Abstract
Cocaine stimuli often trigger relapse of drug-taking, even following periods of prolonged abstinence. Here, electrophysiological recordings were made in rats (n = 29) to determine how neurons in the prelimbic (PrL) or infralimbic (IL) regions of the medial prefrontal cortex (mPFC) encode cocaine-associated stimuli and cocaine-seeking, and whether this processing is differentially altered after 1 month of cocaine abstinence. After self-administration training, neurons (n=308) in the mPFC were recorded during a single test session conducted either the next day or 1 month later. Test sessions consisted of three phases during which (i) the tone–houselight stimulus previously paired with cocaine infusion during self-administration was randomly presented by the experimenter, (ii) rats responded on the lever previously associated with cocaine during extinction and (iii) the tone–houselight was presented randomly between cocaine-reinforced responding during resumption of cocaine self-administration. PrL neurons showed enhanced encoding of the cocaine stimulus and drug-seeking behavior (under extinction and self-administration) following 30 days of abstinence. In contrast, although IL neurons encoded cocaine cues and cocaine-seeking, there were no pronounced changes in IL responsiveness following 30 days’ abstinence. Importantly, cue-related changes do not represent a generalized stimulus-evoked discharge as PrL and IL neurons in control animals (n=4) exhibited negligible recruitment by the tone–houselight stimulus. The results support the view that, following abstinence, neural encoding in the PrL but not IL may play a key role in enhanced cocaine-seeking, particularly following re-exposure to cocaine-associated cues.
Keywords: electrophysiology, behavior, self-administration, relapse, craving
Introduction
Cocaine addiction is characterized by cycles of drug use, abstinence and relapse (Koob & Le Moal, 2008; Koob & Volkow, 2010). During abstinence, addicts frequently experience feelings of craving evoked by stress, drug-associated cues or drug re-exposure that often leads to relapse (Gawin, 1991; O’Brien, 1997; Volkow et al., 2010). In cocaine addicts, reports show both reduced brain activity (Goldstein and Volkow 2002, 2011) and reduced gray matter volume (Franklin et al., 2002; Matochik et al., 2003; Tanabe et al., 2009) in the prefrontal cortex (PFC), but heightened PFC and nucleus accumbens (NAc) activation to drug-associated stimuli (Bonson et al., 2002, Maas et al., 1998, Goldstein & Volkow, 2011). Importantly, this enhanced activation to cues is positively correlated with feelings of ‘craving’ before self-administering cocaine (Risinger et al., 2005).
In rodent models, the medial PFC (mPFC)–NAc circuit appears to play a key role in drug-seeking behavior (Kalivas, 2008; Gipson et al., 2013). In support, electrophysiological recordings revealed that distinct populations of NAc (Peoples et al., 1997; Carelli et al., 2000; Carelli & Wondolowski, 2003) and mPFC (Chang et al., 2000) neurons encode the important features of cocaine-seeking behavior including response initiation, completion and reinforcement delivery. Likewise, NAc neurons are activated by noncontingent ‘probes’ of the tone–houselight previously paired with cocaine infusion during self-administration (Carelli, 2000; Carelli & Ijames, 2001). Indeed, it has been postulated that cocaine-associated cues initiate cocaine-seeking by increasing synaptic strength in the NAc (i.e., spine diameter and AMPA/NMDA current ratio), and this effect is dependent on mPFC activity (Gipson et al., 2013).
The mPFC consists of discrete subregrions that appear to mediate different aspects of the addiction cycle. In the rat, the mPFC is comprised of the dorsal (prelimbic; PrL) and ventral (infralimbic; IL) areas (Ongur & Price, 2000; Heidbreder & Groenewegen, 2003) that preferentially project to the NAc core and shell, respectively. The PrL is important for cue-elicited drug-seeking (Lasseter et al., 2010) while the IL appears to play a more critical role in the inhibition of cocaine-seeking in extinction (Peters et al., 2008). As such, it has been postulated that the mPFC exerts control over subcortical regions to drive motivated behavior, particularly following periods of abstinence (Koya et al., 2009).
Here, we used electrophysiological recording procedures in behaving rats to determine whether the responsiveness of neurons in the PrL or IL, elicited by cocaine-associated stimuli alone and during drug-seeking (extinction and self-administration), is altered after 1 month’s abstinence from cocaine self-administration. Prior work has indicated that cocaine abstinence selectively recruits neurons in the NAc core during the presentation of cocaine cues (Hollander & Carelli, 2005; 2007; Cameron & Carelli, 2012; Guillem et al., 2013, but see Ghitza et al., 2003), and increased synaptic strength in the NAc core to cocaine cues is dependent on PrL activity (Gipson et al., 2013). As such, we predicted that a greater population of cells that encode task-relevant information will be observed in the PrL but not IL during presentation of cocaine-associated cues and during drug-seeking behavior following extended abstinence.
Materials and methods
Subjects
Male Sprague–Dawley rats (Harlan), aged 90–120 days and weighing ~ 275–350 grams at the beginning of the study, were used (n = 29). Animals were housed individually in polypropylene cages and maintained on a standard 12:12 hour light–dark cycle with lights on at 07.00 h. Food and water were available ad libitum during the 1-week period of adaptation to the vivarium before surgery. During behavioral training and abstinence, animals were restricted to no less than 85% of their preoperative body weight by limiting water access to ~ 30 ml per day. Animal procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee (IACUC).
Surgery
A subset of animals (n = 25) were prepared for both intrajugular catheter and microwire electrode array implantation in the same surgery. Rats were anesthetized with a ketamine hydrochloride (100 mg/kg) and xylazine hydrochloride (10 mg/kg) cocktail (i.m.). The catheter was custom-made by Access Technologies and Norfolk Medical (Skokie, IL, USA) and implanted in the jugular vein using established procedures (Wheeler et al., 2008; Owesson-White et al., 2009). Each array was custom-designed, purchased from a commercial source (NB Labs, Denison, TX, USA) and described in detail elsewhere (Carelli et al., 2000). Briefly, arrays consisted of eight microwires (50 μm diameter) and were permanently implanted in a subset of animals (n=14) into the PrL (AP, +2.7; ML, ±0.6; DV, −4.0 mm) in one hemisphere and the IL (AP, +2.7; ML, ±0.5; DV, −5.0 mm) in the contralateral hemisphere. The location of PrL and IL arrays were counterbalanced by hemispheric side across animals. In the remaining animals, arrays were implanted bilaterally in either the IL cortex (n=7) or the PrL cortex (n=4) using the same coordinates as above. All placements were relative to bregma on a level skull (Paxinos and Watson, 2005). The remaining animals (n = 4) were implanted with microwire arrays (PrL in one hemisphere and IL in the other hemisphere) and used as control subjects (see below).
Cocaine self-administration training
Behavioral procedures were adapted from Hollander & Carelli (2007). One week following surgery, experimental animals (n = 25) were placed on water restriction (~30 ml of water per day) for at least 3 days before training began. Cocaine self-administration training consisted of daily 2-h sessions conducted in a 43 × 43 × 53 cm3 Plexiglas chamber (Med. Associates, Inc., St. Albans, VT, USA) housed within a commercial sound-attenuated cubicle (Fibrocrete, Inc., Crandall, GA, USA). The beginning of the self-administration session was signaled by the onset of a cue light positioned 6.5 cm above a lever and lever extension. Lever depression on an FR1 schedule resulted in intravenous cocaine delivery (0.33 mg per infusion, ~1 mg/kg per infusion, over 6 s) paired with termination of the cue light, simultaneous retraction of the lever and onset of a tone–houselight conditioned stimulus (CS; 20 s). Following the 20-s CS period, the lever extended into the chamber and the cue light was illuminated. Training was complete after 14 days. Rats were then randomly divided into two groups: 1 day (n=12) or 30 days (n=13) of abstinence during which time rats remained in their home cages (no drug).
Experimental test sessions
PrL and IL neurons were recorded during a single test session conducted either 1 or 30 days following the last training session (Figure 1A). The test session consisted of three phases: (1) CS probes, (2) extinction and (3) self-administration and CS probes (Figure 1B). In Phase 1, the cue light above the lever was illuminated but the lever was not extended. Ten pseudorandom presentations of the tone–houselight CS (5 s) were presented over 15 minutes. The cue light above the lever remained on throughout Phase 1. Next (Phase 2), the lever associated with cocaine during training was extended into the chamber and extinction was initiated. During the extinction phase each lever depression resulted in the presentation of the CS (20 s) only (no drug), termination of the cue light and simultaneous retraction of the lever. Following the 20-s CS without drug infusions, the lever was extended into the chamber and the cue light illuminated to initiate another trial. After 2 h, Phase 3 was initiated via a priming infusion of cocaine (0.33 mg per infusion; 6 s) paired with the CS (20 s). Lever presses during Phase 3 resulted in cocaine infusions, as described in the training session. Additionally, the computer presented 18 CS probes (5 s) pseudorandomly interspersed between lever presses. The test session was completed 2 h after the start of Phase 3.
Figure 1.

Schematic diagram of (A) experimental timeline and (B) test session (see Materials and Methods for details).
Control sessions
To examine whether cue-related firing represents a generalized stimulus-evoked discharge independent of associative learning, other rats (n = 4) were implanted with electrodes into the PrL and IL cortex and underwent similar water restriction as experimental subjects. Following 3 days of water restriction, animals were tested in two control phases. In the first phase, neurons were recorded during 20 probe trials of the tone–houselight (5 s each over 30 minutes). These cues (identical to those used in the self-administration) were never paired with cocaine. Immediately thereafter, each rat received an intraperitoneal injection of cocaine (20 mg/kg). Ten minutes later, the animals were presented with an additional 20 tone–houselight probes (5 s each over 30 minutes).
Electrophysiological recordings
Electrophysiological procedures have been described in detail previously (Carelli, 2000; Hollander & Carelli, 2005). Before the start of each session, the subject was connected to a flexible recording cable attached to a commutator (Med Associates Inc., St Albans, VT, USA), which allowed virtually unrestrained movement within the chamber. The headstage of each recording cable contained 16 miniature unity-gain field-effect transistors. Neurons were recorded differentially between each active and the inactive (reference) electrode from the permanently implanted microwires. The inactive electrode was examined before the start of the session to verify the absence of neuronal spike activity and served as the differential electrode for other electrodes with cell activity. Online isolation and discrimination of neuronal activity was accomplished using a neurophysiological system commercially available (multichannel acquisition processor, MAP System, Plexon, Inc., Dallas, TX, USA). Multiple window-discrimination modules and high-speed analog-to-digital signal processing in conjunction with computer software enabled isolation of neuronal signals based on waveform analysis. The neurophysiological system incorporated an array of digital signal processors (DSPs) for continuous spike recognition. The DSPs provided a continuous parallel digital output of neuronal spike events to a Pentium computer. Another computer controlled behavioral events of the experiment (Med Associates Inc.) and sent digital outputs corresponding to each event to the multichannel acquisition processor box to be time-stamped along with the neural data. Discrimination of individual waveforms began by setting a threshold level (well above background noise) for each wire. Units detected had to display peak voltage at least 20% greater than baseline. Individual waveforms corresponding to a single cell were discriminated using template analysis procedures and time–voltage boxes provided by the neurophysiological software system (MAP system, Plexon, Inc., Dallas, TX, USA). Cell recognition and sorting was finalized after the experiment using the Offline Sorter program (Plexon, Inc.), when neuronal data were further assessed based on the principal component analysis of the waveforms, cell firing characteristics such as autocorrelograms and interspike interval distribution to ensure putative cells showed biologically appropriate firing refractory periods, and cross-correlograms to ensure that multiple cells recorded on the same wires showed firing that was statistically independent of each other. All successfully identified cells within each region were analyzed together (i.e., not discriminated as pyramidal vs non-pyramidal cells).
Data analysis
Behavior
The number of lever presses during self-administration training were compared for the two groups (day 1 vs day 30) using a two-way repeated-measures ANOVA with session (1 through 14) as the within-subject factor and group as the between-subjects factor. Behavioral data during the distinct phases of the test session were also examined. In Phase 1, the total amount of time each rat spent in the quadrant associated with the cocaine-paired lever was recorded. Further, the number of approaches toward this quadrant after each CS presentation was also recorded. Approaches to and time in the quadrant were defined by at least one-half of a body length in the quadrant with the rat’s orientation toward the quadrant. Due to technical issues with video recording, behavioral data was only recorded from a subset of animals (n=10 in the day-1 group and n=11 in the day-30 group). Student’s t-test was used to compare the two groups (day 1 versus day 30) for all of the behavior measures, i.e., amount of time (s) spent in and number of approaches to the cocaine-associated quadrant during Phase 1, the number of extinction presses during Phase 2 and the number of reinforced self-administration presses during Phase 3. Due to catheter failure there were only 10 rats in each of the two groups that completed Phase 3 (self-administration). Behavioral analyses were completed using GraphPad Prism 6 (Graphpad Software, Inc., La Jolla, CA, USA).
Electrophysiology
Changes in neuronal firing patterns relative to task events were analyzed by constructing peri-event histograms (PEHs) and raster displays (bin width 250 ms) surrounding each event (CS presentations and lever presses) using commercially available software (Neuroexplorer for Windows version 4.034; Plexon Inc.). The responsiveness of each cell was examined during three epochs: (i) CS onset (0–2.5 s following CS presentation in Phases 1 and 3); (ii) prior to lever pressing (−2.5 to 0 s before the response); and (iii) following lever pressing (0–2.5 s after response completion). Individual units were categorized as showing either a decrease in firing rate (inhibition) or an increase in firing rate (excitation) compared to baseline (i.e., termed ‘phasic’) or no difference from baseline (termed ‘nonphasic’). Specifically, cells were classified as phasic if during one of these epochs the firing rate was greater than or less than the 99.9% confidence interval projected from the baseline period (10 to 0 s before CS onset or 10 to −2.5 s before a lever press) for at least one 250-ms time bin. This confidence interval was selected such that only robust responses were categorized as excitatory or inhibitory following established procedures (Day et al., 2011). Some neurons in this analysis exhibited low baseline firing rates, and the 99.9% confidence interval included zero. These cells were classified as inhibitory if the number of consecutive bins with zero spikes in the event epoch was at least double the number of consecutive zero-spike time bins during the baseline period. Units that exhibited both excitations and inhibitions within the same epoch were classified by the response that was most proximal to the event in question, unless the most proximal response was ongoing when the event occurred. Fisher’s exact test was used to compare the number of phasic and nonphasic cells between 1-day and 30-day rats during each phase of the test session for both subregions of the mPFC (PrL and IL). Statistical analyses of neural data were done using GraphPad Quickcalcs (Graphpad Software, Inc. La Jolla, CA, USA).
Histology
Upon completion of the experiment, rats were deeply anesthetized intramuscularly with a ketamine and xylazine mixture (100 and 10 mg/kg, respectively). To mark the placement of electrode tips, a 13.5-μA current was passed through each microwire electrode for 5 s. Transcardial perfusions were then performed using physiological saline and 3% potassium ferricyanide in 10% formalin, and brains were removed. After post-fixing and freezing, 40-μm coronal brain sections were mounted. The addition of potassium ferricyanide allowed for a blue reaction corresponding to the location of the electrode tip which was viewed under a 1× microscope lens. Placement of an electrode tip within the PrL or IL cortex was determined by examining the relative position of observable reaction product to visual landmarks and anatomical organization of the mPFC represented in a stereotaxic atlas (Paxinos & Watson, 2005).
Results
Behavioral training
Rats displayed increased lever pressing for cocaine across sessions regardless of group (total number of presses in 1-day group, 245 ± 26; 30-day group, 291 ± 27). A two-way repeated-measures ANOVA with session (day 1 through 14) as the within-subject factor and group (day 1 vs day 30) as the between-subjects factor revealed a main effect of session (F13,299 =17.21, P<0.05) but no main effect of group (F1,23 =1.5, P>0.10), nor an interaction (F23,299 =0.73, P>0.10). Thus, any differences seen between groups following drug abstinence could not be attributable to differences in initial self-administration training.
Distribution of neuronal recordings in the PrL and IL cortex
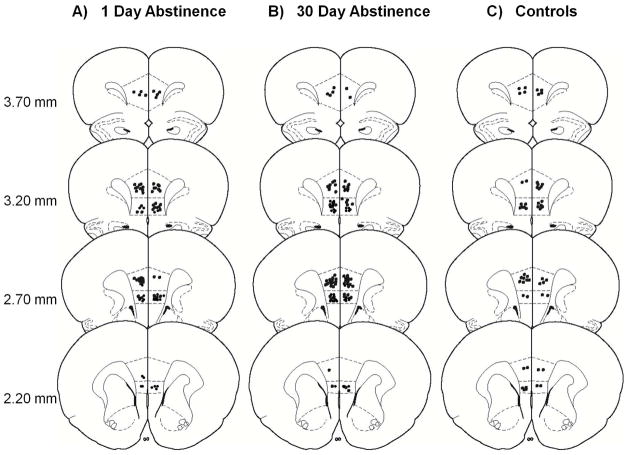
A total of 308 cells were recorded in the PrL or IL cortex from 29 animals (1-day, n = 12 rats; 30-day, n = 13 rats; controls, n = 4). Of 308 neurons, 117 cells were recorded in 1-day-abstinent animals (PrL, 58; IL, 59), 133 cells were recorded in 30-day-abstinent animals (PrL = 67 and IL = 66) and 58 cells were recorded in control animals (PrL, 35; IL, 23). In Phase 3, fewer cells were recorded due to catheter failures. Specifically, 104 cells were recorded in 1-day-abstinent animals (PrL, 55; IL, 49) and 101 cells were recorded in 30-day-abstinent animals (PrL, 60; IL, 41). Figure 2 shows the histological distribution of electrode placements from which neurons were recorded in each cortical region as a function of group.
Figure 2.
Anatomical distribution of electrode wire tip placements in the PrL (dorsal) and IL (ventral) subregions of the mPFC in (A) 1-day-abstinent, (B) 30-day-abstinent and (C) control animals.
Enhanced behavioral responses and neural encoding in the PrL, but not IL, to cocaine-associated stimuli after 1 month of abstinence
A goal of the present study is to examine whether 30 days of cocaine abstinence altered how neurons in two distinct prefrontal cortical subregions (PrL and IL) encode information about cocaine-associated stimuli before and after extended abstinence (Phase 1). Consistent with prior findings (Hollander & Carelli, 2007), during Phase 1 the 30-day-abstinent animals showed a stronger preference for the area of the chamber that was previously associated with the cocaine lever than did 1-day-abstinent animals. Specifically, compared to the 1-day-abstinent group, the 30-day animals spent significantly more time in the quadrant in which the previous cocaine-associated lever was positioned (t(19) = 4.11, P < 0.01) and made significantly more approaches into this area (t(19) = 4.95, P < 0.01) during CS probes (Figure 3A).
Figure 3.
(A) Behavioral and neuronal responses to CS presentations during Phase 1. Rats in the 30-day-abstinent group spent more time in the cocaine-associated quadrant of the experimental chamber (white bars) and made significantly more approaches to the retracted lever (black bars) during CS probe presentations. *P < 0.05. (B) PEHs show examples of individual prefrontal cortex neurons that exhibited an excitation (CSe, top) or inhibition (CSi, bottom) in cell firing relative to CS onset (gray dashed line) during Phase 1. CS duration (5 s) is indicated by the horizontal gray line below PEHs. Each PEH contains 250 bins here and in subsequent figures. (C) Piecharts show distribution of phasic and nonphasic neurons across population of recorded cells. There was a significantly greater number of phasic neurons in PrL (gray) activated by the CS in the 30-day group than in the 1-day group. *P < 0.05. (D) Piecharts of population responses show no difference in the number of phasic cells in the IL across abstinence periods.
This enhanced behavioral responding to the cocaine-associated cue following 30 days of cocaine abstinence was also accompanied by a greater number and percentage of cells displaying phasic changes in activity in response to CS presentations in the PrL, but not the IL. Specifically, during stimulus probe presentations in Phase 1, distinct populations of neurons exhibited either an increase (Figure 3B top; CS excitation, termed CSe) or decrease (Figure 3B bottom; CS inhibition, termed CSi) in firing rates at CS probe onset and thus were classified as ‘phasic’ to CS presentation. In the PrL region there was a significantly greater number of cells that displayed phasic changes to the CS probes in rats that underwent 30 days of abstinence (30 out of 67; 45%) compared to rats that underwent only 1 day of abstinence (14 out of 58; 24%), as shown in Figure 3C (Fisher’s exact test, P<0.05). Interestingly, this result was due to increases in both types of phasic neurons (CSe and CSi), as shown in Table 1. In contrast, in the IL no significant differences were observed in the number of phasic cells between day-1 (15 out of 59; 25%) and day-30 rats (17 out of 66; 26%), as shown in Figure 3D (Fisher’s exact test, P > 0.10). This finding was evident across both CSe and CSi cell types in the IL (Table 1). Further, there was no difference before abstinence (i.e., day-1 rats) in the percentage of phasic cells between the IL and the PrL to cue presentations as shown by a Fisher’s exact test, P > 0.10 (Fig 3C top versus D top).
Table 1.
Numbers and percentages of cells exhibiting an excitation (CSe) or inhibition (CSi) in firing rate during CS probes (Phase 1) in the PrL versus IL as a function of days of abstinence
| Abstinence | n cells | CSe
|
CSi
|
NP
|
|||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| PrL | |||||||
| 1-day | 58 | 7 | 12 | 7 | 12 | 44 | 74 |
| 30-day | 67 | 14 | 21 | 16 | 24 | 37 | 55 |
| IL | |||||||
| 1-day | 59 | 11 | 18 | 4 | 6 | 44 | 75 |
| 30-day | 66 | 13 | 20 | 4 | 7 | 49 | 74 |
NP, nonphasic.
Enhanced behavioral responding and neural encoding in the PrL, but not IL, to lever presses under extinction after 1 month of abstinence
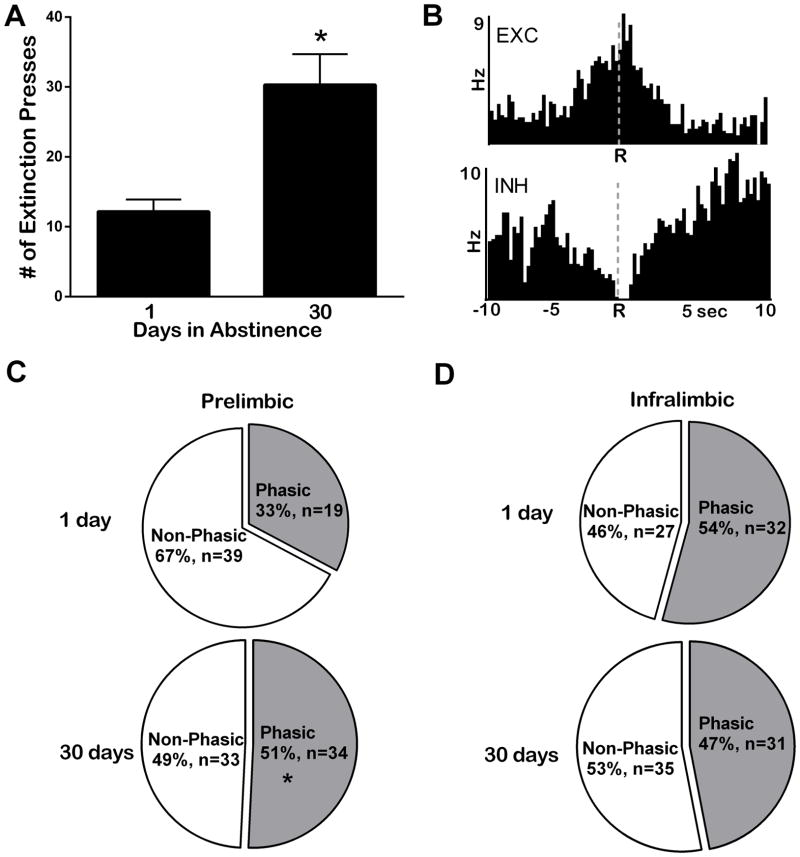
Another goal of this study was to examine whether 30 days of cocaine abstinence altered how the PrL and IL neurons encoded cocaine-seeking behavior under extinction conditions (Phase 2), before and following abstinence. Rats that underwent 30 days of abstinence made significantly more lever presses than did 1-day animals (t(19) = 3.7, P < 0.05; Figure 4A), consistent with an ‘incubation of craving’ effect (Pickens et al., 2011). This increase in behavioral responding was accompanied by an increase in the recruitment of phasic cells in the PrL but not the IL. As illustrated in Figure 4B, phasic neurons were classified as exhibiting an increase (excitation in firing rate, termed EXC; top) or decrease (inhibition in firing rate, termed INH, bottom) within seconds of the unreinforced response. While neurons in both regions showed phasic responses relative to lever press responding during extinction, only neurons in the PrL (Figure 4C) displayed a significantly greater number of type EXC and INH cells following 30 days of abstinence (34 out of 67; 51%) compared to 1 day (19 out of 58; 33%) as determined by Fisher’s exact test (P <0.05) and shown in Table 2. In contrast, there was no difference in the number of phasic cells in the IL (Figure 4D) for the 30-day-abstinent (31 out of 66; 47%) and 1-day-abstinent (32 out of 59; 54%) groups (Fisher’s exact test, P >0.10), across cell types (Table 2). It is important to note, however, that before abstinence (i.e. day-1 rats) there were more phasic cells under extinction in the IL than in the PrL as shown by a Fisher’s exact test, P <0.05 (Figure 4C top versus D top).
Figure 4.
Behavioral and neural responses during extinction (Phase 2). (A) Rats that underwent 30 days of abstinence lever-pressed under extinction significantly more than rats that underwent 1 day of abstinence (black bars). (B) PEHs show examples of individual prefrontal cortex neurons that exhibited an excitation (EXC, top) or inhibition (INH, bottom) in cell firing relative to the non-reinforced response. (C) Piecharts show that in the PrL there were more cells exhibiting phasic activity (gray) in the 30-day group than in the 1-day group *P < 0.05. (D) IL neurons showed no difference in activation during extinction presses between 1-day and 30-day groups.
Table 2.
Number and percentage of cells exhibiting an excitation (EXC) or inhibition (INH) in firing rate during extinction (Phase 2) in the PrL versus IL as a function of days of abstinence
| Abstinence | n cells | EXC
|
INH
|
NP
|
|||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| PrL | |||||||
| 1-day | 58 | 11 | 19 | 8 | 14 | 39 | 67 |
| 30-day | 67 | 17 | 25.5 | 17 | 25.5 | 33 | 49 |
| IL | |||||||
| 1-day | 59 | 20 | 34 | 12 | 20 | 27 | 46 |
| 30-day | 66 | 22 | 33 | 9 | 14 | 35 | 53 |
NP, nonphasic.
Enhanced recruitment of PrL (but not IL) neurons during resumption of cocaine self-administration and presentation of cocaine-associated cues
The present study also determined whether 30 days of cocaine abstinence altered how mPFC neurons in the PrL and the IL encode the resumption of cocaine self-administration following abstinence (Phase 3). Behaviorally, the number of cocaine-reinforced lever presses did not differ between the groups (t(19) = 0.95, P > 0.10,; Figure 5A) in Phase 3. Even though there were no differences in lever pressing between the two groups, there was an increase in the recruitment of phasic cells in the PrL, but not IL, following 30 days of abstinence. Figure 5B shows examples of the types of neuronal patterned discharges observed within seconds of the cocaine-reinforced response in the mPFC. These phasic cells were classified as exhibiting either an increase in firing within seconds preceding the response (termed type pre-response or PR, top), or an increase (termed type reinforcement excitation, or RFe, middle) or decrease (termed type reinforcement inhibition, or RFi, bottom) in firing rate immediately after response completion. Importantly, all three types of neuronal firing patterns were observed in both the PrL and IL. However, in the PrL there were significantly more phasic cells exhibiting one of the three types of patterned discharges following 30 days (37 out of 60; 62%) than following 1 day of abstinence (22 out of 55; 40%; Fisher’s exact test, P <0.05; Figure 5C, also Table 3). In contrast, as observed during Phases 1 and 2, there was no difference between the number of phasic IL neurons in the 1-day (27 out of 49; 55%) versus 30-day groups (19 out of 41; 46%; Fisher’s exact test, P >0.10; Figure 5D) in Phase 3, nor were differences observed across cell types (PR, RFe, RFi; Table 3).
Figure 5.
Behavioral and neural responses during cocaine self-administration (Phase 3). (A) There were no differences in the number of self-administration presses between rats that underwent 1 day or 30 days of cocaine abstinence (black bars). (B) PEHs show examples of representative patterned discharges relative to the cocaine-reinforced response (indicated by ‘R’ at gray dashed line). Medial prefrontal cortex neurons exhibited either increases in firing rate within seconds preceding the response (top; PR) or increases (middle; RFe) or decreases (bottom; RFi) in activity seconds following response completion. Cocaine delivery is indicated by the horizontal gray lines below PEHs. (C) Piecharts show that in the PrL there were more phasic cells (gray) in the 30-day group compared to the 1-day group. *P < 0.05. (D) IL neurons showed no difference.
Table 3.
Number and percentage of cells exhibiting patterned discharges (type PR, RFe or RFi) during self-administration (Phase 3) in the PrL versus IL cortex as a function of days of abstinence
| Abstinence | n cells | PR
|
RFe
|
RF1
|
NP
|
||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| PrL | |||||||||
| 1-day | 55 | 5 | 9 | 11 | 20 | 6 | 11 | 33 | 60 |
| 30-day | 60 | 8 | 13 | 16 | 27 | 13 | 22 | 23 | 38 |
| IL | |||||||||
| 1-day | 49 | 4 | 8 | 11 | 23 | 12 | 24 | 22 | 45 |
| 30-day | 41 | 4 | 10 | 4 | 10 | 11 | 26 | 22 | 54 |
NP, nonphasic.
Further, the recruitment of cells exhibiting distinct patterns of neuronal firing to the CS probes within the PrL region following abstinence was maintained following resumption of cocaine self-administration in Phase 3. Specifically, in the PrL region there was a significantly greater number of cells that displayed phasic changes to the CS probe during Phase 3 in rats that underwent 30 days of abstinence (30 out 60; 50%) than in rats that underwent only 1 day of abstinence (16 out of 55; 29%) as determined by Fisher’s exact test (P<0.05). No difference in the number of phasic cells between 1-day rats (14 out 49; 29%) and 30-day rats (12 out of 41; 29%) was observed in the IL (Fisher’s exact test, P > 0.10; data not shown).
Neural responses across phases in the PrL and IL
Interestingly, during the test sessions on day 1 or day 30, significantly more neurons were phasic in the IL during instrumental actions (i.e., extinction presses in Phase 2 and reestablishment of self-administration in Phase 3) compared to Pavlovian cues (i.e., CS probes in Phase 1), as determined by Fisher’s exact tests (Figs 4D and 5D compared to 3D; P <0.05). While distinct subsets of PrL neurons encoded all phases of the task on day 1, PrL cells showed elevated encoding for all phases of the test day following the 30-day abstinence period, including both instrumental and Pavlovian components (Fisher’s exact tests, all P < 0.05; Figures 3C, 4C and 5C). The latter finding indicates that once PrL neurons are recruited by abstinence this effect remains evident across all phases.
To investigate this finding further, we examined whether the same neurons track task events (i.e., show patterned discharges across all three phases) in the PrL and IL as a function of abstinence. Figure 6 shows the percentage of the phasic cells in the PrL (Fig. 6A) and the IL (Fig. 6B) that selectively responded during each distinct phase (CS probes, extinction or self-administration) versus the percentage of phasic cells that responded during all three phases of the task, after 1 or 30 days of abstinence. Before abstinence, the majority of neurons in the PrL differently encode each task event, with only a small percentage showing phasic responses across all three phases. However, following 1 month abstinence the majority of phasic cells were responsive across all three phases of the task. These findings indicate that the enhanced recruitment of PrL neurons within each phase as illustrated in Figures 3–5 following abstinence probably reflects the same population of cells throughout the session. Interestingly, this effect was not observed in the IL.
Figure 6.
The percentage of (A) PrL and (B) IL cells that were selectively responsive during only Phase 1 (CS only), Phase 2 (EXT only) or Phase 3 (SA only), or were responsive during all three phases (All Phases), as a function of abstinence period. Percentages are of the total phasic cells recorded. Note that these numbers do not match those reported in Figures 3–5 as the latter does not discriminate selective neural responses, as shown here.
Cue-related neuronal responses are dependent on associative learning
Finally, to examine whether cue-related firing represents a generalized stimulus-evoked discharge independent of associative learning, PrL and IL neurons were recorded in control rats (n = 4) given 20 presentations (5 s each) of the tone–houselight used as the CS. Immediately thereafter, each rat received an intraperitoneal injection of cocaine (20 mg/kg). Ten minutes later, an additional 20 CS probe presentations were given. Of 35 cells recorded in the PrL region and 23 recorded in the IL region, only four neurons (11%) in the PrL and two neurons (9%) in the IL were responsive to the stimulus, which remained unchanged following experimenter administered cocaine.
Discussion
In the present study, neurons in both the PrL and IL were responsive to drug-associated cues and instrumental contingencies before and following abstinence. However, 1 month of abstinence from cocaine self-administration resulted in enhanced recruitment of PrL neurons that encode cocaine-associated stimuli and drug-seeking behavior, under both extinction and self-administration conditions. This enhanced recruitment appears to include the same population of neurons that track task events throughout the three phases of the session. In contrast, while more IL neurons encoded instrumental behaviors (during both extinction and self-administration) rather than Pavlovian cues before abstinence, the recruitment of IL neurons across the three phases of the test session did not significantly change after 30 days of cocaine abstinence. Finally, the changes in cell firing to cues do not represent a generalized stimulus-evoked alteration as PrL and IL neurons were not responsive to cue probes in control animals that had not learned the cue–cocaine association. Collectively, our data are consistent with a differential role of the PrL and IL cortices in cue-guided drug-seeking behavior, before and following extended cocaine abstinence. The primary findings of the present study are discussed in detail below.
Enhanced recruitment of PrL but not IL neurons to drug cues after abstinence
During Phase 1, both PrL and IL neurons were activated by cocaine-associated cues before abstinence (day 1 rats), suggesting that both regions are tracking this information. However, a significant increase in the encoding of cue-associated information (CSe and CSi cells) was observed in the PrL, but not IL, following 1 month of abstinence (Figure 3; Table 1). These findings are in accordance with prior studies implying a role of the PrL in cue-elicited drug-seeking (McFarland et al., 2003; McLaughlin & See, 2003; Zavala et al., 2008; Lasseter et al., 2010; Gipson et al., 2013). In addition, our results support the view that the enhanced recruitment of neural signaling in the PrL to drug cues may be, in part, driving increased cocaine-seeking following abstinence (Pickens et al., 2011).
Interestingly, the PrL and IL play different roles in drug-seeking behavior in the presence of cocaine-associated cues, and those distinct roles extend to their respective projections in the NAc. Specifically, the PrL preferentially sends glutamatergic projections to the NAc core (McGeorge & Faull, 1989; Sesack & Pickel, 1992; Pennartz et al., 1994), and both brain regions are necessary for cue-induced reinstatement (McLaughlin & See, 2003; Fuchs et al., 2004). In contrast, the IL predominantly sends projections to the NAc shell, and neither of those brain regions appears necessary for this behavior (Fuchs et al., 2004; Ball & Slane, 2012). More recently, it has been shown that cues associated with cocaine may drive drug-seeking by inducing changes in dendritic spine size and synaptic strength in the NAc core, and this is dependent on mPFC activity (Gipson et al., 2013). Further, PrL inputs to NAc core are necessary for cue-guided drug-seeking behavior (Stefanik et al., 2013). Collectively, these findings support a critical role of the PrL–NAc core circuit in drug-seeking evoked by cocaine cues.
An important question regarding the PrL–NAc circuit is how this system becomes engaged following extended periods of drug abstinence. Given the aforementioned findings, it is possible that the PrL exerts control over limbic and striatal regions (e.g. NAc) to directly enhance cue-evoked motivated behavior following abstinence. In support, we have previously shown that NAc core neurons display heightened activation during presentation of cocaine-associated stimuli after 1 month of abstinence (Hollander & Carelli, 2005; 2007; see also Guillem et al., 2013), similar to the alterations in PrL activity observed in the present study. As the PrL sends unidirectional projections to the NAc core (McGeorge & Faull, 1989; Sesack & Pickel, 1992; Pennartz et al., 1994; McFarland et al., 2003; LaLumiere & Kalivas, 2008), the cue-evoked changes in neuronal activity in the core following abstinence could arise from these projections.
It is likely that the enhanced recruitment of PrL neurons by cocaine-associated stimuli is a direct consequence of cellular neuroadapations that occur in the mPFC following repeated drug exposure and abstinence (Trantham et al., 2002; Kalivas, 2007; Schwendt et al., 2007; Zavala et al., 2008; Berglind et al., 2009; Koya et al., 2009; Lasseter et al., 2009). An early study by Robinson et al. (2001) showed increased dendritic branching and density of spines along the dendrites within the NAc and PFC following 1 month of cocaine abstinence. Changes in dendrite morphology were not observed in control animals allowed to self-administer food (Robinson et al., 2001). Additional studies have examined neuroadapations following cocaine exposure and abstinence related to, for example, the extracellular signal-regulated kinase signaling pathway, brain-derived neurotrophic factor, regulators of G-protein signaling, glutamate receptor changes, synaptic plasticity and glutamate transmission (Conrad et al., 2008; Thomas et al., 2008; McGinty et al., 2010; Moussawi et al., 2010; Wolf & Ferrario, 2010). Further, it was shown that cocaine self-administration led to long-term changes in synaptic potentiation and glutamate homeostasis in the projection from mPFC to NAc, and this is mechanistically linked to group II metabotropic glutamate signaling (Moussawi et al., 2011). These neuroadapations alter glutamate signaling, transmission and receptor expression in the NAc and PrL. As the glutamatergic pathway between the PrL and NAc is critical for guiding drug-seeking in rats (Stefanik et al., 2013), alterations in glutamatergic transmission may contribute to our finding that both PrL and NAc recruit more phasic neurons following abstinence. Although the precise mechanism(s) are still being determined, these findings suggest that cocaine abstinence appears to alter neuronal connectivity within the corticolimbic circuit that may underlie the enhanced activation of PrL neurons reported here.
Enhanced recruitment of PrL, but not IL, phasic neurons during extinction
In Phase 2 we demonstrated that both PrL and IL neurons encoded information about extinction before abstinence (day 1 animals; Figure 4). Interestingly, in the 1-day group, IL neurons showed more phasic cells under extinction than PrL neurons, suggesting a broader role for the IL in tracking extinction learning before abstinence. However, PrL neurons in the abstinence group showed increased phasic responsiveness during extinction than did those in the 1-day-abstinent group (Figure 4A; Table 2). This enhanced recruitment of phasic neurons was not observed for IL neurons following abstinence. As rats were responding to the cocaine-associated cue under extinction in our study, this heightened recruitment of phasic neurons in the PrL but not IL following abstinence may be due to enhanced encoding of cue-associated information described above. Consistent with previous studies, we have shown that under extinction there is an ‘incubation of craving effect’, in that 30-day rats pressed significantly more under extinction than did 1-day rats. This may represent an enhancement in motivation to seek the drug following abstinence that is reflected in an increase in neural firing in the PrL.
In addition, our data support a role for the IL in drug-directed behaviors. Koya et al. (2009) showed that disruption of IL activity in 30-day-abstinent rats decreased incubation of craving (i.e., extinction responding) while increased IL activity in 1-day-abstinent rats enhanced this behavior. In our study, while the percentage of phasic cells in IL neurons did not change as a function of abstinence, IL neurons showed more phasic activity during lever pressing under extinction and self-administration than did IL neurons responding to CS probes. Also, a larger proportion of IL neurons responded under extinction than the proportion of PrL neurons responding before abstinence (i.e., in 1-day-abstinent rats). This finding is consistent with studies showing that the IL is not involved in cue-guided drug-seeking behavior but may be involved in guiding instrumental actions more generally.
It has been proposed that distinctly different neural circuits underlie reinstatement of cocaine-seeking in animals that experienced repeated extinction versus forced abstinence. Specifically, Kalivas (2008) proposed that the circuit involving repeated extinction engages various cortical (mPFC) and subcortical (e.g. NAc, basolateral amygdala) regions while the abstinent circuit engages the substantia nigra, dorsal striatum and sensory–motor cortex. However, our data show increased recruitment of phasic neurons in both the PrL (present study) and the NAc core (Hollander & Carelli, 2005; 2007) following abstinence to cues and during drug-seeking in extinction and reinstatement. Therefore, although we have not examined neural signaling in the dorsal striatum in extinction or abstinence models, our current data suggest a more complex neurocircuitry (i.e., including NAc core and PrL) underlying drug-seeking following abstinence than was proposed by Kalivas (2008).
Enhanced recruitment of PrL, but not IL, neural encoding during resumption of cocaine self-administration after abstinence
Neurons in both the PrL and IL encoded information about drug-seeking during the reestablishment of cocaine self-administration during Phase 3 in 1-day-abstinent rats (Figure 5). While there was generally greater activity in the IL than in the PrL during the resumption of self-administration, only PrL neurons showed increased recruitment of all cell types (PR, RFe, RFi) after abstinence (Figure 5; Table 3), consistent with its role in drug-seeking.
Behaviorally, rats did not show differences in self-administration responses in Phase 3. Thus, it is possible that the enhanced behavioral responding following abstinence observed in Phase 1 (CS probes) and Phase 2 (extinction) is due to a global cue-induced recruitment of PrL neurons following abstinence that extends to all the phases of this task. Also, the heightened encoding by PrL neurons is most probably due to the neuroadaptations described above, in spite of a lack of behavioral difference between groups. The later is not surprising as rats typically maintain an optimal level of cocaine and dopamine in the brain during self-administration (Pettit & Justice, 1989). Importantly, our study suggests optimal cocaine levels are probably not altered by extended abstinence, consistent with our prior reports (Hollander & Carelli, 2005; 2007). Regardless, neural recordings obtained in Phase 3 of our study again support differential roles of the mPFC in drug-seeking behavior following abstinence, in this case following resumption of cocaine self-administration behavior.
PrL neurons track task-relevant information across phases
The increased recruitment of phasic cells following abstinence appears to reflect the tracking of task-related information by the same population of PrL cells throughout the three phases of session (Figure 6). That is, following 1 month of abstinence, there were more phasic PrL cells responsive across all three phases of the task than in the 1-day-abstinent rats. This increase in overlapping encoding in the PrL (but not IL) as a function of abstinence may indicate that the same population of cells are recruited following abstinence in each phase (Figures 3–5). This is consistent with previous work in our lab using a multiple schedule task that showed an increase in overlapping encoding by NAc neurons following abstinence (Cameron & Carelli, 2012). Together, these findings present an interesting possibility that one consequence of abstinence is an increase in encoding of task-relevant information in the PrL–NAc core circuit. This heightened circuit encoding may be one mechanism by which drug-related stimuli can elicit craving and trigger relapse in drug-abstinent individuals, though future studies will be required to establish this putative mechanism.
Concluding remarks
The present findings support unique roles for the PrL and IL cortical networks to the NAc core and shell in discrete aspects of drug-seeking behavior, before and following extended cocaine abstinence. Generally, our data are consistent with prior findings showing that, while the PrL-NAc core circuit appears critical for cue-elicited drug-seeking (McFarland et al., 2003; McLaughlin & See, 2003; Zavala et al., 2008; Lasseter et al., 2010; Gipson et al., 2013), the IL–NAc shell system may play an important role in the inhibition of cocaine-seeking in extinction (Kalivas, 2008; Peters et al., 2008; LaLumiere et al., 2012). However, further investigation is needed to better model and apply this information to cocaine addiction in humans. For example, while our current study employed 2 weeks of short-access cocaine self-administration prior to abstinence, other studies have focused on extended, long-access cocaine self-administration that may better model compulsive drug-seeking behavior (Wee et al., 2007; George et al., 2008). Further, models employing punishment procedures revealed that a subset of animals (perhaps the more ‘addicted’ subgroup) exhibit undeterred drug-seeking in spite of footshock (Pelloux et al., 2007; Pelloux et al., 2013), and the prefrontal cortex plays a key role in this behavior (Chen et al., 2013). Additionally, studies incorporating choice procedures also tease apart distinct populations of animals that may better model compulsive cocaine users (Ahmed et al., 2013; Lenoir et al., 2013). Thus, future studies are needed to examine neural activity in mPFC–NAc circuits that better represent the loss of control over drug-directed behaviors that is characteristic of the addicted state in humans.
Acknowledgments
The authors thank Jessica Briley, Laura Ciompi and Fei Fei Wang for their technical support. This research was supported by DA014339 to RMC.
Abbreviations
- CS
conditioned stimulus
- CSe
CS excitation
- CSi
CS inhibition
- EXC
excitation in firing rate
- IL
infralimbic cortex
- INH
inhibition in firing rate
- mPFC
medial PFC
- NAc
nucleus accumbens
- PEH
peri-event histogram
- PFC
prefrontal cortex
- PR
pre-response (cell type)
- PrL
prelimbic cortex
- RFe
reinforcement excitation (cell type)
- RFi
reinforcement inhibition (cell type)
Footnotes
The authors report no conflict of interest.
References
- Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Current opinion in neurobiology. 2013;23:581–587. doi: 10.1016/j.conb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Ball KT, Slane M. Differential involvement of prelimbic and infralimbic medial prefrontal cortex in discrete cue-induced reinstatement of 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) seeking in rats. Psychopharmacology. 2012;224:377–385. doi: 10.1007/s00213-012-2762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biological psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CM, Carelli RM. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. The European journal of neuroscience. 2012;35:940–951. doi: 10.1111/j.1460-9568.2012.08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM. Activation of accumbens cell firing by stimuli associated with cocaine delivery during self-administration. Synapse. 2000;35:238–242. doi: 10.1002/(SICI)1098-2396(20000301)35:3<238::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Selective activation of accumbens neurons by cocaine-associated stimuli during a water/cocaine multiple schedule. Brain research. 2001;907:156–161. doi: 10.1016/s0006-8993(01)02604-x. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Selective encoding of cocaine versus natural rewards by nucleus accumbens neurons is not related to chronic drug exposure. J Neurosci. 2003;23:11214–11223. doi: 10.1523/JNEUROSCI.23-35-11214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Neuronal and behavioral correlations in the medial prefrontal cortex and nucleus accumbens during cocaine self-administration by rats. Neuroscience. 2000;99:433–443. doi: 10.1016/s0306-4522(00)00218-9. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Carelli RM. Nucleus accumbens neurons encode predicted and ongoing reward costs in rats. The European journal of neuroscience. 2011;33:308–321. doi: 10.1111/j.1460-9568.2010.07531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23:7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Ahmed SH, Peoples LL. Escalation of Cocaine Intake and Incubation of Cocaine Seeking Are Correlated with Dissociable Neuronal Processes in Different Accumbens Subregions. Biological psychiatry. doi: 10.1016/j.biopsych.2013.08.032. (in press) [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience and biobehavioral reviews. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacology. 2005;30:1464–1474. doi: 10.1038/sj.npp.1300748. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci. 2008;28:5088–5098. doi: 10.1523/JNEUROSCI.0253-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialogues in clinical neuroscience. 2007;9:389–397. doi: 10.31887/DCNS.2007.9.4/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotoxicity research. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual review of psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. The European journal of neuroscience. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. The European journal of neuroscience. 2009;30:1370–1381. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci. 2010;3:101–117. doi: 10.1007/7854_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Augier E, Vouillac C, Ahmed SH. A choice-based screening method for compulsive drug users in rats. Current protocols in neuroscience/editorial board, Jacqueline N. Crawley… [et al.] 2013;Chapter 9(Unit 9):44. doi: 10.1002/0471142301.ns0944s64. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. NeuroImage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain research. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences of the United States of America. 2010;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Wightman RM, Carelli RM. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. The European journal of neuroscience. 2009;30:1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier; New York: 2005. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ. Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. The European journal of neuroscience. 2013 doi: 10.1111/ejn.12289. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Progress in neurobiology. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Uzwiak AJ, Gee F, West MO. Operant behavior during sessions of intravenous cocaine infusion is necessary and sufficient for phasic firing of single nucleus accumbens neurons. Brain research. 1997;757:280–284. doi: 10.1016/s0006-8993(97)00299-0. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacology, biochemistry, and behavior. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Hearing MC, See RE, McGinty JF. Chronic cocaine reduces RGS4 mRNA in rat prefrontal cortex and dorsal striatum. Neuroreport. 2007;18:1261–1265. doi: 10.1097/WNR.0b013e328240507a. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. The Journal of comparative neurology. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Brown RM, Kalivas PW. Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. J Neurosci. 2013;33:13654–13662. doi: 10.1523/JNEUROSCI.1570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British journal of pharmacology. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:4859. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Trantham H, Szumlinski KK, McFarland K, Kalivas PW, Lavin A. Repeated cocaine administration alters the electrophysiological properties of prefrontal cortical neurons. Neuroscience. 2002;113:749–753. doi: 10.1016/s0306-4522(02)00246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GB, Zhang XL, Zhao LY, Sun LL, Wu P, Lu L, Shi J. Drug-related cues exacerbate decision making and increase craving in heroin addicts at different abstinence times. Psychopharmacology. 2012;221:701–708. doi: 10.1007/s00213-011-2617-5. [DOI] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. The Journal of pharmacology and experimental therapeutics. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35(2):185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, Neisewander JL. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking Behavior. Synapse. 2008;62:421–431. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]