetics make M. truncatula ideal for legume functional genomics (Young & Udvardi 2009).
M. truncatula (Medicago) is a plant that occurs naturally in the arid and semi-arid environments of the Mediterranean Basin area and which has been developed into an annual legume forage crop in Australia. It can, therefore, be considered as a drought-adapted species and an ideal experimental model to advance our understanding of the underlying molecular mechanisms of drought adaptation and tolerance in legumes, and in plants in general. In the study presented here, 24-day-old Medicago plants grown in soil were subjected to progressive long-term drought and rewatering treatments in order to mimic what plants experience in their natural environment. Changes in gene transcript and metabolite levels were measured at multiple time points during drought stress, over a 15-day period. We present a comprehensive and detailed overview of the transcriptome and metabolome changes associated with the progression of drought stress, tightly linking our data with physiological measurements of the plant water status during the time-course of drought progression. This will enable future comparative analysis (i.e. between different germplasm and/or multiple stress combinations). Our goal was also to provide candidate genes that might prove useful in translational approaches for legume crop improvement.
Materials and Methods
Plant growth, drought stress, physiological measurement and sampling
Plant growth conditions and sampling stages were following the experiment onset for Medicago Gene Expression Atlas (Benedito et al. 2008) with appropriate modification for a gradual drought-stress imposition. In brief, the M. truncatula Gaertn. cv. Jemalong line A17 seeds were scarified in concentrated anhydrous sulfuric acid for ∼8 min, followed by sterilization with 30% bleach plus 0.1% Tween-20 solution for 10 min. The resulting seeds were pre-germinated at 4 °C for 3 d on wet filter paper before planting.
Germinating seeds were then selected and sown in 6.5-inch plastic pots (16 cm diameter at top and 12 cm at bottom and 11 cm high, with 1450 mL in volume) filled with pre-autoclaved mixture of turface and washed sand (2:1 in volume). The plants were grown in a Conviron (Walk-in chambers, Conviron, Winnipeg, Manitoba, Canada) growth room set at 22–26 °C around canopy with a 16 h-day/8 h-night photo cycle and 40% relative humidity. The photon flux density at soil level was 200–250 μM m−2 s−1 supplied mainly with cool light. Plants were watered from the soil top daily in the early morning with half strength B&D nutrient solution (Broughton & Dilworth 1971) plus 2 mM KNO3 and 2 mM NH4NO3 until 24 d after planting. Most of the plants at that time showed the fourth shoot emerging out and the main shoot could be coded as m7.9–m9.9 (Bucciarelli et al. 2006). Half of the plants were put under drought-stress imposition by holding watering while another half were kept with regular watering until harvesting or used for physiological measurement.
Physiological measuring to one set of plants and taking tissue samples for RNA isolation and metabolite analysis to another set of plants were performed simultaneously at the mimic mid-day time period (1300–1500 h). Leaf water potential (Ψw), relative leaf water content (%) of the up-most fully expanded leaves and the absolute water holding in pots (gram/pot) or percentage of the maximum field capacity have been used to monitor the drought-stress intensity in the preliminary experiments and the final design, although only Ψw will be showed here. The soil mixture used in this experiment made the water loss very gradual until 2 d after watering was ceased (data not shown). Ψw was measured using a thermocouple psychrometer (HR-33T Dew Point Microvoltmeter, and the small sample chambers from Wescor, Logan, UT, USA) and calculated MPa as: Ψ = ΔV/−7.5. Triplicate biological materials were collected for both control and drought-stressed samples at all time points. The whole shoot and root samples were separated, cleaned quickly, frozen in liquid nitrogen and stored at −80 °C prior to RNA isolation and metabolite analysis. Relative water content (RWC) of leaves was calculated using the formula [(FW-DW):(TW-DW)] × 100, where FW corresponds to the fresh weight of leaf samples, TW the turgid weight after 24 h rehydrating of leaf samples at 4 °C in the dark and DW, the dry weight of leaf samples after 24 h at 85 °C
Gene expression analysis
Total RNAs were isolated with TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA; http://www.lifetechnologies.com/us/en/home.html) following the manufacturer's guide. Samples were evaluated for purity with a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA; http://www.home.agilent.com/). The Affymetrix GeneChip® – Medicago Genome Array (Affymetrix, Santa Clara, CA, USA; http://www.affymetrix.com/) was used for expression analysis. Probe labelling, array hybridization and scanning were performed according to the manufacturer's instructions (Affymetrix) for eukaryotic RNA, using a one-cycle protocol for cDNA synthesis. The RNAs from all drought treatment/time points and two well-watered controls were analysed as showed in the Supporting information (Supporting Information Fig. S1 & S2). Based on these results and other physiological measurement, shoot (S) and root (R) RNA samples of three independent biological replicates of selected drought-stress treatment at day 2, 3, 4, 7, 10, 14 (named as D2/3/4/7/10/14D-S/R, respectively), 24 h post-rewatering of the 14-day drought-stressed plants (named as D14RW-S/R, respectively), and two well-watered controls at day 2 and day 4 (equalling to 26- or 28-day-old plants, named as D2W-S/R and D4W-S/R, respectively) were analysed. Data extraction and normalization was performed as previous described (Benedito et al. 2008).
Metabolite analysis
Whole shoot or root materials were lyophilized to be completely dry before they were homogenized into fine powder. Extraction and metabolite analysis was performed as reported by Broeckling et al. (2005) with minor modifications. Briefly, 6 mg of samples were used for extraction with chloroform for non-polar fraction, followed with water for polar fraction, both incubated at 50 °C for 45 min in the same vial. Docosanol and ribitol were added in the extraction buffers for internal standards, respectively. The polar extracts were dried down and resuspended, methoximated with 15 mg mL−1 methoxyamine hydrochlorides solution in pyridine. The non-polar extracts were resuspended with chloroform and hydrolyzed with HCl at 50 °C for 4 h, followed by drying down and resuspension in pyridine. All the extracts were derivatized through the addition of appropriate volume of methyltrimethylsilyltrifluoroacetamide (MSTFA) 1% N-methyl-N-trimethylsilyltrifluoroacetamide (TMSC) and incubated at 50 °C for 1 h.
One microlitre of the resulting solution was injected at 15:1 split ratio for the polar and 1:1 split ratio for the non-polar extracts onto a Hewlett Packard Agilent 6890 Gas Chromatograph System (HP 6890 GC, Agilent Technologies, Santa Clara, CA, USA) equipped with a 60 M DB-5-MS column (J&W Scientific, Folsom, CA, USA) coupled to a HP 5973 MS. The injection port and transfer arm was held at 280 °C. Separation was achieved with a temperature programme of 80 °C for 2 min, then ramped at 5 °C per min to 315 °C and held for 12 min and constant flow of 1.0 mL per min. The MS source was held at 250 °C and the quadruple at 150 °C and scanned from ratio of mass to charge (m/z) of 50 to 650. Compound derivative identification and quantification were conducted using the Metabolomics Ion-based Data Extraction Algorithm (MET-IDEA) developed and described by Broeckling et al. (2006). Due to visible chromatography difference, shoot and root samples were analysed separately.
Triplicate biological samples were used for the assay. Chromatography peaks presenting in all three replicates were extracted and quantified by selected single ions of the mass spectrums, with known or unknown derivatives/compounds/pools described as m/z of the selected ion, retention time of the compound (min) and identifier. The relative abundance of each derivatives/compounds/pool was normalized to the internal standards. Principal component analysis (PCA) was performed on Log10 transformed dataset of the mean values with Spotfire (TIBCO, Somerville, MA, USA) software.
Results
Experimental design and drought-stress physiology
Care was taken to make drought-stress treatments as realistic as possible. First, we tested various soil mixtures to find a substrate that dried slowly in the absence of watering and would allow easy harvesting of roots with minimal damage. Ultimately, we chose 16 cm diameter, 11 cm deep pots containing approximately 1.50 kg of a 2:1 mixture of sand:turface (v/v), which held declining amounts of water for 14 d during water withholding. We grew plants, one per pot, for 24 d prior to subjecting them to water withholding for various lengths of time, to establish the limits of drought beyond which plants would not recover. This limit was approximately 18 d. Ψw and RWC of well-watered 24-day-old plants taken at mid-day was typically around −0.8 MPa and 76.3%, respectively (Fig. 1). Following water withholding, plants maintained this level of Ψw and RWC for 2 d, after which Ψw decreased linearly for 4 d to −2.6 MPa at day 6. RWC also started to decline at day 3 and continued declining to 55.6% at day 5. Interestingly, a new steady-state Ψw above −3.0 MPa and RWC higher than 50% was maintained between days 6 and 10, after which water potential and RWC collapsed to −4.7 MPa and 37.7%, respectively, by day 14 after water withholding. Plants rewatered on day 14 recovered fully, with Ψw increasing to −1.9 MPa and RWC reaching 79.1% within just 24 h (Fig. 1).
Figure 1.
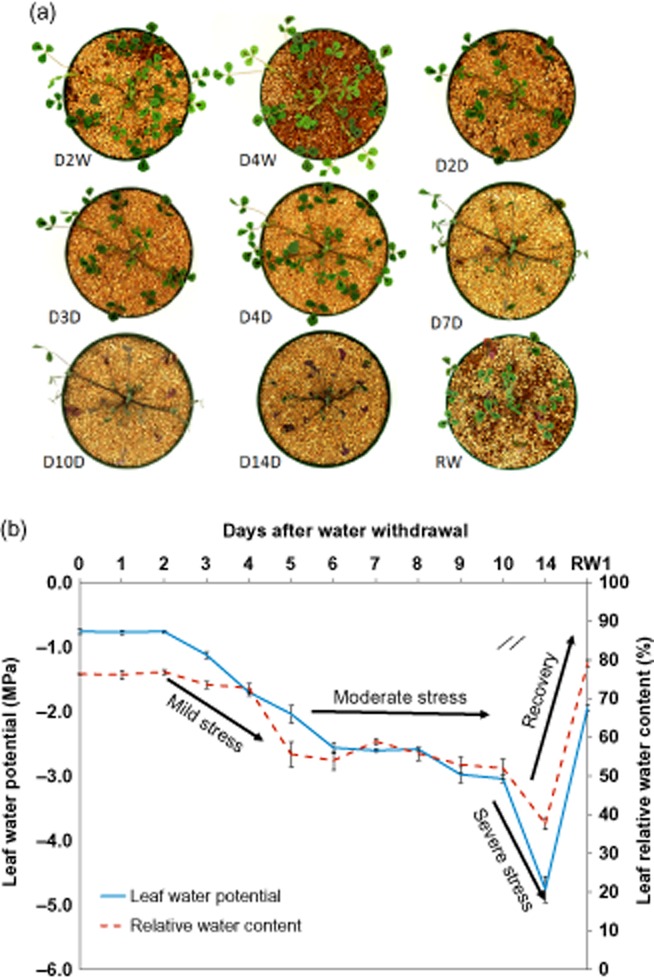
Drought stress time-course of Medicago truncatula plants. (a) Well-watered and drought-stressed M. truncatula plants throughout drought-stress progression and upon rewatering. (b) Effects of drought on leaf water potential (Ψw) and relative water content (RWC). D2W, 2-day well watered; D4W, 4-day well watered; D2D, 2-day drought; D3D, 3-day drought; D4D, 4-day drought; D7D, 7-day drought; D10D, 10-day drought; D14D, 14-day drought; RW1, 1 d after rewatering. Error bars represent SE (n = 3 plants).
We defined three phases of drought stress, based on Ψw, RWC and shoot phenology: Mild stress (days 2–5) during which Ψw declined progressively (−0.8 > Ψw > −2.0 MPa) without visible signs of stress apart from cessation of growth by day 4; moderate stress (days 6–10) during which Ψw stabilized above −3.0 MPa and RWC remained higher than 50.0%, but leaves withered; and severe but non-lethal stress (day 14) marked by shrivelling of leaves and very low Ψw (−4.7 MPa) and RWC (37.7%). Remarkably, most of the above-ground organs recovered fully within 24 h of rewatering, even after 14 days of drought (Fig. 1).
Transcriptome variation of drought-stressed Medicago plants
Plants were grown for 24 d with daily watering before being subjected to drought stress, as described above. Roots and shoots were harvested separately at 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 and 14 d after water withholding, or 24 h after rewatering of plants deprived of water for the full 14 d. Besides the 0-day control, additional control plants were watered for 2 or 4 more days and were harvested at days 26 and 28 after planting, respectively as illustrated in Fig. 1. All plant materials were sampled between 0900 and 1100 h, 3–5 h after the beginning of the day cycle, to avoid as much as possible diurnal variation in gene expression that would obscure the effects of drought or rewatering. Total RNA was isolated from roots and shoots and subjected to Affymetrix GeneChip analysis. The Affymetrix Medicago GeneChip contains 50 900 partially redundant probe sets designed to detect transcripts for the vast majority of Medicago genes. To determine the most informative time points for statistical analysis of transcriptome responses to drought stress and to avoid the cost of measuring three biological replicates for each time point, GeneChip analysis was initially performed on a single biological replicate of shoots and roots separately for all time points (Supporting Information Fig. S1 & S2, respectively). Transcript levels of 32.3% of all genes represented by probe sets on the GeneChip increased or decreased at least twofold in drought-stressed shoots compared with levels in the watered control (drought day 0), at one or more time points (Supporting Information Fig. S1). Similarly, 34.8% of genes in roots were induced or repressed at least twofold, respectively, in response to drought (Supporting Information Fig. S2). Based on the number of differentially expressed genes at each time point of drought stress, we chose the following time points for further analysis using two additional biological replicates: 2, 3, 4, 7, 10 and 14 d of drought (water withholding) and 24 h following rewatering. Plants that continued to receive water for an additional 2 and 4 d, that is 26- and 28-day-old plants, were used as controls because they matched the growth/development of the drought-stressed plants, which ceased growth soon after 26 d but before 28 d. There were very few differentially expressed genes in plants watered for 26 d compared with watered plants harvested at 28 d, so the 26-day-old well-watered plants served as the sole control for the drought-stressed and rewatered plants.
Gene transcripts were detected in shoots and roots by 38 516 (75.7% of total) and 39 547 (77.7%) probe sets, respectively, with the majority of genes (corresponding to 35 720 probe sets) expressed in both organs. Genes corresponding to a total of 8994 probe sets for shoots and 10 466 for roots, were identified as drought-regulated (Fig. 2a). Among these, 724 and 785 were drought-specific in shoots and roots, respectively, and 221 were common to shoots and roots (Fig. 2a). By using the highest transcript level change among all time points to categorize every probe set, genes corresponding to 5458 and 5164 probe sets were significantly induced by drought in shoots and roots, respectively, when compared with controls (>2-fold, P < 0.05) (Fig. 2b). Among these, 2768 were categorized as commonly induced genes in both shoot and root. In shoots, the number of induced genes was greater than the number of repressed genes at each drought-stress time point. However, in roots, the opposite trend occurred with the number of repressed genes being greater than the number of induced genes at all time points except for two at the beginning of drought imposition (Fig. 3). Globally, genes corresponding to only 1757 probe sets were categorized as commonly repressed by drought in both organ types.
Figure 2.
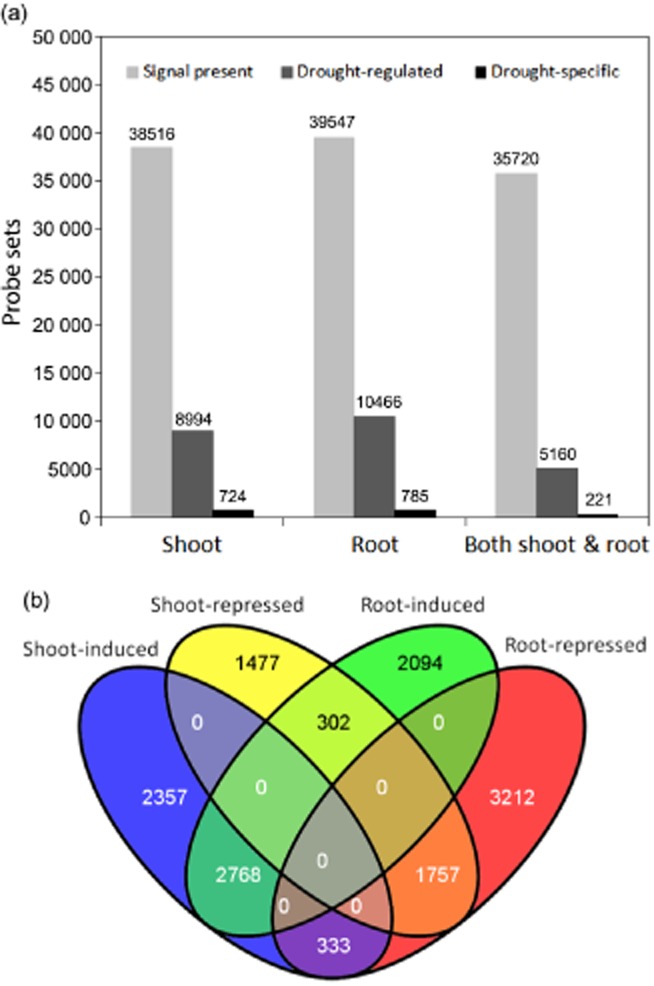
Global transcriptomic changes during drought stress in Medicago truncatula. (a) Global number of probe sets that were regulated by drought or exclusively expressed under drought stress. (b) Venn diagram of drought-regulated genes in shoots and roots. The cut-off limit was twofold change and P-value < 0.05.
Figure 3.
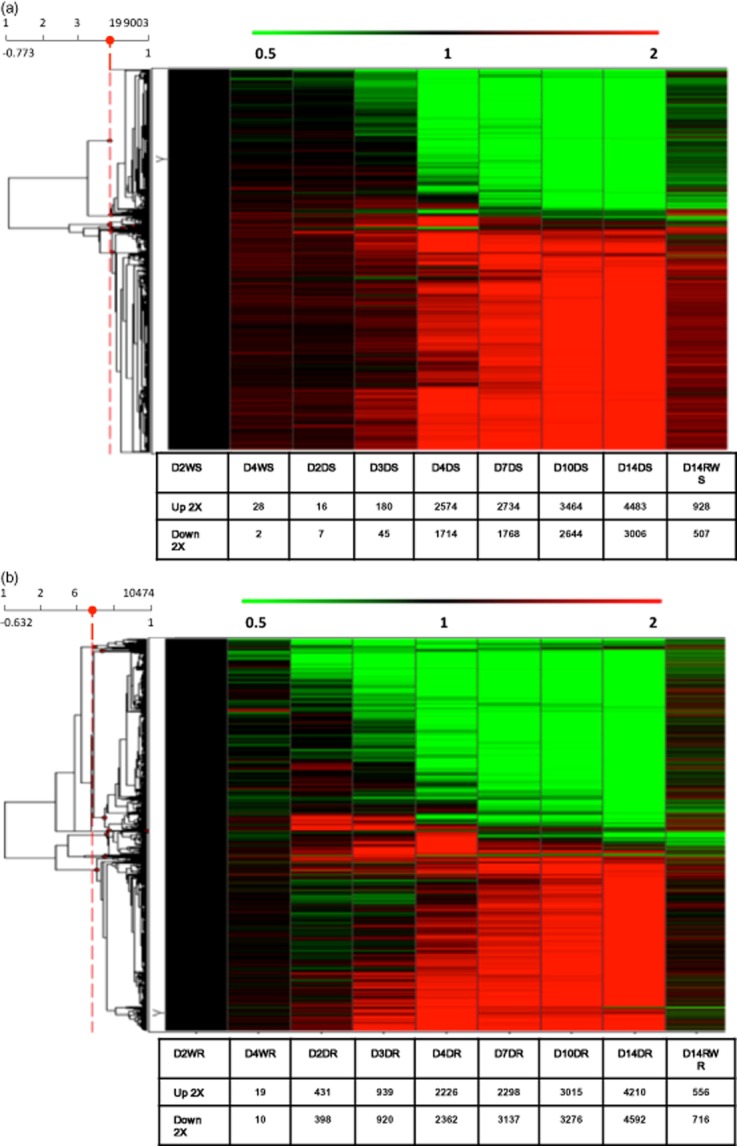
Hierarchical clustering analysis of drought-induced changes in gene expression in (a) shoots and (b) roots. Data from D14RWR were not used for clustering. D2W, 2-day well watered; D4W, 4-day well watered; D2D, 2-day drought; D3D, 3-day drought; D4D, 4-day drought; D7D, 7-day; D10D drought, 10-day drought; D14D, 14-day drought; D14RW, 1 d after rewatering; S, shoots; R, roots.
In shoots, a cumulative total of 14.2% of the genes were induced and 9.2% repressed in response to drought, while in the roots, 13.1% were induced and 13.4% repressed. Thus, although root-gene expression responded faster at the early drought stages, the global transcriptome response was of the same order of amplitude in both shoots and roots.
Top 100 most responsive genes in shoots and roots during drought stress
Based on the expression ratio at any drought-stress time point relative to well-watered controls (day 2), the top 100 most drought-induced and repressed genes in the shoots and in roots were selected (Supporting Information Tables S1–S4). Most of these in shoots and roots showed a very low expression level under well-watered conditions and at the onset of the drought treatment (day 2 without watering; Fig. 3; Supporting Information Figs S1 & S2). In shoots, most genes on the top 100 list were induced to the highest level of expression at day 4, corresponding to late mild stress with a Ψw of −1,70 MPa and RWC of 72% (Fig. 1; Supporting Information Table S1). In the roots, gene induction happened earlier, at day 3, when Ψw was −1.13 MPa and RWC of 73.5% (Fig. 1; Supporting Information Table S3).
Present in the list of the top 100 most drought-activated genes in shoots was one encoding a Δ1-pyrroline-5-carboxylate synthetase gene (P5CS, TC94074) for proline synthesis, one NAD-dependent aldehyde dehydrogenase family protein gene (TC108200) and multiple genes related to galactose metabolism, such as an alkaline alpha galactosidase I (TC107085), an alkaline alpha galactosidase II (TC95539) and a galactinol synthase (BG451003; Supporting Information Table S1). Several transcription factor genes were also found to be highly activated in the shoots, for example, genes encoding a homeobox associated leucine zipper protein (IMGAG|1047.m00031), a nuclear transcription factor Y subunit B3 (AJ501814) and an ethylene-responsive transcription factor (ERF1, TC105911), a R2R3myb transcription factor (BF635572) and two NAC-domain transcription factor genes (TC94915, IMGAG|739.m00012). A myb transcription factor (IMGAG|1070.m00005), a NAC transcription factor-like protein (IMGAG|739.m00012) and a BZip transcription factor gene (TC103857) were present in the root top 100 most induced gene list (Supporting Information Table S3).
Among the top 100 genes induced in shoots and roots, 39 were common to both organs (Table 1). Six of these encode late embryogenesis abundant (LEA) proteins, six are annotated as heat-shock protein genes, two are cold-inducible genes and 15 have no functional annotation. Interestingly, two genes were annotated as defensins, which commonly respond to biotic stress responses (Hanks et al. 2005; Stotz et al. 2009). Others include one expansin gene involved in cell-wall loosening, a ferritin gene for ion storage, and one benzodiazepine receptor gene. In total, there were four enzymatic genes present in this list, including a gene for 1-cys peroxiredoxin, one for aldehyde dehydrogenase, one for phosphatase type 2C precursor and one 9-cis-epoxycarotenoid dioxygenase (NCED). One gene categorized as a NAC transcription factor-like protein (IMGAG|739.m00012) was found to be highly induced in both shoots and roots (Table 1).
Table 1.
Common top 40 most drought-responsive genes in both shoots and roots
| Expression | Rank | Probe sets | Gene annotation and locus | Representative Public ID |
|---|---|---|---|---|
| Up-regulated | 1 | Mtr.8651.1.S1_at | Medtr6g0846401 | dehydrin | HC | chr63178867631786630 | 20130731 | TC100921 |
| 2 | Mtr.35625.1.S1_s_at | IMGA|contig_55282_11 Unknown protein contig_55282 1127618 F PREDN 20111014 | TC108850 | |
| 3 | Mtr.45188.1.S1_at | IMGA|contig_81759_11 Defensin contig_81759 863339 F PREDN 20111014 | TC99014 | |
| 4 | Mtr.11503.1.S1_at | Medtr1g1006271 | hypothetical protein | HC | chr14555204145547838 | 20130731 | TC110135 | |
| 5 | Mtr.44594.1.S1_at | Medtr2g0794301 | Defensin MtDef21 | HC | chr23343044933431563 | 20130731 | TC97678 | |
| 6 | Mtr.10877.1.S1_at | Medtr3g0781701 | NAD-dependent aldehyde dehydrogenase family protein | HC | chr33521329535209217 | 20130731 | TC108200 | |
| 7 | Mtr.21257.1.S1_at | IMGA|Medtr2g0140401 Late embryogenesis abundant protein chr2 41529914154570 E EGN_Mt100125 20111014 | IMGAG|1147.m00027 | |
| 8 | Mtr.36679.1.S1_s_at | IMGA|Medtr5g0200601 hypothetical protein chr5 73896707388452 F EGN_Mt100125 20111014 | BQ145052 | |
| 9 | Mtr.11099.1.S1_at | Medtr4g0947201 | 1cys peroxiredoxin PER1 | HC | chr43884344738842477 | 20130731 | TC108877 | |
| 10 | Mtr.41871.1.S1_at | IMGA|Medtr7g0349401 227 kDa class IV heat shock protein chr7 1005556910055006 F EGN_Mt100125 20111014 | TC110284 | |
| 11 | Mtr.29531.1.S1_at | IMGA|Medtr8g1051901 hypothetical protein chr8 3118080131181202 E EGN_Mt100125 20111014 | TC105266 | |
| 12 | Mtr.41906.1.S1_at | Medtr4g0993701 | expansinB1like protein | HC | chr44123206341233559 | 20130731 | TC94511 | |
| 13 | Mtr.43091.1.S1_s_at | Medtr7g0931601 | seed maturation protein | HC | chr73699506636993553 | 20130731 | IMGAG|1101.m00002 | |
| 14 | Mtr.37831.1.S1_at | IMGA|AC233577_251 hypothetical protein AC2335775 108210103914 E EGN_Mt100125 20111014 | TC101513 | |
| 15 | Mtr.17894.1.S1_at | IMGA|Medtr1g0141101 hypothetical protein chr1 36597133659180 F EGN_Mt100125 20111014 | IMGAG|1019.m00005 | |
| 16 | Mtr.12358.1.S1_at | IMGA|Medtr7g0931601 Seed maturation protein LEA chr7 2970494429703431 F EGN_Mt100125 20111014 | TC94509 | |
| 17 | Mtr.19279.1.S1_at | IMGA|Medtr4g1305401 Heat shock protein chr4 4585175445849633 E EGN_Mt100125 20111014 | IMGAG|978.m00004 | |
| 18 | Mtr.23672.1.S1_at | Medtr4g1239501 | group 3 LEA protein | HC | chr45111324851111106 | 20130731 | 1688.m00031 | |
| 19 | Mtr.51178.1.S1_at | IMGA|Medtr4g1038401 hypothetical protein chr4 3600742236008323 E EGN_Mt100125 20111014 | IMGAG|877.m00013 | |
| 20 | Mtr.11070.1.S1_s_at | IMGA|Medtr3g0924602 Chloroplast small heat shock protein chr3 3168876631689882 F EGN_Mt100125 20111014 | TC108781 | |
| 21 | Mtr.15417.1.S1_at | Medtr8g0937901 | NAC transcription factor-like protein | HC | chr83924284239241213 | 20130731 | IMGAG|739.m00012 | |
| 22 | Mtr.27693.1.S1_at | IMGA|Medtr3g0850201 hypothetical protein chr3 2769531427694918 F EGN_Mt100125 20111014 | BE942288 | |
| 23 | Mtr.15895.1.S1_s_at | Medtr1g0834402 | dormancy auxin associated protein | HC | chr13713575837137709 | 20130731 | IMGAG|849.m00019 | |
| 24 | Mtr.13436.1.S1_at | Medtr1g0283001 | protein phosphatase 2Clike protein | HC | chr195020769505086 | 20130731 | TC98007 | |
| 25 | Mtr.37609.1.S1_at | Medtr8g0700151 | Lipid transfer protein | HC | chr82970650029707286 | 20130731 | TC101042 | |
| 26 | Mtr.41130.1.S1_at | IMGA|Medtr3g0924602 Chloroplast small heat shock protein chr3 3168876631689882 F EGN_Mt100125 20111014 | TC108780 | |
| 27 | Mtr.41655.1.S1_at | IMGA|Medtr2g0264201 hypothetical protein chr2 83546338355298 F EGN_Mt100125 20111014 | TC109832 | |
| 28 | Mtr.39929.1.S1_at | IMGA|contig_115871_11 class II heat shock protein contig_115871 124869 F PREDN 20111014 | TC106102 | |
| 29 | Mtr.19818.1.S1_at | Medtr7g0699801 | ferritin | HC | chr72579481925791891 | 20130731 | IMGAG|1091.m00001 | |
| 30 | Mtr.8790.1.S1_at | IMGA|Medtr7g1086501 hypothetical protein chr7 3422464434225432 F EGN_Mt100125 20111014 | TC101400 | |
| 31 | Mtr.12321.1.S1_at | IMGA|contig_88317_11 MtN19like protein contig_88317 1541621 E PREDN 20111014 | TC94372 | |
| 32 | Mtr.11203.1.S1_at | Medtr4g0176501 | phosphatidylethanolamine binding protein | HC | chr455428115544084 | 20130731 | TC109200 | |
| 33 | Mtr.41622.1.S1_at | Medtr3g0677201 | cold regulated protein putative | HC | chr33036086830361438 | 20130731 | TC109750 | |
| 34 | Mtr.5918.1.S1_at | Medtr4g0850701 | DnaJ heat shock amine terminal domain protein | HC | chr43325166933250524 | 20130731 | BG452391 | |
| 35 | Mtr.27969.1.S1_at | IMGA|Medtr5g0200601 hypothetical protein chr5 73896707388452 F EGN_Mt100125 20111014 | BF635147 | |
| 36 | Mtr.12214.1.S1_at | IMGA|Medtr5g0640601 class I heat shock protein chr5 2586810725868924 F EGN_Mt100125 20111014 | TC93983 | |
| 37 | Mtr.12327.1.S1_at | IMGA|contig_69549_11 Late embryogenesis abundant protein contig_69549 11172381 F PREDN 20111014 | TC94389 | |
| 38 | Mtr.45327.1.S1_at | IMGA|Medtr5g0263201 Omega-hydroxypalmitate O-feruloyl transferase chr5 1056343910564838 E EGN_Mt100125 20111014 | TC99383 | |
| 39 | Mtr.35044.1.S1_at | IMGA|contig_70576_11 9cisepoxycarotenoid dioxygenase contig_70576 16823514 E PREDN 20111014 | CX531529 | |
| Down-regulated | 1 | Mtr.12822.1.S1_at | IMGA|Medtr6g0219501 Pectate lyase chr6 48288254825330 F EGN_Mt100125 20111014 | TC96079 |
On the list of top 100 most repressed genes in the shoots was a gene for a plant lipid transfer/seed storage/trypsin-alpha amylase inhibitor gene that responded negatively between moderate to severe drought stress but recovered close to normal expression after 24 h rewatering (Supporting Information Table S4). Most of the top 100 most repressed genes in the roots responded to drought as early as day 2 (beginning of mild stress with Ψw of −0.76 MPa and RWC of 76.81%) and the expression was repressed to the lower levels more gradually than the top-repressed genes in shoots (Supporting Information Table S4). The top three most highly repressed expressed genes showed the highest expression level at 24 h after rewatering (higher than under normal growth conditions). In the roots, one gene encoding a chalcone synthase (TC95902) seemed to be suppressed by mild drought stress and was induced by moderate and severe drought stress (Supporting Information Tables S3 & S4). Several repressed genes encoded hydrolases such as xyloglucan endotransglucosylase, glycosyl hydrolases, pectate lyase, or encoded transporters such as two nitrate transporters (BE318511, BE205238) in roots, or photosynthesis-related genes such as photosystem II type I chlorophyll a/b-binding protein precursor gene (TC100145) in shoots. Among the top 100 most repressed genes in shoots and roots, there was only one that was common to both organs, which was annotated as a probable pectate lyase P18 precursor and (TC96079; Table 1).
Strikingly, the majority of the genes that were induced or repressed in roots (93.6% and 93.2%, respectively) and shoots (84.1% and 87.5%, respectively) in response to drought reacted oppositely to the addition of water at day 14, returning back to their pre-stress steady-state levels.
Relation between drought-stress intensity and the magnitude of gene expression
For the genes that responded to drought stress, generally there was a strong correlation between the magnitude of gene expression variation (induction or repression) and the degree of drought stress, as measured by the Ψw (Fig. 4). The correlation between transcript level change and Ψw was >0.8 (absolute value) for 59.6% of the drought-responsive genes in shoots and <0.5 for only 10% of such genes. Similarly, 46.6% of the drought-responsive genes in the roots had correlation coefficients (transcript × Ψw) > 0.8 and only about 19% < 0.5. Interestingly, the majority of genes that highly correlated with Ψw were induced rather than repressed by drought stress in both shoots (40.2% versus 19.4% of all regulated genes, respectively) and roots (31.8% versus 14.8%, respectively) (Fig. 4). In shoots and roots, 15.6% and 15.1%, respectively, of the repressed genes whose expression change showed correlation coefficients >0.8, were related to carbohydrate metabolism based on Gene Ontology (GO) annotation. There were 7.3% and 7.1% induced genes in the shoots and roots, respectively. A small set of drought-induced genes exhibited Pearson's correlation coefficients > 0.99 (transcript level versus −Ψw), making them potentially interesting markers for drought stress (Table 2). Genes for which the correlation between transcript level change and Ψw was relatively low (<0.5 or lower), exhibited various types of response to drought; transient induction or repression, a delayed or threshold response, or a plateau or flat-valley response (Supporting Information Figs S3 & S4).
Figure 4.
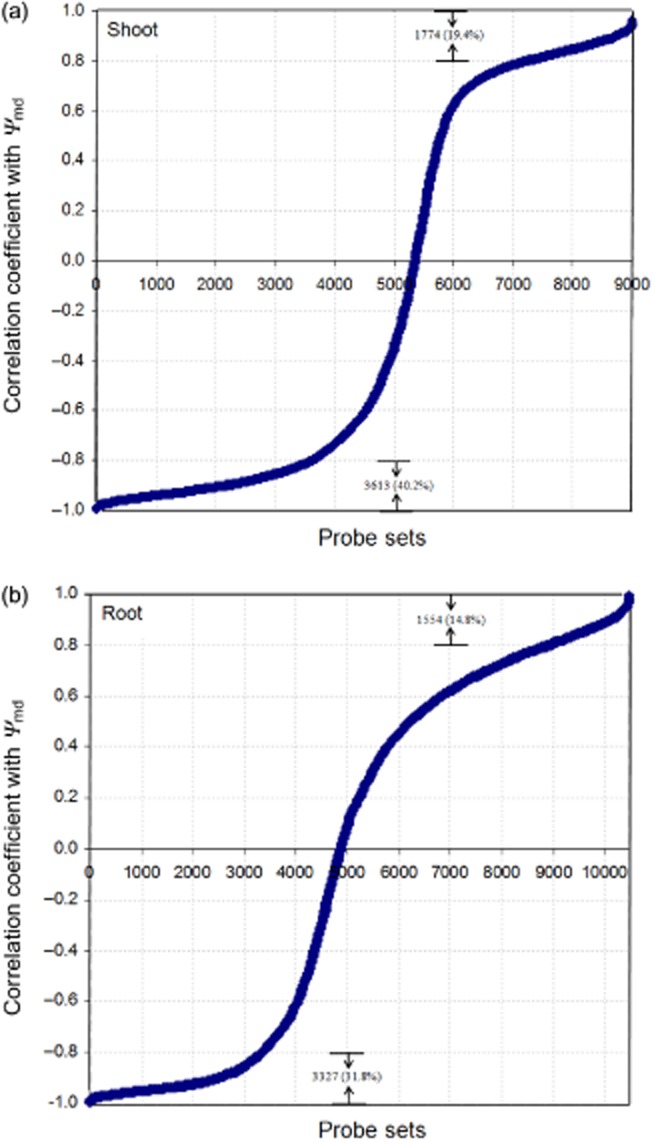
Correlation between leaf water potential (Ψw) and transcript level of drought-affected genes in shoots (a) and roots (b).
Table 2.
Genes showing high correlation coefficient of expression level change and leaf water potential
| Probe set | Gene annotation and locus | Tissue type | Correlation Coefficient | Highest ratio change |
|---|---|---|---|---|
| Mtr.42865.1.S1_at | TC93979 /Alternative oxidase (Fragment), partial (10%) | Shoot | −0.9955 | 7.58 |
| Mtr.31045.1.S1_at | Medtr0703s00201 | hypothetical protein | HC | scaffold070358457647 | 20130731 | Shoot | −0.9941 | 2.81 |
| Mtr.2667.1.S1_at | IMGA|Medtr6g0444701 Protein complex coatmer beta subunit chr6 86322398630922 H EGN_Mt100125 20111014 | Shoot | −0.9937 | 9.00 |
| Mtr.50791.1.S1_s_at | IMGA|Medtr6g0710901 Translationally controlled tumour protein-like protein chr6 1455150314551988 H EGN_Mt100125 20111014 | Shoot | −0.9906 | 3.83 |
| Mtr.9692.1.S1_at | IMGA|Medtr7g0922001 DNA binding protein SMUBP2 chr7 2923336229226076 E EGN_Mt100125 20111014 | Shoot | −0.9886 | 5.78 |
| Mtr.23608.1.S1_at | Medtr7g0732601 | PPR containing plant-like protein | HC | chr72736229927360390 | 20130731 | Shoot | −0.9883 | 3.27 |
| Mtr.40657.1.S1_at | TC107767 /1-aminocyclopropane-1-carboxylic acid oxidase, partial (8%) | Shoot and root | −0.9879 | 6.61 |
| Mtr.37441.1.S1_s_at | Medtr3g0899771 | zinc binding alcohol dehydrogenase family protein | HC | chr34090312940906106 | 20130731 | Shoot | −0.9878 | 5.68 |
| Mtr.12232.1.S1_x_at | IMGA|Medtr4g1185801 hypothetical protein chr4 4090274740906982 I EGN_Mt100125 20111014 | Shoot | −0.9872 | 8.30 |
| Mtr.37444.1.S1_at | N/A | Shoot | −0.9867 | 5.30 |
| Mtr.8630.1.S1_at | IMGA|Medtr8g0378001 hypothetical protein chr8 87786608777233 F EGN_Mt100125 20111014 | Shoot | −0.9858 | 6.29 |
| Mtr.44072.1.S1_at | Medtr5g0099702 | endo14betaglucanase | HC | chr525626342565277 | 20130731 | Shoot | −0.9857 | 8.26 |
| Mtr.43253.1.S1_at | N/A | Shoot | −0.9856 | 7.47 |
| Mtr.44922.1.S1_at | IMGA|contig_76403_11 Cold shock protein1 contig_76403 19932942 E PREDN 20111014 | Shoot | −0.9851 | 3.16 |
| Mtr.10364.1.S1_at | IMGA|Medtr8g0454001 MLP-like protein chr8 1175437111753092 E EGN_Mt100125 20111014 | Shoot | −0.9838 | 19.71 |
| Mtr.7580.1.S1_x_at | AA660448 /FEA=mRNA /DEF=similar to PIR|C61615|C61615 sericin MG-2 – greater wax moth (fragments) {Galleria mellonella;}, partial (8%) | Shoot | −0.9824 | 3.53 |
| Mtr.40811.1.S1_at | IMGA|AC233662_161 Ribosome production factor AC2336621 7204277318 E EGN_Mt100125 20111014 | Shoot | −0.9812 | 4.43 |
| Mtr.9656.1.S1_at | Medtr5g0364801 | two-component response regulator ARR3like protein | HC | chr51591829415920037 | 20130731 | Shoot | −0.9806 | 4.54 |
| Mtr.43227.1.S1_at | IMGA|contig_52784_11 RING finger protein contig_52784 304248 F PREDN 20111014 | Shoot | −0.9802 | 4.06 |
| Mtr.20096.1.S1_at | Medtr7g1132501 | hypothetical protein | LC | chr74662885146631118 | 20130731 | Root | −0.995694 | 3.35 |
| Mtr.37975.1.S1_at | Medtr4g0069701 | CBL interacting kinase | HC | chr4882035879328 | 20130731 | Root | −0.994203 | 7.18 |
| Mtr.32750.1.S1_at | Medtr2g0789701 | MATE efflux family protein | HC | chr23306789733072195 | 20130731 | Root | −0.990034 | 20.68 |
| Mtr.18757.1.S1_at | Medtr1g0715301 | sulfate bicarbonate oxalate exchanger and transporter sat1 | HC | chr13174411131751380 | 20130731 | Root | −0.989965 | 21.96 |
| Mtr.21271.1.S1_at | Medtr3g0719901 | cation H exchanger 3 | HC | chr33234414432340399 | 20130731 | Root | −0.988743 | 4.55 |
| Mtr.41483.1.S1_at | IMGA|Medtr4g0495001 DNA repair protein RAD5 chr4 1442998514438493 E EGN_Mt100125 20111014 | Root | −0.988537 | 3.06 |
| Mtr.8872.1.S1_at | IMGA|Medtr2g0451201 hypothetical protein chr2 1611035216108774 H EGN_Mt100125 20111014 | Root | −0.988160 | 3.37 |
| Mtr.17829.1.S1_at | Medtr5g0091601 | UDPDglucose UDPDgalactose 4epimerase | HC | chr521295232125184 | 20130731 | Root | −0.987361 | 4.84 |
| Mtr.10685.1.S1_at | IMGA|Medtr4g0647501 hypothetical protein chr4 2043435920437282 F EGN_Mt100125 20111014 | Root | −0.987331 | 5.11 |
| Mtr.11963.1.S1_s_at | Medtr1g0715301 | sulfate bicarbonate oxalate exchanger and transporter sat1 | HC | chr13174411131751380 | 20130731 | Root | −0.986725 | 14.70 |
| Mtr.40657.1.S1_at | TC107767 /FEA=mRNA /DEF=similar to UP|Q84L58 (Q84L58) 1-aminocyclopropane-1-carboxylic acid oxidase, partial (8%) | Root and shoot | −0.986689 | 3.99 |
| Mtr.42178.1.S1_at | IMGA|Medtr3g0915701 Soluble starch synthase chr3 3117741531184709 E EGN_Mt100125 20111014 | Root | −0.986264 | 4.00 |
| Mtr.43205.1.S1_at | IMGA|Medtr1g0256201 hypothetical protein chr1 83180188315560 F EGN_Mt100125 20111014 | Root | −0.985650 | 4.00 |
| Mtr.25503.1.S1_at | Medtr7g1098001 | WRKY family transcription factor | HC | chr74497335444975284 | 20130731 | Root | −0.985551 | 4.57 |
| Mtr.39305.1.S1_at | IMGA|CU571152_10151 Heat stress transcription factor A3 CU5711525 7052866484 E EGN_Mt100125 20111014 | Root | −0.984654 | 5.78 |
| Mtr.3434.1.S1_at | N/A | Root | −0.984504 | 3.96 |
| Mtr.43557.1.S1_at | N/A | Root | −0.983875 | 7.12 |
| Mtr.50233.1.S1_at | Medtr4g1305302 | translation initiation factor IF3 | HC | chr45439356254389216 | 20130731 | Root | −0.983717 | 4.24 |
| Mtr.43557.1.S1_x_at | N/A | Root | −0.982991 | 7.19 |
| Mtr.38430.1.S1_at | IMGA|contig_57466_11 CBL interacting protein kinase contig_57466 3932028 F PREDN 20111014 | Root | −0.982434 | 9.90 |
| Mtr.40073.1.S1_at | IMGA|Medtr2g0429001 Protein TIFY 10B chr2 1560499715607468 F EGN_Mt100125 20111014 | Root | −0.982421 | 5.02 |
| Mtr.30102.1.S1_at | N/A | Root | −0.982287 | 4.00 |
| Mtr.44497.1.S1_at | Medtr1g1074902 | stress enhanced protein | HC | chr14879720648799645 | 20130731 | Root | −0.981902 | 4.16 |
| Mtr.50976.1.S1_at | Medtr3g0775701 | glutaredoxin C4 | HC | chr33486633034869834 | 20130731 | Root | −0.981623 | 6.38 |
| Mtr.32833.1.S1_at | BE325397 /FEA=mRNA /DEF=weakly similar to UP|Q69K08 (Q69K08) Lingual lipase-like, partial (6%) | Root | −0.981611 | 12.63 |
| Mtr.15473.1.S1_s_at | IMGAG|742.m00002 /FEA=mRNA /DEF=Amino acid/polyamine transporter II AC122169.23.11 4547 9560 mth2-9m5 01/13/05 | Root | −0.981566 | 5.14 |
| Mtr.28127.1.S1_at | IMGA|AC151525_291 hypothetical protein AC15152518 135373136092 L EGN_Mt100125 20111014 | Root | −0.981373 | 3.25 |
| Mtr.9959.1.S1_at | N/A | Root | −0.980908 | 9.14 |
| Mtr.47159.1.S1_s_at | Medtr1g0121901 | hypothetical protein | HC | chr123802772379844 | 20130731 | Root | −0.980771 | 5.59 |
| Mtr.36849.1.S1_at | IMGA|contig_68666_11 Receptor-like protein kinase contig_68666 5413838 E PREDN 20111014 | Root | −0.980583 | 3.84 |
| Mtr.48549.1.S1_at | Medtr4g1186571 | hypothetical protein | HC | chr44915389849154167 | 20130731 | Root | −0.980569 | 3.93 |
| Mtr.13647.1.S1_at | Medtr4g0748602 | general transcription factor 3Clike protein | HC | chr42848503928503815 | 20130731 | Root | −0.980330 | 3.06 |
| Mtr.6506.1.S1_at | N/A | Root | −0.980052 | 3.28 |
| Mtr.34978.1.S1_at | IMGA|Medtr3g0925802 RNA binding protein chr3 3175856731753379 F EGN_Mt100125 20111014 | Root | −0.980048 | 4.39 |
N/A, No annotation.
An Affymetrix programme, dCHIP was used to determine whether a gene transcript was present or absent in each sample, which resulted in the identification of genes corresponding to 693 probe sets that were expressed exclusively during drought stress in roots, 609 in shoots and 228 in both roots and shoots, that is transcripts detected in at least two of three biological replicates (Table 3). The majority of these genes, 564 in roots and 503 in shoots and 221 in both organs, were not expressed in unstressed plants as shown by a previous study (Benedito et al. 2008) indicating that they may have evolved specialized roles for drought-stress adaptation. Included among these were genes encoding pyrroline-5-carboxylate synthetase (P5CS) enzymes, a zinc metalloprotease (FtsH protease) and a chalcone reductase (CHR), all of which were induced in both roots and shoots.
Table 3.
Genes exclusively expressed in the present drought-stress experiment
| Shoot | Root | Shoot and root | Sub-total | |
|---|---|---|---|---|
| Drought stress condition | ||||
| Present call ≥ 2 | 503 | 564 | 221 | 1288 |
| Present call ≥ 3 | 50 | 66 | 70 | 186 |
| Well watered 26- and 28-day-old plants | ||||
| Present call ≥ 2 | 106 | 129 | 7 | 242 |
| Present call ≥ 3 | 5 | 10 | 2 | 17 |
| Both well watered and drought stress condition | ||||
| Present call ≥ 2 | 350 | |||
| Present call ≥ 3 | 127 | |||
| Total | ||||
| Present call ≥ 2 | 1880 | |||
| Present call ≥ 3 | 330 | |||
Early transcriptome responses to drought stress
The total number of drought-regulated genes was tightly correlated to drought-stress imposition length and intensity (Fig. 3; Supporting Information Figs S1 & S2). Two days after water withholding, very few genes were induced (corresponding to 16 probe sets) or repressed (seven probe sets) in shoots, compared with watered controls of the same age. Among the genes induced by this very mild drought stress in shoots were one or two CpABA1-like genes encoding zeaxanthin epoxidase (ZEP), an up-stream enzyme of the abscisic acid (ABA) biosynthesis pathway (Table 4). These corresponded to two probe sets representing non-overlapping regions of either the same or two different CpABA1-like genes. This gene(s) was different to others in the group in that its expression was induced and kept high only at the early stages of the drought stress. It was listed among early transient responsive genes in drought-stressed shoots (Supporting Information Table S6) and will be described further. Genes corresponding to the other 14 probe sets maintained high expression levels during drought progression until plants were rewatered. These included three ferritin-encoding genes, two legume-specific genes with unknown function, one histidine-containing phosphotransfer protein gene (with two probe sets), a small signal peptidase gene, a protein kinase gene and a cation transporter gene. Interestingly, gene corresponding to eight probe sets in this group were previously found to be expressed mainly in flower tissues (Medicago Gene Expression Atlas, http://bioinfo.noble.org/gene-atlas/v2/). Among the seven genes repressed as early as day 2 after water withholding, three were peroxidase genes, one a carboxylate oxidase gene, one a thioredoxin gene and two had unknown functions.
Table 4.
Early-responsive genes in drought-stressed shoots
| Probe sets | Gene annotation and locus | Representative Public ID | Expression change in ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D2WS | D4WS | D2DS | D3DS | D4DS | D7DS | D10DS | D14DS | D14RWS | |||
| Mtr.33852.1.S1_at | Medtr5g0173501 | zeaxanthin epoxidase | HC | chr563135606323118 | 20130731 | BI271514 | 1 | 1.7076 | 2.0546 | 5.9089 | 6.0337 | 1.5695 | 0.7919 | 0.7639 | 0.8126 |
| Mtr.1857.1.S1_at | IMGA|Medtr5g0173301 Zeaxanthin epoxidase chr5 60566986060213 E EGN_Mt100125 20111014 | BE204779 | 1 | 1.9801 | 2.4014 | 7.4664 | 8.0392 | 1.8765 | 0.8100 | 0.8173 | 0.8279 |
| Mtr.45371.1.S1_at | N/A | TC99492 | 1 | 2.3633 | 2.7004 | 3.9066 | 7.0092 | 10.5298 | 10.6059 | 11.3689 | 3.0113 |
| Mtr.49088.1.S1_at | Medtr8g0754201 | rhodanese related sulfur transferase | HC | chr83189814731896095 | 20130731 | IMGAG|726.m00008 | 1 | 1.6006 | 2.0694 | 3.6752 | 3.4273 | 5.2127 | 5.2575 | 5.5416 | 2.2310 |
| Mtr.2090.1.S1_at | N/A | BF519327 | 1 | 2.2711 | 2.1017 | 7.7580 | 32.0803 | 30.1453 | 30.7151 | 35.8098 | 4.9058 |
| Mtr.27132.1.S1_at | Medtr2g1008801 | histidine phosphotransfer protein | HC | chr24339584143394459 | 20130731 | AW561075 | 1 | 2.1733 | 2.4844 | 4.5350 | 6.3434 | 6.4350 | 7.0647 | 7.1753 | 1.9299 |
| Mtr.27132.1.S1_s_at | Medtr2g1009001 | histidine phosphotransfer protein | HC | chr24340798543406861 | 20130731 | AW561075 | 1 | 2.3600 | 2.9214 | 4.7801 | 6.2354 | 6.0902 | 7.1425 | 6.9854 | 1.9733 |
| Mtr.19818.1.S1_at | Medtr7g0699801 | ferritin | HC | chr72579481925791891 | 20130731 | IMGAG|1091.m00001 | 1 | 1.0728 | 2.1563 | 4.6703 | 45.8855 | 54.9684 | 51.0670 | 49.8691 | 3.1585 |
| Mtr.42339.1.S1_at | IMGA|Medtr2g1008801 Histidine phosphotransfer protein chr2 3225151932250137 F EGN_Mt100125 20111014 | TC111274 | 1 | 2.1123 | 2.5241 | 4.6124 | 5.8058 | 6.6711 | 7.7121 | 7.7093 | 2.0289 |
| Mtr.38411.1.S1_at | Medtr4g1043701 | KDEL-tailed cysteine endopeptidase CEP1 | HC | chr44318181443180214 | 20130731 | TC102742 | 1 | 2.1640 | 2.5370 | 7.9693 | 51.4026 | 12.2821 | 17.4863 | 24.0820 | 1.1213 |
| Mtr.5824.1.S1_at | IMGA|Medtr4g0145401 Ferritin3 chr4 32420453244769 F EGN_Mt100125 20111014 | BG447918 | 1 | 0.8987 | 2.1797 | 1.4741 | 2.7457 | 2.7383 | 2.4946 | 2.1554 | 0.8920 |
| Mtr.48899.1.S1_at | Medtr4g0145401 | ferritin | HC | chr441108784113850 | 20130731 | IMGAG|1018.m00018 | 1 | 0.7696 | 2.0582 | 1.4596 | 2.9716 | 3.1232 | 2.5401 | 1.9641 | 0.6123 |
| Mtr.8666.1.S1_s_at | Medtr4g0887701 | plant-specific domain TIGR01615 family protein | HC | chr43537456735372760 | 20130731 | TC100959 | 1 | 1.8028 | 2.2378 | 3.1163 | 19.3778 | 11.3059 | 23.3458 | 32.5770 | 2.5965 |
| Mtr.38045.1.S1_at | IMGA|Medtr3g0996701 hypothetical protein chr3 3475120234752285 F EGN_Mt100125 20111014 | TC101962 | 1 | 1.1265 | 2.0256 | 1.7574 | 2.2566 | 1.5401 | 2.3782 | 2.6234 | 1.0667 |
| Mtr.13183.1.S1_at | IMGA|Medtr3g0268301 hypothetical protein chr3 77814137788057 I EGN_Mt100125 20111014 | TC97194 | 1 | 2.5103 | 2.1194 | 2.1319 | 2.8021 | 1.6950 | 1.2759 | 1.3123 | 0.7486 |
| Mtr.38011.1.S1_s_at | IMGA|Medtr8g0117801 Serine threonine protein kinase OXI1 chr8 20287182030325 F EGN_Mt100125 20111014 | TC101889 | 1 | 1.7217 | 2.1427 | 2.1321 | 2.0858 | 1.7319 | 1.7165 | 1.7856 | 1.6237 |
| Mtr.12311.1.S1_at | IMGA|Medtr1g1068701 Peroxidase chr1 3148123931483015 H EGN_Mt100125 20111014 | TC94347 | 1 | 0.5533 | 0.2980 | 0.2600 | 0.1968 | 0.1831 | 0.1613 | 0.2173 | 0.3912 |
| Mtr.25950.1.S1_at | Medtr6g0926201 | 1aminocyclopropane1carboxylate oxidase | HC | chr63487849334880015 | 20130731 | 1471.m00036 | 1 | 0.8684 | 0.4049 | 0.3364 | 0.0913 | 0.1759 | 0.1143 | 0.1913 | 0.2642 |
| Mtr.12857.1.S1_at | Medtr5g0379301 | thioredoxin | HC | chr51657270116571711 | 20130731 | TC96215 | 1 | 0.9411 | 0.4845 | 0.2762 | 0.1275 | 0.1240 | 0.1114 | 0.1171 | 0.2305 |
| Mtr.1900.1.S1_at | Medtr2g0842151 | hypothetical protein | HC | chr23537452635374016 | 20130731 | BE315767 | 1 | 5.1857 | 0.4215 | 0.4690 | 0.0933 | 0.2904 | 0.3310 | 0.1955 | 3.5458 |
| Mtr.40611.1.S1_at | IMGA|Medtr5g0838601 Peroxidase chr5 3517780635180052 F EGN_Mt100125 20111014 | TC107670 | 1 | 0.5880 | 0.4678 | 0.6134 | 0.5563 | 0.4297 | 0.3341 | 0.4158 | 0.7070 |
| Mtr.36469.1.S1_at | IMGA|Medtr5g0903201 Cell division control protein-like protein chr5 3828865438297961 I EGN_Mt100125 20111014 | BG457318 | 1 | 1.9555 | 0.4316 | 0.4241 | 0.1575 | 0.7177 | 1.0139 | 0.8562 | 1.9398 |
| Mtr.10356.1.S1_at | IMGA|Medtr2g0297501 Peroxidase chr2 1000268910004662 F EGN_Mt100125 20111014 | TC106484 | 1 | 0.4947 | 0.4728 | 0.5576 | 0.7908 | 1.0295 | 1.7203 | 1.9780 | 0.4378 |
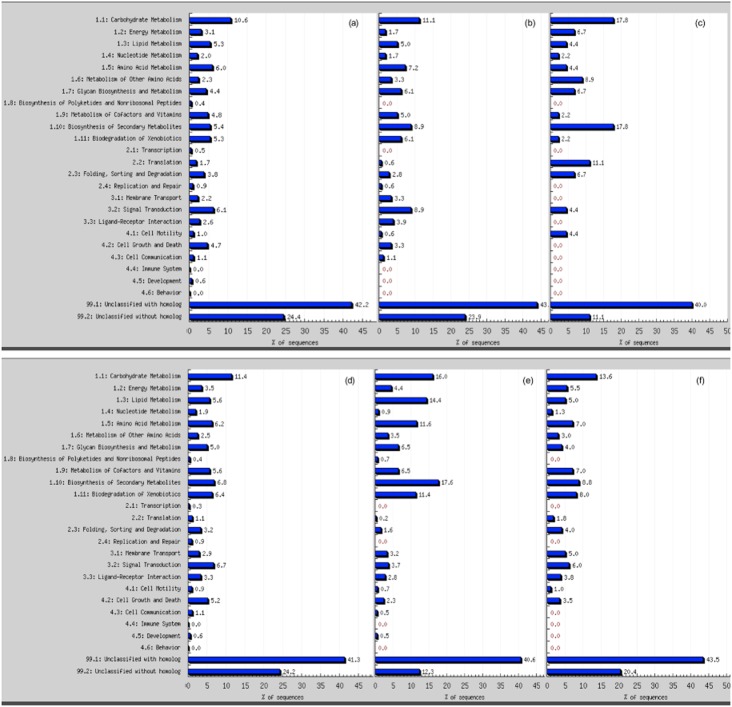
In contrast to the shoots, genes corresponding to 431 probe sets were induced and 398 were repressed in the roots after 2 d drought, indicating that roots were more responsive to drought and/or that shoots were somewhat buffered against the effects of drought by the activities of the roots (Fig. 3; Supporting Information Table S5). Compared with GO annotation categories of all genes expressed in the root tissues in this experiment, the 2-day drought stress induced a disproportionate number of genes related to secondary metabolite biosynthesis (17.6%), lipid metabolism (14.4%), amino acid metabolism (11.6%) and biodegradation of xenobiotics (11.4%) (Fig. 5). In contrast, no overrepresentation of signal transduction related genes was found among induced or repressed at this early drought-stress stage.
Figure 5.
Gene GO categories in shoots (top panel) and roots (bottom panel). (a) genes present in shoot/root tissues; (b) drought-responsive genes; (c) early drought-induced genes (day 3 for shoot and day 2 for root); (d) early drought-repressed genes (day 3 for shoot and day 2 for root); (e) mild (day 4) drought-induced genes; (f) mild (day 4) drought-repressed genes.
The number of genes induced or repressed in shoots by the third day of drought was an order of magnitude greater than at day 2, corresponding to 180 and 45 probe sets, respectively, while the numbers of such genes doubled in roots between days 2 and 3 to approximately 900 probe sets (Fig. 2b,c). In the shoots, day 3 was marked by a higher percentage of induced signal transduction related genes (8.9%) when compared with all the other genes present at all the other time points examined. However, among all repressed genes in 3-day drought-stressed shoots, carbohydrate metabolism and secondary metabolite biosynthesis related genes accounted for 17.8% each of the total (Fig. 5).
Early transient responsive genes
Early transient responsive genes were defined as those that were up- or down-regulated 2–3 d after water withdrawal, and that returned to control levels by day 7. In the shoots, there were a total of 189 probe sets for genes that were up-regulated at day 2 and 3, while 48 were repressed. Among these, only 50 were categorized as early transient responsive genes (Supporting Information Table S6). Among the 41 transiently up-regulated genes that were annotated as enzyme encoding, two were CpABA1-like (ZEP) protein genes as mentioned earlier, three encoded for polygalacturonase-like proteins, two trehalose-6-phosphate phosphatases, one beta-galactosidase, a myo-inositol 1-phoshate-synthase and one encoded a periaxin-like protein. Others were annotated as genes coding for regulator proteins, including a histidine-containing phosphotransfer protein, a receptor-like protein kinase, a protein kinase-like protein, a Zn-finger-RING protein, a nuclear transcription factor Y subunit gamma (NF-Y protein chain C, CCAAT-binding transcription factor subunit C), a MADS box protein and an AP2/ERF transcription factor. On the other hand, only nine genes were found to be transiently repressed, including peroxidase 1B precursor, curculin-like lectin, fibrillarin, globulin-like protein, firrV-1-B58 precursor, isoflavone reductase, response regulator and response regulator receiver genes. Several genes of unknown function were also found to be shoot early transient responsive genes.
In the roots, genes corresponding to 1108 and 965 probe sets were up- or down-regulated and were categorized as early-responsive genes (Supporting Information Table S5). Among these, 268 were found to be transiently regulated (Supporting Information Table S7). Again, two ZEP genes (CpABA1-like) were found as early transient responsive genes in the roots, one corresponding to that detected in the shoots, as described above. This indicates a potential role for ABA as one of the early signal molecules that modulate physiological responses to drought. Another gene for a myo-inositol 1-phoshate synthase that is likely to be different from the one detected in shoots was found as an early transient responsive gene in the roots. There were also a high number of genes (11) encoding cytochrome P450 and one for cytochrome b that were classified as early transient responsive genes. Transiently activated transcription factors in the roots encode a MYB-related protein, a NAC domain protein, an AP2/ERF protein, a Zn-finger (CCHC) type, a RING-H2 finger protein RHB1a and a bHLH protein. Several signal transduction related genes were also found in this category, such as two regulators of chromosome condensation-like protein-3, a histidine-containing phosphotransfer protein, a protein kinase homolog, a brassinosteroid Leucine Rich Repeat (LRR) receptor kinase, two diphosphonucleotide phosphatase-like proteins and five serine/threonine protein kinases. Enzymatic genes in this category were more diversified with a purple acid phosphatase, an acid phosphatase type 5 precursor, a NADH dehydrogenase, an xyloglucan endo-1,4-beta-D-glucanase, a xyloglucan endotransglucosylase/hydrolase protein, two genes for anthranilate N-benzoyltransferase-like protein, two genes for N-hydroxycinnamoyl/benzoyltransferase-like protein, two peroxidase, two xyloglucan endo-1,4-beta-D-glucanase and one exo-beta-glucanase, two fatty acid elongase 3-ketoacyl-CoA synthase, two lipid transfer proteins, one Arg decarboxylase, chalcone isomerase, glutathione S-transferase and five genes for O-methyltransferase. Others included on the list of transiently activated genes encoded an antihaemostatic protein and a stigma/style ABC transporter. Only one gene encoding a LEA protein was found transiently induced in roots (Supporting Information Table S7).
Early transiently repressed genes in roots corresponding to 137 probe sets were found, encoding three zinc finger proteins, three MYBs, a dof type Zn-finger and a C2H2 Zn-finger, TAZ finger, two DnaJ-like protein, a trihelix, a NAM-like protein, a WRKY, a homeodomain leucine zipper protein, AT-hook DNA-binding protein, one regulator of chromosome condensation, an ATP-NAD kinase protein, S-receptor kinase, a calcium-dependent protein kinase, protein phosphatase 2C, among others. Three coded for a proline dehydrogenase gene, five nodulin-like proteins, one phosphoribulokinase, a cyclin, a chlorophyll a-oxygenase, a glucuronosyl transferase, a coproporphyrinogen oxidase, one cytochrome P450 and two cytochrome b, four glycoside hydrolases, one glucosyltransferase, a pyruvate decarboxylase, a chalcone synthase, an UDP-glycosyltransferase, a steroid sulfotransferase-like protein, a flavonol sulfotransferase, two ribosomal proteins, a mitochondrial elongation factor, a patatin-like protein, a heat shock protein, a geranyl diphosphate synthase, a respiratory burst oxidase and an alpha-mannosidase. A histidine amino acid transporter and a nitrite transport protein were also found transiently repressed in root (Supporting Information Table S7).
Transcription factor gene expression changes during progressive drought
Given the massive, coordinated changes in gene expression during drought stress (Fig. 2), we sought to identify transcription factor (TF) genes that responded to drought that are likely to regulate the expression of other genes. A total of 1659 probe sets were identified corresponding to putative TF genes, using the published criteria (Kakar et al. 2008). Of these, 417 and 507 probe sets detected significant changes in transcript levels in shoots and roots, respectively, during drought stress (Fig. 6a). In total, genes corresponding to 692 probe sets encode putative TFs that are drought-stress regulated. These were classified into myb, AP2/EREBP, bHLH, NAC, bZIP, homeodomain contain proteins, C2C2 and C2H2 zinc finger proteins, WRKY, and other transcription factor families and subfamilies (Fig. 6b, Supporting Information Tables S8 & S9). There were 232 putative TFs found induced or repressed by drought stress in both shoots and roots at any one or more time points (Fig. 6a).
Figure 6.
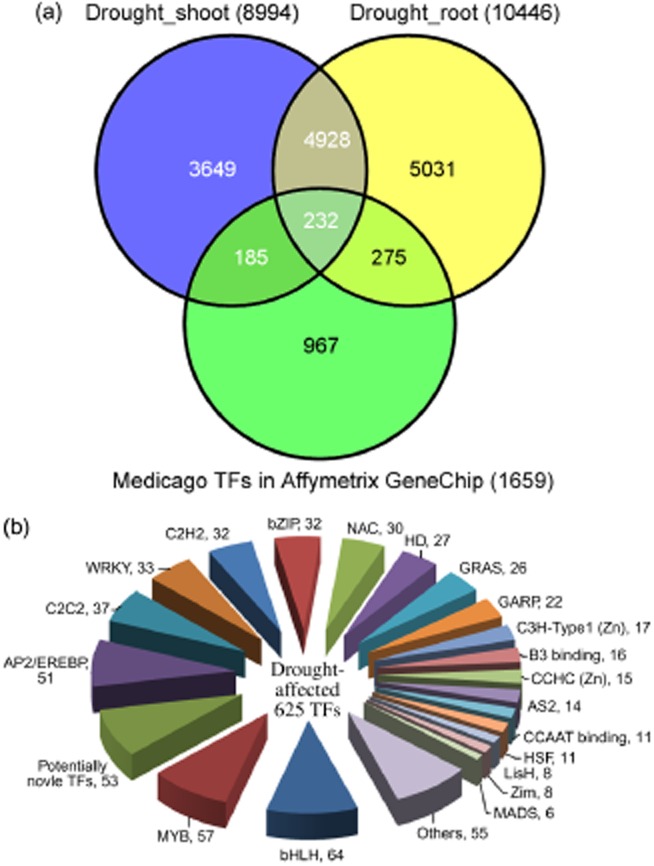
Drought responsive transcription factor genes in Medicago truncatula. (a) Venn diagram of drought-responsive genes in shoots and/or roots and the proportion of all TF genes in these categories. (b) Classification of the drought-responsive TFs in M. truncatula.
There were 86 TFs found exclusively induced in the shoots and 37 repressed. The corresponding numbers in root tissues were 108 and 99, respectively. By days 2 and 3 of drought, 18 and 63 TF genes were induced in roots, respectively, including eight NACs, eight MYBs, six AP2/EREBPs, six bZIPs, five HDs, four bHLHs and other TFs. These rapidly induced TF genes may be direct targets of early/mild drought-stress signalling and presumably orchestrate transcription of appropriate early-responsive genes in roots (Supporting Information Table S5). Seventeen and 43 TFs were repressed in roots in the same period, including nine bHLHs, seven WRKYs, six AP2/EREBPs, four bZIPs, four C2C2 zinc finger proteins and others (Supporting Information Table S9). Relatively few putative TFs were induced or repressed in shoots by day 2 of drought, including only four induced TFs (two CpABA1-like proteins, one WRKY4-like protein and a phaseolin G-box binding protein). By day 3, there were 45 TFs found induced (10 NACs, six bZIPs, five AP2/EREBPs, five MADSs, four CCAAT TFs, etc.). There was only one repressed (ethylene-responsive element binding protein) TF gene. Again, these induced TF genes presumably regulate the expression of appropriate early drought-response genes in shoots (Table 4).
Transcriptome changes during the transition from mild to moderate drought stress
With the present drought experimental procedure, 4 d after water withdrawal was the point when plants started to present morphological drought-stress responses (leaf rolling, cessation of growth) and when Ψw and RWC dropped to −1.70 MPa and 72 %, respectively. At this point, approximately 2000 probe sets representing nearly 6% of all genes measured were induced and a similar percentage were repressed in both roots and shoots. No specific category of genes was significantly induced or repressed at this time point (Fig. 5e) when compared with the GO annotation categories of all time points (Fig. 5b), both in shoots and roots. Virtually none of the affected genes were regulated by development alone (compared with well-watered controls at day 4). In other words, essentially all of these genes responded to drought stress rather than to developmental cues.
Three NCED genes were highly induced in the roots from day 3 to day 10. Five ZEP (CpABA-like) genes were temporarily induced by stress, with two of them induced at day 3 and the others at day 4. The expression level of all of these decreased to normal level by day 7 (moderate stress). In shoots, five NCED genes were identified. Among them, one was induced and maintained a high level of expression from day 10 until day 14 of drought, two were induced by mild and severe stress, and another two were repressed by moderate and severe stress (day 7 to 14). The expression pattern of the five ZEP genes showed differences between shoots and roots at day 10. One gene remained highly induced from day 10 until severe stress while another was repressed over the same period.
Despite the decline in Ψw, leaf shrivelling and withering between days 4 and 14 of drought progression, the identity of the genes induced or repressed changed little during this period although their numbers swelled to 8–12% of all detected genes (Fig. 3). A large number of genes that were previously found to be expressed specifically in flowers, seeds or nodules were found to be induced by moderate and severe drought stress in our experiments. These were mostly induced during the transition from mild to moderate stress, around day 4. Among the late drought-induced genes in shoots and roots were genes encoding cysteine proteinase, proteinase inhibitor, mannitol dehydrogenase, beta-amylase, IMP dehydrogenase/GMP reductase, plant invertase/pectin methylesterase inhibitor, glucose-6-phosphate/phosphate-translocator precursor and some legume-specific proteins. A flower- and seed-specific myb-like transcription factor and a seed-specific homeodomain transcription factor genes were also found induced in shoots from day 4 onwards.
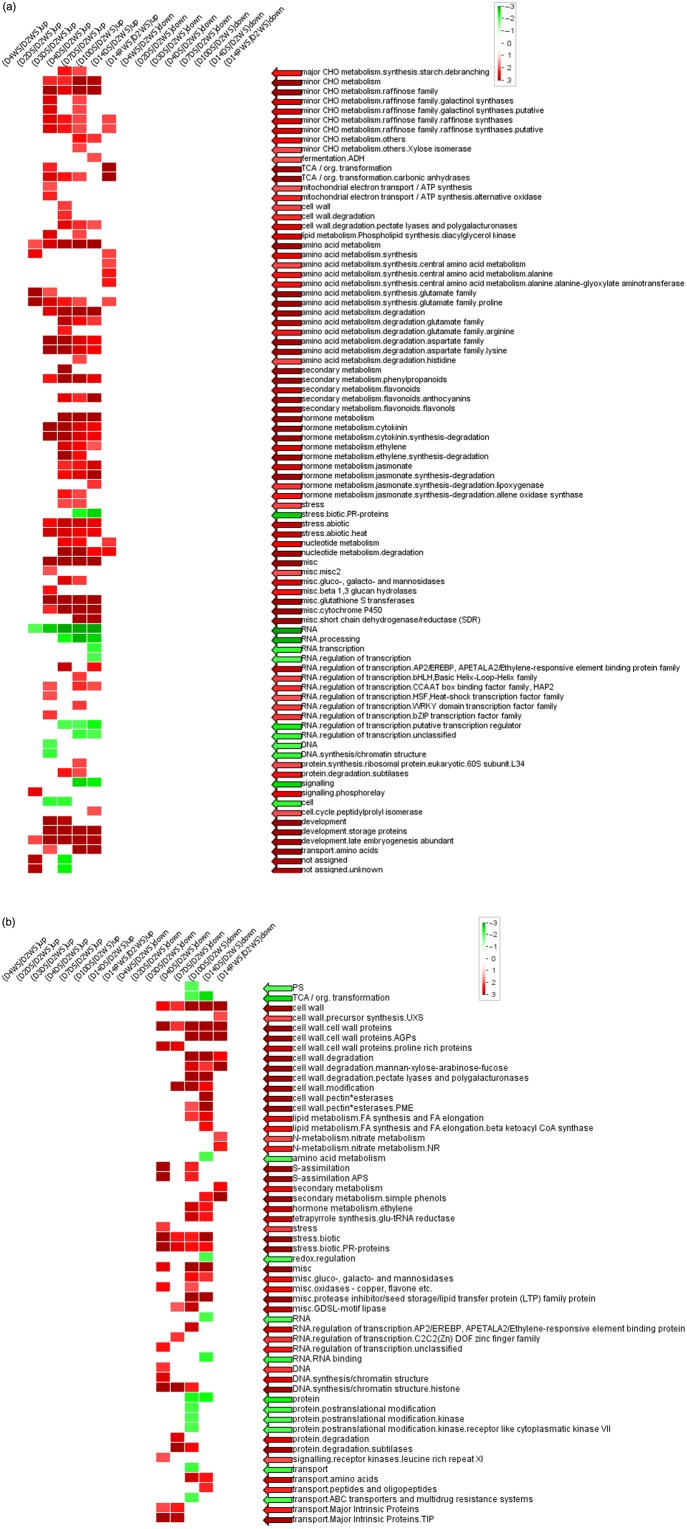
A PageMan analysis of genes whose expression was strongly regulated by Ψw revealed several overrepresented gene groups (Fig. 7). Among the gene groups that were up-regulated as drought stress intensified were sugar metabolism, amino acid metabolism, cell-wall degradation, secondary metabolism and hormone metabolism genes including cytokinin, ethylene and jasmonate and various families of TFs (Fig. 7a). The gene groups that were down-regulated were mostly cell-wall biosynthesis and degradation related, some abiotic stress coding genes and a few TFs (Fig. 7b).
Figure 7.
PageMan analysis of genes whose expression in the shoots was strongly and positively (a) (correlation coefficient > 0.8, 1774 genes) or negatively (b) (correlation coefficient < −0.8, 3613 genes) correlated to Ψw. Log2-transformed ratios were used. Overrepresentation analysis was performed using Fisher's exact test and the cut-off log2 ratio was 2. D2W, 2-day well watered; D4W, 4-day well watered; D2D, 2-day drought; D3D, 3-day drought; D4D, 4-day drought; D7D, 7-day; D10D drought, 10-day drought; D14D, 14-day drought; D14RW, 1 d after rewatering; S, shoots.
Metabolome analysis of drought-stressed Medicago plants
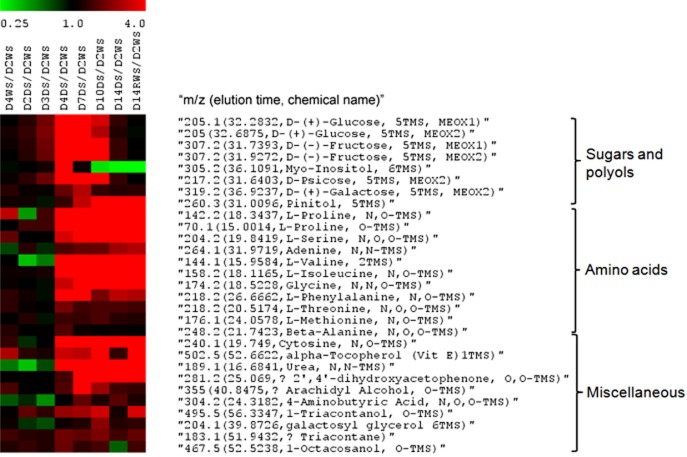
To detect drought stress-associated metabolites in shoots and roots of Medicago and to examine their accumulation/degradation trends during the progression of drought stress and recovery, a GC-MS analysis was performed on the samples used for RNA isolation and microarray profiling experiments described earlier. Over 300 metabolites were detected in shoot and root samples, including 135 polar compounds of which 100 were identified as known, and 165 non-polar compounds of which 70 were identified as known. To determine which metabolites responded most to drought in shoot and root tissues, a PCA was performed. The mean values of all samples were applied to a PCA after log10-transformation. The first three principal components derived from this data matrix encompassed 98.2% of the total variance, which assigned total eigenvalue of 283.5 to differences between shoot and root, 9.3 to drought-stress treatment versus non-stressed conditions and 2.5 to growth/development differences during the 2 weeks of experiment (Supporting Information Fig. S5; Supporting Information Table S10). The first component accounted for 94.3% of the variance (Fig. 8). Among the 300 metabolites used for analysis, 294 compounds were used to differentiate the samples. Compounds differing in amount between shoots and roots were evenly distributed among polar and non-polar extractions from small to large molecular weight. Compounds that showed the biggest eigenvalue included 182.3 (49.007 min, nonacosane), 183.1 (51.9432 min, triacontane), 292.2 (37.3309 min, unknown), 174.2 (29.1318 min, L-Putrescine, N,N,N,N-TMS′)″, 218.1 (26.455 min, L-Phenylalanine, N,O-TMS″)″, 283.1 (22.6378 min, cycloheptasiloxane, tetradecamethyl″)″, 161.1 (23.5094 min, 3,6,9-Trioxa-2,10-disilaundecane, 2,2,10,10-tetramethyl-″)″, 218.2 (20.5149 min, L-Threonine, N,O,O-TMS″)″, 177 (12.1498 min, glycolic acid, O,O-TMS″)″, which were all non-polar extractions.
Figure 8.
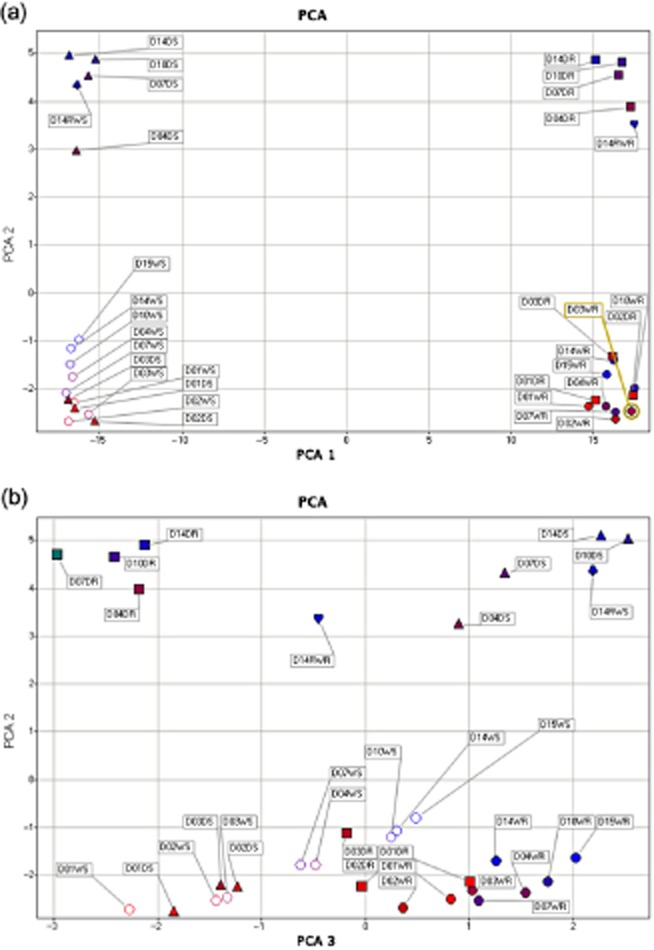
Principle component analysis (PCA) of metabolites from both well-watered and drought-stressed samples. Metabolic fingerprinting using: (a) PCA analysis – PCA1 and 2; and (b), PCA2 and 3.
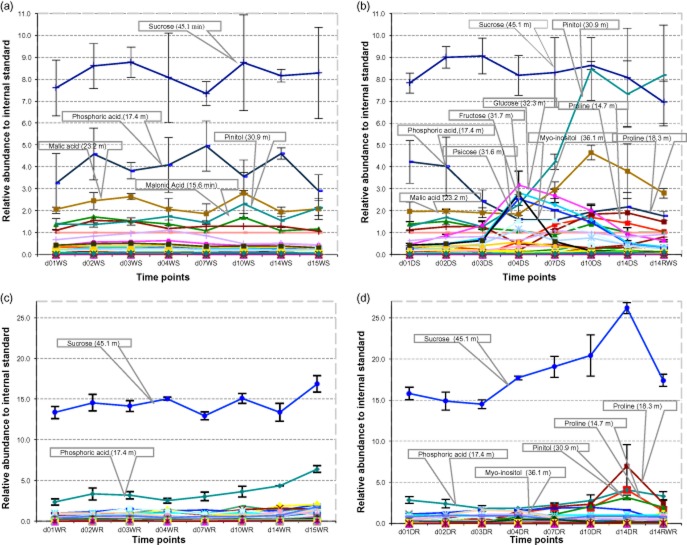
Metabolites that responded to drought stress both in shoots and roots were mainly small water-soluble molecules including proline, cytosine, L-Isoleucine, malic acid, L-Valine and citric acid (Fig. 9). In shoots especially, some well-known osmoprotectants accumulated early and transiently such as myo-inositol, glucose, fructose, psicose, ononitol, while others started to accumulate later such as proline and ribose. Pinitol accumulated early in shoots and its amount peaked when plants were subjected to moderate-severe stress (Fig. 9b). There were two unknown compounds that showed similar pattern and similar abundance to ribose. Metabolites that accumulated significantly from day 3 to day 4 (i.e. when Ψw dropped from −1.13 to −1.70 MPa) were selected and used to build a heat map (Fig. 10). Three classes of metabolites fell into this category; amino acids (including proline), sugars (including myo-inositol) and other miscellaneous.
Figure 9.
Relative abundance of polar compounds during normal development (a,c) and drought stress (b,d), in shoots (a,b) and roots (c,d). W, well watered; D, drought stressed; S, shoots; R, roots.
Drought stress heat map of the metabolites whose abundance in shoots increased over 1.5-fold on the transition between day 3 (Ψw = −1.13 MPa) and day 4 (Ψw = −1.70 MPa).
In roots, the sucrose amount increased progressively from the end of mild water stress (day 4, Ψw = −1.7 MPa) until rewatering after severe water stress (Fig. 9d). Phosphoric acid and pinitol also accumulated early and continuously during drought. The malic acid amount increased as early as day 3 but at a slower rate. Proline started to accumulate at day 4 and greatly from moderate and severe drought stress (from days 10 to 14), as in shoots. Again, myo-inositol and several other carbohydrates including fructose and mannose transiently accumulated and peaked at day 3–4. L-asparagine accumulated only in response to moderate (day 7, Ψw = −2.6 MPa) to severe drought stress (day 14, Ψw = −4.76 MPa). Citric acid and pyroglutamic acid both decreased in amount during drought stress.
Discussion
Our experimental setup was designed to impose a progressive drought stress to mimic what plants experience in the field. By applying water deficit gradually, the plant has time to adjust its metabolism and better deploy its adaptive responses. Therefore, this slowly developing drought stress increases the physiological relevance of the transcriptomic and metabolomic changes observed in this study. The present work also goes beyond previous related transcriptomic studies by assessing the water stress actually endured by plants. This important information was obtained by recording the leaf water status, and in particular the Ψw. This parameter provides precise information on the drought intensity occurring in the plant, therefore enabling an accurate correlation between transcriptomic (or metabolomic) variation and drought-stress progression. In order to get a cross-germplasm or cross-experiment comparison of the drought responses, a well-defined stress intensity measure is extremely important. Standardizing the measure, staging and description of plant drought stress makes physiological and molecular findings in reference plants more valuable for data comparisons or for translating the findings to target crops. Even though time points were used in the figures and tables to simplify the description of the drought time-course, terms of mild, moderate and severe drought stress were used as reference to reflect stress intensity and these were carefully defined according to a specific Ψw.
For over a decade and a half, the use of Arabidopsis as a model plant has revealed many key pathways related to drought-stress responses, namely the ABA-dependent and ABA-independent signalling pathways (Shinozaki & Yamaguchi-Shinozaki 1997). The existence of a vast collection of Arabidopsis T-DNA insertion mutants has further enabled the discovery of multiple genes involved in those pathways and has shed some light on the intrinsic gene networks involved in the drought-stress response (Liu et al. 1998; Haake et al. 2002; Aharoni et al. 2004; Tran et al. 2004; Umezawa et al. 2004; Xiong et al. 2006; Wohlbach et al. 2008; Yoshida et al. 2010). Nevertheless, the multitude of complex traits that account for plant drought resistance cannot be grasped by focusing on Arabidopsis alone. Arabidopsis is a rather drought-sensitive species that cannot tolerate low Ψw. Therefore, its drought responses will be more related to stress avoidance than stress tolerance (Verslues & Juenger 2011). Strikingly, the majority of available transcriptomic data (and GO annotations) for drought and osmotic treatments relies on the A. thaliana Columbia accession, a long-time laboratory line, which most likely misses many drought-relevant genes (Des Marais et al. 2012).
Here, we promote the use of M. truncatula as a new model for the study of the molecular basis of drought resistance. It is a plant that can be considered to be drought-tolerant, surviving and resuming growth when Ψw reaches values below −4 MPa (Fig. 1). Another advantage of using M. truncatula is that it shows greater genetic relatedness and genomic synteny to important legume forages such as alfalfa (M. sativa), white clover (Trifolium repens L.) and crops such as soybean (G. max), pea (Pisum sativum) and bean (Phaseolus vulgaris) than Arabidopsis and is therefore, a more suitable model for translational genomics for these plants (Zhu et al. 2003; Choi et al. 2004a,b; Eujayl et al. 2004; Kaló et al. 2004; Sledge et al. 2005; George et al. 2008; Hougaard et al. 2005; Li et al. 2008).
With the time-course design of this experiment, many genes were found whose expression was specifically affected by the stress duration and severity. Transcript variation showed stress-dependent expression patterns, which could be observed by the simultaneous assessment of the leaf water status (RWC and Ψw) at each sampling time point, and were validated by a statistic approach. Compared with two previous studies on the physiological (Nunes et al. 2008) and molecular (Iyer et al. 2013) drought responses of M. truncatula cv Jemalong, the drought stress imposed in this study was both more extensive and intensive, going from very mild to severe water stress, over a 14-day time-course. At the last drought stage of the present study, the RWC and Ψw dropped as low as 37.7% and −4.76 MPa, respectively, which was far more extreme than the two previous studies (Nunes et al. 2008; Iyer et al. 2013). This was defined as severe drought stress although the plants had not yet reached the permanent wilting point (reached approximately after 5 more days) and were able to fully recover upon rehydration. This further supports the high level of drought tolerance in M. truncatula, which was thoroughly exploited in the present analysis.
The genes operating for drought avoidance in Medicago, both at the whole plant level and at the cellular level, are likely to be detected during mild stress and those for tolerance at later stages, from moderate to severe drought stress. It cannot be excluded however, that drought tolerance-related genes could be activated earlier, in order to prepare the plant to a developing water deficit. Nevertheless, the majority of the early-responsive genes detected in this study most likely participate in water deficit signalling cascades and in drought-avoidance strategies employed by Medicago as the primary response to a developing drought stress. The ZEP genes detected in the roots and shoots as early as day 2 after water withholding (–0.76 MPa), fall into this category, highlighting and further supporting the fundamental role of ABA as an early stress-responsive hormone are included. ABA is a major regulator of drought responses, responsible for triggering several avoidance mechanisms such as stomatal closure and osmoregulation, as well regulating the expression of genes that confer cellular tolerance to low water potentials (Cutler et al. 2010; Hubbard et al. 2010). In Medicago, ABA induction immediately after water withdrawal is likely to be related to the activation of drought avoidance pathways. A previous study using the same cultivar (M. truncatula cv Jemalong) revealed putative drought-avoidance mechanisms in Medicago that probably helped to maintain a high RWC even when soil water content (SWC) was reduced to 30% (Nunes et al. 2008). The authors suggested that these avoidance processes were independent of stomata closure as reduction in leaf conductance only occurred when SWC decreased to 17%. In the present work, there was a faster reduction of Ψw than of RWC, especially at the beginning of the drought treatment (Fig. 1b), suggesting the occurrence of osmolyte accumulation. Although no measurements of osmotic potential were made to confirm this, our (metabolomics) data together with previous physiological data (Nunes et al. 2008), support the occurrence of osmoregulation in Medicago as an early drought-avoidance strategy.
As drought stress progressed, a significant and constant increase on the expression level of the FtsH protease gene was detected in shoots, from day 4 (end of mild drought stress) to day 14 (severe drought stress). The expression of this gene was specifically regulated by drought stress and it was among the top 100 highly expressed genes in the shoots (Supporting Information Table S1). The FtsH protease is an ATP-dependent zinc metalloprotease that has been suggested to be involved on the proteolysis of the photosystem II reaction centre D1 protein (Lindahl et al. 2000). One of the cellular consequences of drought stress is the overproduction of reactive oxygen species because of stomata closure, leading to oxidative stress (reviewed in Cruz de Carvalho 2008). Under oxidative stress, the D1 protein is prone to irreversible damage and needs to be degraded and replaced in order to keep the photosystem II operational (Lindahl et al. 2000). The FtsH protease has been suggested to specifically recognize and cleave the oxidized damaged forms of the D1 protein under abiotic stress (reviewed in Yamamoto et al. 2008). Hence, our results suggest that the FtsH protease is likely to have a role in the cellular response to drought-induced oxidative stress, by participating in the active and vital repair of photosystem II under moderate to severe water deficits. This repair mechanism could account, at least partially, for Medicago's high drought tolerance given the occurrence of the high expression levels detected in the shoots under very low leaf water potentials.
Metabolite profiling has the potential to provide not only deeper insight into complex regulatory processes but also to determine the phenotype of specific chemical compounds and to identify chemical signatures for specific phenotypes (Fiehn et al. 2000). Here, we have provided Medicago's biochemical compound composition variation in response to progressive drought stress. Our work has gone one step further by comparing the metabolomic data with the transcriptomic data, hence providing further insights into the regulation of metabolic pathways throughout a progressive drought stress. Among the metabolites detected in drought-stressed Medicago plants, myo-inositol and proline had striking regulatory profiles worth highlighting.
Myo-inositol is a versatile cellular compound, precursor of several other compounds such as phosphatidylinositol, myo-inositol polyphosphate and several compatible solutes such as galactinol, pinitol, raffinose-family oligosaccharides and cell-wall polysaccharides (reviewed in Valluru & Van den Ende 2011). Several of these compounds seem to have roles in abiotic stress responses, notably through signalling pathways and/or by direct reactive oxygen species (ROS) scavenging (Valluru & Van den Ende 2011). In Medicago, metabolic profiling showed a peak in myo-inositol accumulation at day 4, in both shoots and roots (Fig. 9). As drought intensified, myo-inositol levels decreased until it was undetectable by the end of severe stress (day 14). A direct correlation between myo-inositol accumulation and the expression of two myo-inositol 1-phoshate-synthase genes was found; one in roots, Mtr.15285.1.S1_at and one in shoots, Mtr.35794.1.S1_s_at. These genes were classified as early transient genes, with their expression rapidly declining as drought progressed (Supporting Information Table S6 & S7). This regulation of myo-inositol production, detected both at the expression level and the cellular metabolite level in both organs, suggests a precise role for myo-inositol at a turning point of drought development (day 4; Ψw = −1.70 MPa).
Proline has been widely recognized as a drought-inducible proteinogenic amino acid with an osmoprotective role. As an osmotic agent, proline accumulation decreases the cellular osmotic potential (hence lowering the cellular Ψw) thus enabling the cell to retain more water, favouring dehydration avoidance. In many plants, proline accumulation has been found to be correlated with drought-stress tolerance, although in some cases no correlation could be found (reviewed in Szabados & Savouré 2010). In Medicago, proline accumulation was detected as soon as day 4, but its accumulation increased with drought-stress intensity, with a peak detected at severe stress, by day 14, both in shoots and roots (Fig. 9). Besides its role as an osmotic agent, proline has also been shown to directly act as a ROS scavenger (Smirnoff & Cumbes 1989; Szabados & Savouré 2010) and as a regulator of the cellular redox-status (Hare et al. 1998; Sharma et al. 2011). Therefore, proline seems to be a key metabolite under drought stress, acting both on cellular dehydration avoidance processes through osmoregulation and on cellular tolerance through the maintenance of cellular redox homeostasis under low water potentials. Our study revealed that Medicago tightly regulates proline production and accumulation during drought-stress progression by the up-regulation of several genes encoding P5CS, a key enzyme in proline synthesis, and concomitantly repressing genes coding for proline degrading enzymes such as proline dehydrogenase (PDH). The expression level of one of the P5CS genes (Mtr.11510.1.S1_at) was induced by drought stress very slightly, the second one (Mtr.33511.1.S1_at) was induced seven to ninefolds by drought in shoots and roots, while another one (Mtr.42902.1.S1_s_at) increased as drought stress progressed with a peak expression at severe drought stress to about 150-folds higher in shoots (included in the top 100 most highly induced genes in shoots; Supporting Information Table S3) and 44-folds higher in roots. The down-regulation of several PDH genes (Mtr. 12290.1.S1_at; Mtr. 12290.1.S1_s_at; Mtr. 12291.1.S1_s_at and Mtr. 42984.1.S1_at) occurred as soon as day 3 and transcripts remained low until plants were rewatered. This variation of proline anabolism and catabolism gene expression can be directly related to the high proline accumulation detected by metabolic profiling in both shoots and roots, initiated at day 4, with peak accumulation at day 14 (Fig. 9b,d). No proline accumulation was detected under well-watered, controlled conditions (Fig. 9a,c) implying that proline accumulation was specifically induced by low water potential. Furthermore, proline's peak accumulation was coincident to the time point when other well-known metabolites such as myo-inositol, glucose, fructose and psicose started to decline in amount after an initial accumulation (Fig. 9).
Interestingly, root proline content under drought stress was several folds higher than in shoots, despite the higher expression levels detected for P5CS in the shoots (Supporting Information Table S1). This suggests that the bulk of proline synthesis may occur in the shoots from which it is probably transported to the roots. This is supported by recent evidence that suggests that roots function as sink organs for proline, which is needed to sustain root growth at low water potentials (Sharma et al. 2011). Other previously identified P5CS genes in Medicago, such as the housekeeping MtP5CS1 (Mtr.12994.1.S1_at) and salt stress-inducible MtP5CS2 (Mtr.44607.1.S1_at) (Armengaud et al. 2004), as well as another P5CS candidate gene (Mtr.42847.1.S1_at), were not drought inducible. They are, however, induced in shoots and/or roots by exogenous application of phytohormones, salt stress, nodulation or mycorrhizal inoculation (http://mtgea.noble.org).
Other metabolites besides proline and myo-inositol also accumulated in parallel with stress intensity. These include cytosine, maleic acid, pinitol (a myo-inositol derivative), L-aspartic acid, pipecolic acid, sucrose, glucose, fructose, psicose, phosphoric acid, malic acid and some unknown compounds. The present metabolite profiling revealed novel drought stress accumulating compounds, especially non-polar compounds, which opens doors for the discovery of new pathways contributing to drought adaptation and tolerance in plants. These findings together with the present stress staging characterization associated with water potential information will make comparison between experiments, ecotypes and species possible.
Translational genomics is a promising approach to transfer genomics-based findings in model organisms to target crop species (Zhang et al. 2004; Stacey & VandenBosch 2005; Tester & Bacic 2005; Valliyodan & Nguyen 2006; Young & Udvardi 2009). In recent years, success stories have been reported when appropriate methods have been used to reveal the mechanisms conserved between species (Zhang et al. 2005; Nelson et al. 2007; Castiglioni et al. 2008; Karaba et al. 2007; Hu et al. 2006). With this work, we provide a thorough characterization of the transcriptomic variation induced by different stages of progressive drought stress, which has been included in the Medicago Gene Atlas server (http://mtgea.noble.org). Furthermore, we provide different sets of data showing positive and negative correlation of gene expression with drought stress that can be exploited by the scientific community and be used for translational genomics approaches in order to improve crop drought resistance.
Acknowledgments
We would like to thank Jitender Cheema from Dept. Computational and System Biology, JIC, for technical assistance. This work was supported by The Samuel Roberts Noble Foundation. The authors declare no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site
Hierarchical clustering analysis of shoot gene expression at all stress time points.
Hierarchical clustering analysis of root gene expression at all stress time points.
Expression pattern clusters of drought-regulated shoot genes.
Expression pattern clusters of drought-regulated root genes.
Eigenvectors of all polar and non-polar GC/MS derivatives.
List of top 100 most drought-induced genes in shoots.
Table S2. List of top 100 most drought-repressed genes in shoots.
Table S3. List of top 100 drought-induced genes in roots.
Table S4. List of top 100 most drought-repressed genes in roots.
Early drought-responsive genes in drought-stressed roots.
Early transient drought-responsive genes in drought-stressed shoots.
Early transient drought-responsive genes in drought-stressed roots.
Drought stress-regulated putative transcription factors genes in shoots.
Drought stress-regulated putative transcription factors genes in roots.
eigenvalues of compounds that contributed to tissue type, stress treatment and growth.
References
- Aharoni A, Dixit S, Jetter R, Thoenes E, van Arkel G. Pereira A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. The Plant Cell. 2004;16:2463–2480. doi: 10.1105/tpc.104.022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengaud P, Thiery L, Buhot N, Grenier-de March G. Savoure A. Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiologia Plantarum. 2004;120:442–450. doi: 10.1111/j.0031-9317.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- Barker D, Bianchi S, Blondon F, Dattée Y, Duc G, Essad S. Huguet T. Medicago truncatula, a model plant for studying the molecular genetics of the rhizobium-legume symbiosis. Plant Molecular Biology Reporter. 1990;8:40–49. [Google Scholar]
- Benedito VA, Torres-Jerez I, Murray JD, Andriankaja A, Allen S, Kakar K. Udvardi MK. A gene expression atlas of the model legume Medicago truncatula. The Plant Journal. 2008;55:504–513. doi: 10.1111/j.1365-313X.2008.03519.x. [DOI] [PubMed] [Google Scholar]
- Benjamin JG. Nielsen DC. Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crops Research. 2006;97:248–253. [Google Scholar]
- Boominathan P, Shukla R, Kumar A, Manna D, Negi D, Verma PK. Chattopadhyay D. Long term transcript accumulation during the development of dehydration adaptation in Cicer arietinum. Plant Physiology. 2004;135:1608–1620. doi: 10.1104/pp.104.043141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Bray EA. Molecular responses to water deficit. Plant Physiology. 1993;103:1035–1040. doi: 10.1104/pp.103.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA. Plant responses to water deficit. Trends in Plant Science. 1997;2:48–54. [Google Scholar]
- Brodribb TJ. Holbrook NM. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology. 2003;132:2166–2173. doi: 10.1104/pp.103.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckling CD, Huhman DV, Farag MA, Smith JT, May GD, Mendes P. Sumner LW. Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. Journal of Experimental Botany. 2005;56:323–336. doi: 10.1093/jxb/eri058. [DOI] [PubMed] [Google Scholar]
- Broeckling CD, Reddy IR, Duran AL, Zhao X. Sumner LW. MET-IDEA: data extraction tool for mass spectrometry-based metabolomics. Analytical Chemistry. 2006;78:4334–4341. doi: 10.1021/ac0521596. [DOI] [PubMed] [Google Scholar]
- Broughton WJ. Dilworth MJ. Control of leghaemoglobin synthesis in snake beans. Biochemical Journal. 1971;125:1075–1080. doi: 10.1042/bj1251075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce WB, Edmeades GO. Barker TC. Molecular and physiological approaches to maize improvement for drought tolerance. Journal of Experimental Botany. 2002;53:13–25. [PubMed] [Google Scholar]
- Bucciarelli B, Hanan J, Palmquist D. Vance CP. A standardized method for analysis of Medicago truncatula phenotypic development. Plant Physiology. 2006;142:207–219. doi: 10.1104/pp.106.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell T, Bassie L. Christou P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9909–9914. doi: 10.1073/pnas.0306974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni P, Warner D, Bensen RJ, Anstrom DC, Harrison J, Stoecker M. Heard JE. Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiology. 2008;147:446–455. doi: 10.1104/pp.108.118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H-K, Kim D, Uhm T, Limpens E, Lim H, Mun J-H. Cook DR. A sequence-based genetic map of Medicago truncatula and comparison of marker colinearity with M. sativa. Genetics. 2004a;166:1463–1502. doi: 10.1534/genetics.166.3.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H-K, Mun J-H, Kim D-J, Zhu H, Baek J-M, Mudge J. Cook DR. Estimating genome conservation between crop and model legume species. Proceedings of the National Academy of Sciences of the United States of America. 2004b;101:15289–15294. doi: 10.1073/pnas.0402251101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DR. Medicago truncatula – a model in the making! Current Opinion in Plant Biology. 1999;2:301–304. doi: 10.1016/s1369-5266(99)80053-3. [DOI] [PubMed] [Google Scholar]
- Cruz de Carvalho MH. Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signaling and Behavior. 2008;3:156–165. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar-Ortiz SM, Arrieta-Montiel MD, Acosta-Gallegos J. Covarrubias AA. Relationship between carbohydrate partitioning and drought resistance in common bean. Plant, Cell & Environment. 2008;31:1399–1409. doi: 10.1111/j.1365-3040.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR. Abrams SR. Abscisic acid: emergence of a core signaling network. Annual Review of Plant Biology. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- Des Marais DL, McKay JK, Richards JH, Sen S, Wayne T. Juenger TE. Physiological genomics of response to soil drying in diverse Arabidopsis accessions. The Plant Cell. 2012;24:893–914. doi: 10.1105/tpc.112.096180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyholos MK. Making the most of drought and salinity transcriptomics. Plant, Cell & Environment. 2010;33:648–654. doi: 10.1111/j.1365-3040.2009.02092.x. [DOI] [PubMed] [Google Scholar]
- Eujayl I, Sledge M, Wang L, May G, Chekhovskiy K, Zwonitzer J. Mian M. Medicago truncatula EST-SSRs reveal cross-species genetic markers for Medicago spp. Theoretical and Applied Genetics. 2004;108:414–422. doi: 10.1007/s00122-003-1450-6. [DOI] [PubMed] [Google Scholar]
- Fiehn O, Kopka J, Dörmann P, Altmann T, Trethewey RN. Willmitzer L. Metabolite profiling for plant functional genomics. Nature Biotechnology. 2000;18:1157–1161. doi: 10.1038/81137. [DOI] [PubMed] [Google Scholar]
- George J, Sawbridge TI, Cogan NOI, Gendall AR, Smith KF, Spangenberg GC. Forster JW. Comparison of genome structure between white clover and Medicago truncatula supports homoeologous group nomenclature based on conserved synteny. Genome. 2008;51:905–911. doi: 10.1139/G08-076. [DOI] [PubMed] [Google Scholar]
- Graham PH. Vance CP. Legumes: importance and constraints to greater use. Plant Physiology. 2003;131:872–877. doi: 10.1104/pp.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake V, Cook D, Riechmann JL, Pineda O, Thomashow MF. Zhang JZ. Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiology. 2002;130:639–648. doi: 10.1104/pp.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks JN, Snyder AK, Graham MA, Shah RK, Blaylock LA, Harrison MJ. Shah DM. Defensin gene family in Medicago truncatula: structure, expression and induction by signal molecules. Plant Molecular Biology. 2005;58:385–399. doi: 10.1007/s11103-005-5567-7. [DOI] [PubMed] [Google Scholar]
- Hazen SP, Pathan MS, Sanchez A, Baxter I, Dunn M, Estes B. Nguyen HT. Expression profiling of rice segregating for drought tolerance QTLs using a rice genome array. Functional & Integrative Genomics. 2005;5:104–116. doi: 10.1007/s10142-004-0126-x. [DOI] [PubMed] [Google Scholar]
- Hougaard BK, Madsen LH, Sandal N, de Carvalho Moretzsohn M, Fredslund J, Schauser L. Stougaard J. Legume anchor markers link syntenic regions between Phaseolus vulgaris, Lotus japonicus, Medicago truncatula and Arachis. Genetics. 2008;179:2299–2312. doi: 10.1534/genetics.108.090084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q. Xiong L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED. Schroeder JI. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes & Development. 2010;24:1695–1708. doi: 10.1101/gad.1953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer NJ, Tang Y. Mahalingam R. Physiological, biochemical and molecular responses to a combination of drought and ozone in Medicago truncatula. Plant, Cell & Environnent. 2013;36:706–720. doi: 10.1111/pce.12008. [DOI] [PubMed] [Google Scholar]
- Julia S, Americo R, Angela S, Silvia R, Regina A, Florine D. Pedro LR. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. The Plant Journal. 2009;60:575–588. doi: 10.1111/j.1365-313X.2009.03981.x. [DOI] [PubMed] [Google Scholar]
- Kakar K, Wandrey M, Czechowski T, Gaertner T, Scheible W-R, Stitt M. Udvardi MK. A community resource for high-throughput quantitative RT-PCR analysis of transcription factor gene expression in Medicago truncatula. Plant Methods. 2008;4:1–12. doi: 10.1186/1746-4811-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaló P, Seres A, Taylor S, Jakab J, Kevei Z, Kereszt A. Kiss G. Comparative mapping between Medicago sativa and Pisum sativum. Molecular Genetics and Genomics. 2004;272:235–246. doi: 10.1007/s00438-004-1055-z. [DOI] [PubMed] [Google Scholar]
- Karaba A, Dixit S, Greco R, Aharoni A, Trijatmiko KR, Marsch-Martinez N. Pereira A. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15270–15275. doi: 10.1073/pnas.0707294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K. Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature Biotechnology. 1999;17:287–291. doi: 10.1038/7036. [DOI] [PubMed] [Google Scholar]
- Kohzuma K, Cruz JA, Akashi K, Hoshiyasu S, Munekage YN, Yokota A. Kramer DM. The long-term responses of the photosynthetic proton circuit to drought. Plant, Cell & Environment. 2009;32:209–219. doi: 10.1111/j.1365-3040.2008.01912.x. [DOI] [PubMed] [Google Scholar]
- Lawlor DW. Tezara W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany. 2009;103:561–579. doi: 10.1093/aob/mcn244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt J. Responses of Plants to Environmental Stresses. New York, NY, USA: Academic Press; 1972. [Google Scholar]
- Li L, He H, Zhang J, Wang X, Bai S, Stolc V. Deng X-W. Transcriptional analysis of highly syntenic regions between Medicago truncatula and Glycine max using tiling microarrays. Genome Biology. 2008;9:R57. doi: 10.1186/gb-2008-9-3-r57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M, Spetea C, Hundal T, Oppenheim AB, Adam Z. Andersson B. The thylakoid FtsH protease plays a role in the light-induced turnover of the photosystem II D1 protein. The Plant Cell. 2000;12:419–432. doi: 10.1105/tpc.12.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K. Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. The Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield TA. Davies WJ. Stomata and stomatal mechanisms. In: Aspinall D, editor; Page JR, Aspinall D, editors. The Physiology and Biochemistry of Drought Resistance in Plants. Sydney, Australia: Academic Press; 1981. pp. 315–346. [Google Scholar]
- Marris E. More crop per drop. Nature. 2008;452:273–277. doi: 10.1038/452273a. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Repetti PP, Adams TR, Creelman RA, Wu J, Warner DC. Heard JE. Plant nuclear factor Y(NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16450–16455. doi: 10.1073/pnas.0707193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes C, de Sousa Araujo S, da Silva JM, Fevereiro MPS. da Silva AB. Physiological responses of the legume model Medicago truncatula cv. Jemalong to water deficit. Environmental and Experimental Botany. 2008;63:289–296. [Google Scholar]
- Oono Y, Seki M, Nanjo T, Narusaka M, Fujita M, Satoh R. Shinozaki K. Monitoring expression profiles of Arabidopsis gene expression during rehydration process after dehydration using ca. 7000 full-length cDNA microarray. The Plant Journal. 2003;34:868–887. doi: 10.1046/j.1365-313x.2003.01774.x. [DOI] [PubMed] [Google Scholar]
- Ozturk ZN, Talamé V, Deyholos M, Michalowski CB, Galbraith DW, Gozukirmizi N. Bohnert HJ. Monitoring large-scale changes in transcript abundance in drought- and salt-stressed barley. Plant Molecular Biology. 2002;48:551–573. doi: 10.1023/a:1014875215580. [DOI] [PubMed] [Google Scholar]
- Pennisi E. The blue revolution, drop by drop, gene by gene. Science. 2008;320:171–173. doi: 10.1126/science.320.5873.171. [DOI] [PubMed] [Google Scholar]
- Pnueli L, Hallak-Herr E, Rozenberg M, Cohen M, Goloubinoff P, Kaplan A. Mittler R. Molecular and biochemical mechanisms associated with dormancy and drought tolerance in the desert legume Retama raetam. The Plant Journal. 2002;31:319–330. doi: 10.1046/j.1365-313x.2002.01364.x. [DOI] [PubMed] [Google Scholar]
- Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y. Yamaguchi-Shinozaki K. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiology. 2003;133:1755–1767. doi: 10.1104/pp.103.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AR, Chaitanya KV. Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of Plant Physiology. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Salekdeh GH, Reynolds M, Bennett J. Boyer J. Conceptual framework for drought phenotyping during molecular breeding. Trends in Plant Science. 2009;14:488–496. doi: 10.1016/j.tplants.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Sanchez DH. Physiological and biotechnological implications of transcript-level variation under abiotic stress. Plant Biology. 2013;15:925–930. doi: 10.1111/plb.12075. [DOI] [PubMed] [Google Scholar]
- Seki M, Umezawa T, Urano K. Shinozaki K. Regulatory metabolic networks in drought stress responses. Current Opinion in Plant Biology. 2007;10:296–302. doi: 10.1016/j.pbi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Sharma S, Villamor JG. Verslues PE. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiology. 2011;157:292–304. doi: 10.1104/pp.111.183210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE. Davies WJ. Regulation of growth and development of plants with a restricted supply of water. In: Jones MB, editor; Jones HG, Flowers TJ, Jones MB, editors. Plants Under Stress. Cambridge, England: Cambridge University Press; 1989. pp. 71–94. [Google Scholar]
- Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ. Nguyen HT. Root growth maintenance during water deficits: physiology to functional genomics. Journal of Experimental Botany. 2004;55:2343–2351. doi: 10.1093/jxb/erh276. [DOI] [PubMed] [Google Scholar]
- Shinozaki K. Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiology. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K. Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- Sledge M, Ray I. Jiang G. An expressed sequence tag SSR map of tetraploid alfalfa (Medicago sativa L.) Theoretical and Applied Genetics. 2005;111:980–992. doi: 10.1007/s00122-005-0038-8. [DOI] [PubMed] [Google Scholar]
- Smil V. Nitrogen in crop production: An account of global flows. Global Biogeochemical Cycles. 1999;13:647–662. [Google Scholar]
- Smirnoff N. Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. [Google Scholar]
- Stacey G. VandenBosch K. Translational’ legume biology. Models to crops. Plant Physiology. 2005;137:1173. doi: 10.1104/pp.104.900141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ. Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz HU, Thomson JG. Wang Y. Plant defensins: defense, development and application. Plant Signaling & Behavior. 2009;4:1010–1012. doi: 10.4161/psb.4.11.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabados L. Savouré A. Proline: a multifunctional amino acid. Trends in Plant Science. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Talame V, Ozturk NZ, Bohnert HJ. Tuberosa R. Barley transcript profiles under dehydration shock and drought stress treatments: a comparative analysis. Journal of Experimental Botany. 2007;58:229–240. doi: 10.1093/jxb/erl163. [DOI] [PubMed] [Google Scholar]
- Tester M. Bacic A. Abiotic stress tolerance in grasses. From model plants to crop plants. Plant Physiology. 2005;137:791–793. doi: 10.1104/pp.104.900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L-SP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K. Yamaguchi-Shinozaki K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. The Plant Cell. 2004;16:2481–2498. doi: 10.1105/tpc.104.022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuberosa R. Salvi S. Genomics-based approaches to improve drought tolerance of crops. Trends in Plant Science. 2006;11:405–412. doi: 10.1016/j.tplants.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Turner NC. Drought resistance and adaptation to water deficits in crop plants. In: Staples RC, editor. Stress physiology in crop plants. New York: Wiley Interscience; 1979. pp. 343–372. [Google Scholar]
- Udvardi MK, Kakar K, Wandrey M, Montanari O, Murray J, Andriankaja A. Town CD. Legume transcription factors: global regulators of plant development and response to the environment. Plant Physiology. 2007;144:538–549. doi: 10.1104/pp.107.098061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Yoshida R, Maruyama K, Yamaguchi-Shinozaki K. Shinozaki K. SRK2C, a SNF1-related protein kinase 2, improves drought tolerance by controlling stress-responsive gene expression in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17306–17311. doi: 10.1073/pnas.0407758101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K. Shinozaki K. Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Current Opinion in Biotechnology. 2006;17:113–122. doi: 10.1016/j.copbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Urano K, Kurihara Y, Seki M. Shinozaki K. Omics' analyses of regulatory networks in plant abiotic stress responses. Current Opinion in Plant Biology. 2010;13:1–7. doi: 10.1016/j.pbi.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Valliyodan B. Nguyen HT. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Current Opinion in Plant Biology. 2006;9:189–195. doi: 10.1016/j.pbi.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Valluru R. Van den Ende W. Myo-inositol and beyond–emerging networks under stress. Plant science. 2011;181:387–400. doi: 10.1016/j.plantsci.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Verslues PE. Juenger TE. Drought, metabolites, and Arabidopsis natural variation: a promising combination for understanding adaptation to water-limited environments. Current Opinion in Plant Biology. 2011;14:240–245. doi: 10.1016/j.pbi.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ying J, Kuzma M, Chalifoux M, Sample A, McArthur C. Huang Y. Molecular tailoring of farnesylation for plant drought tolerance and yield protection. The Plant Journal. 2005;43:413–424. doi: 10.1111/j.1365-313X.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- Wohlbach DJ, Quirino BF. Sussman MR. Analysis of the Arabidopsis histidine kinase ATHK1 reveals a connection between vegetative osmotic stress sensing and seed maturation. The Plant Cell. 2008;20:1101–1117. doi: 10.1105/tpc.107.055871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Wang R-G, Mao G. Koczan JM. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiology. 2006;142:1065–1074. doi: 10.1104/pp.106.084632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T. Blumwald E. Developing salt-tolerant crop plants: challenges and opportunities. Trend in Plant Science. 2005;12:615–620. doi: 10.1016/j.tplants.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K. Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annual Review of Plant Biology. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Aminaka R, Yoshioka M, Khatoon M, Komayama K, Takenaka D. Yamamoto Y. Quality control of photosystem II: impact of light and heat stresses. Photosynthesis Research. 2008;98:589–608. doi: 10.1007/s11120-008-9372-4. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J. Yamaguchi-Shinozaki K. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. The Plant Journal. 2010;61:672–685. doi: 10.1111/j.1365-313X.2009.04092.x. [DOI] [PubMed] [Google Scholar]
- Young ND. Udvardi M. Translating Medicago truncatula genomics to crop legumes. Current Opinion in Plant Biology. 2009;12:193–201. doi: 10.1016/j.pbi.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Young ND, Cannon SB, Sato S, Kim D, Cook DR, Town CD. Tabata S. Sequencing the genespaces of Medicago truncatula and Lotus japonicus. Plant Physiology. 2005;137:1174–1181. doi: 10.1104/pp.104.057034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ND, Debellé F, Oldroyd GED, Geurts R, Cannon SB, Udvardi MK. Roe BA. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature. 2011;480:520–524. doi: 10.1038/nature10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Chen X, Hong YY, Wang Y, Xu P, Ke SD. Xiang CB. Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density. The Plant Cell. 2008;20:1134–1151. doi: 10.1105/tpc.108.058263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-Y, Broeckling CD, Blancaflor EB, Sledge MK, Sumner LW. Wang Z-Y. Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa. The Plant Journal. 2005;42:689–707. doi: 10.1111/j.1365-313X.2005.02405.x. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Creelman RA. Zhu J-K. From laboratory to field. Using information from Arabidopsis to engineer salt, cold, and drought tolerance in crops. Plant Physiology. 2004;135:615–621. doi: 10.1104/pp.104.040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Kim D-J, Baek J-M, Choi H-K, Ellis LC, Kuester H. Cook DR. Syntenic relationships between Medicago truncatula and Arabidopsis reveal extensive divergence of genome organization. Plant Physiology. 2003;131:1018–1026. doi: 10.1104/pp.102.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-K. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hierarchical clustering analysis of shoot gene expression at all stress time points.
Hierarchical clustering analysis of root gene expression at all stress time points.
Expression pattern clusters of drought-regulated shoot genes.
Expression pattern clusters of drought-regulated root genes.
Eigenvectors of all polar and non-polar GC/MS derivatives.
List of top 100 most drought-induced genes in shoots.
Table S2. List of top 100 most drought-repressed genes in shoots.
Table S3. List of top 100 drought-induced genes in roots.
Table S4. List of top 100 most drought-repressed genes in roots.
Early drought-responsive genes in drought-stressed roots.
Early transient drought-responsive genes in drought-stressed shoots.
Early transient drought-responsive genes in drought-stressed roots.
Drought stress-regulated putative transcription factors genes in shoots.
Drought stress-regulated putative transcription factors genes in roots.
eigenvalues of compounds that contributed to tissue type, stress treatment and growth.