Abstract
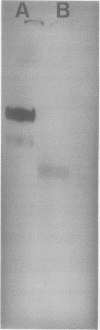
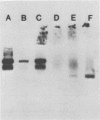
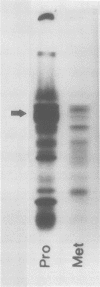
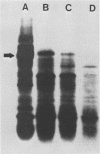
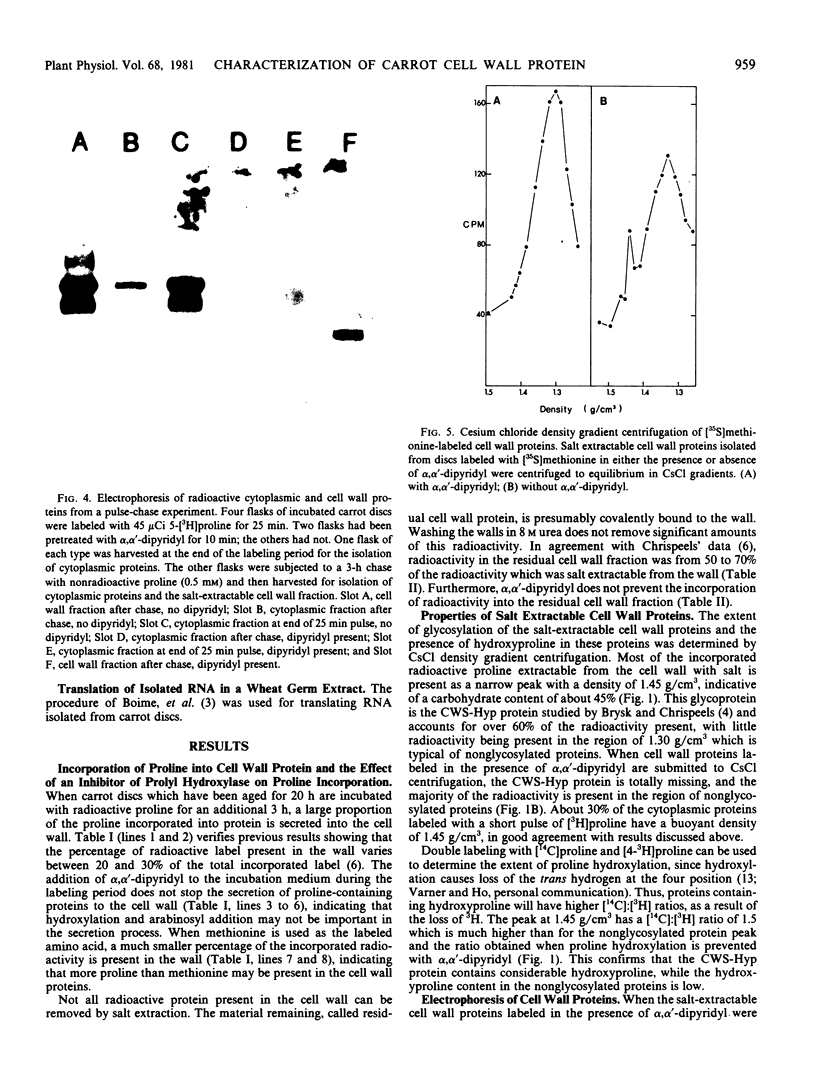
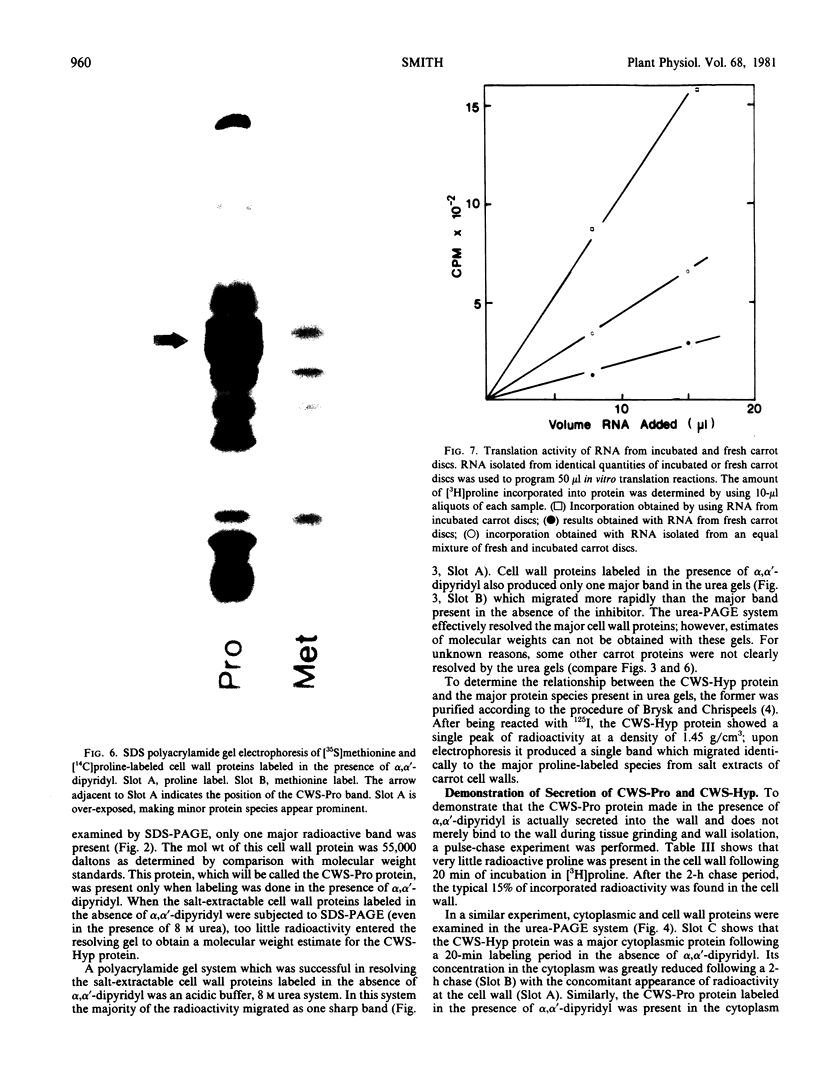
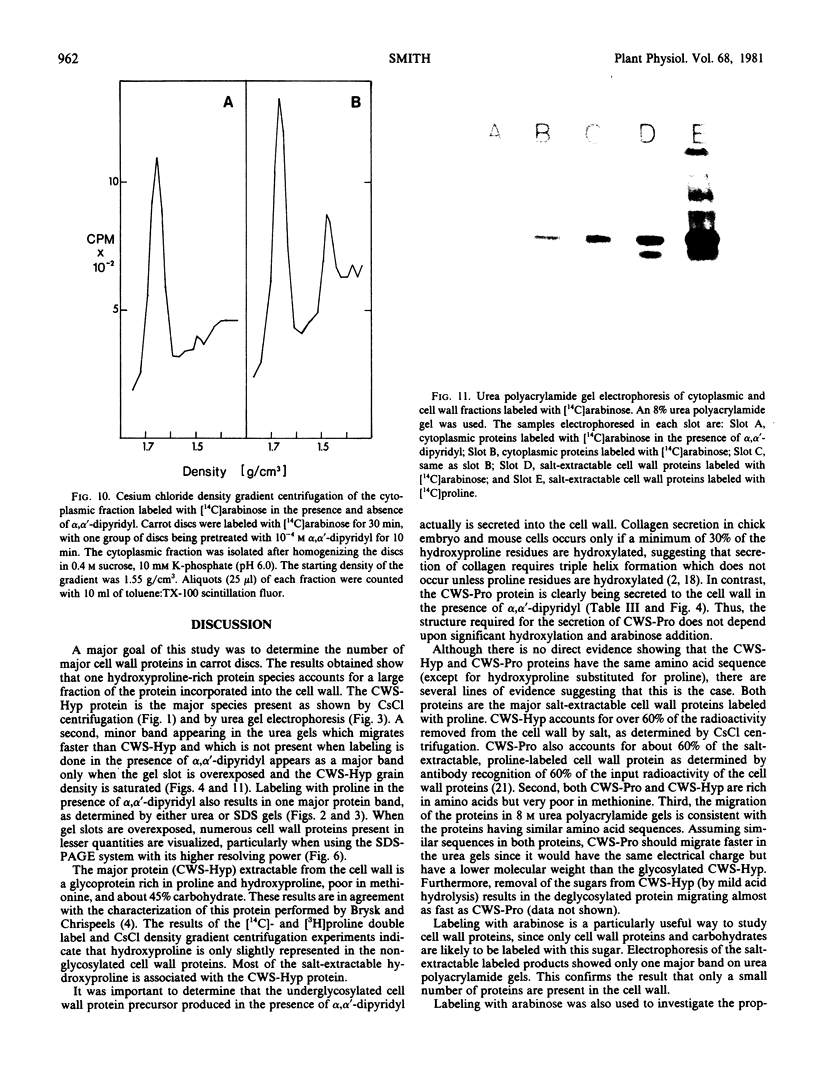
A single glycoprotein accounts for the majority of radioactivity secreted to the cell wall when incubated carrot (Daucus carota) discs are labeled with radioactive proline or arabinose. The ferrous chelator α,α′-dipyridyl prevents the synthesis of this protein. A new proline-labeled protein is made in the presence of α,α′-dipyridyl and is secreted to the cell wall. The protein has little, if any, carbohydrate attached to it and has a molecular weight of 55,000 daltons. This protein appears to be the nonhydroxylated, nonglycosylated form of the major cell wall glycoprotein. α,α′-Dipyridyl does not prevent proline label from becoming tightly (presumably covalently) bound to the cell wall, providing further evidence that hydroxylation and arabinosylation are not required for the covalent attachment of proteins to the cell wall. Messenger RNA extracted from incubated carrot discs produces a product which electrophoreses similarly to the protein made in the presence of α,α′-dipyridyl. The possible use of the carrot disc system to study gene structure and regulation is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanck T. J., Peterkofsky B. The stimulation of collagen secretion by ascorbate as a result of increased proline hydroxylation in chick embryo fibroblasts. Arch Biochem Biophys. 1975 Nov;171(1):259–267. doi: 10.1016/0003-9861(75)90031-4. [DOI] [PubMed] [Google Scholar]
- Boime I., McWilliams D., Szczesna E., Camel M. Synthesis of human placental lactogen messenger RNA as a function of gestation. J Biol Chem. 1976 Feb 10;251(3):820–825. [PubMed] [Google Scholar]
- Brysk M. M., Chrispeels M. J. Isolation and partial characterization of a hydroxyproline-rich cell wall glycoprotein and its cytoplasmic precursor. Biochim Biophys Acta. 1972 Feb 29;257(2):421–432. doi: 10.1016/0005-2795(72)90295-4. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and secretion of hydroxyproline containing macromolecules in carrots. I. Kinetic analysis. Plant Physiol. 1969 Aug;44(8):1187–1193. doi: 10.1104/pp.44.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth J. M., Denborough M. A. The use of equilibrium-density-gradient methods for the preparation and characterization of blood-group-specific glycoproteins. Biochem J. 1970 May;117(5):879–891. doi: 10.1042/bj1170879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashek W. V. Synthesis and Transport of Hydroxyproline-rich Components in Suspension Cultures of Sycamore-Maple Cells. Plant Physiol. 1970 Dec;46(6):831–838. doi: 10.1104/pp.46.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall D. K., Shimbayashi K. Factors Affecting Growth of Tobacco Callus Tissue and Its Incorporation of Tyrosine. Plant Physiol. 1960 May;35(3):396–404. doi: 10.1104/pp.35.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner M., Chrispeels M. J. Involvement of the Golgi Apparatus in the Synthesis and Secretion of Hydroxyproline-rich Cell Wall Glycoproteins. Plant Physiol. 1975 Mar;55(3):536–541. doi: 10.1104/pp.55.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLLAR S. J., JARAI M. Biochemical chlorination in Streptomvces aureofaciens. Nature. 1960 Nov 19;188:665–665. doi: 10.1038/188665a0. [DOI] [PubMed] [Google Scholar]
- LAMPORT D. T. HYDROXYPROLINE BIOSYNTHESIS: LOSS OF HYDROGEN DURING THE HYDROXYLATION OF PROLINE. Nature. 1964 Apr 18;202:293–294. doi: 10.1038/202293a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamport D. T., Miller D. H. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971 Oct;48(4):454–456. doi: 10.1104/pp.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. Regulation of collagen secretion by ascorbic acid in 3T3 and chick embryo fibroblasts. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1343–1350. doi: 10.1016/0006-291x(72)90614-6. [DOI] [PubMed] [Google Scholar]
- Pope D. G. Relationships between Hydroxyproline-containing Proteins Secreted into the Cell Wall and Medium by Suspension-cultured Acer pseudoplatanus Cells. Plant Physiol. 1977 May;59(5):894–900. doi: 10.1104/pp.59.5.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadava D., Chrispeels M. J. Hydroxyproline biosynthesis in plant cells. Peptidyl proline hydroxylase from carrot disks. Biochim Biophys Acta. 1971 Feb 10;227(2):278–287. doi: 10.1016/0005-2744(71)90060-x. [DOI] [PubMed] [Google Scholar]
- Smith M. A. Characterization of Carrot Cell Wall Protein : II. IMMUNOLOGICAL STUDY OF CELL WALL PROTEIN. Plant Physiol. 1981 Oct;68(4):964–968. doi: 10.1104/pp.68.4.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Wasmuth J. J., Caskey C. T. Biochemical characterization of azetidine carboxylic acid-resistant Chinese hamster cells. Cell. 1976 May;8(1):71–77. doi: 10.1016/0092-8674(76)90187-2. [DOI] [PubMed] [Google Scholar]