Abstract
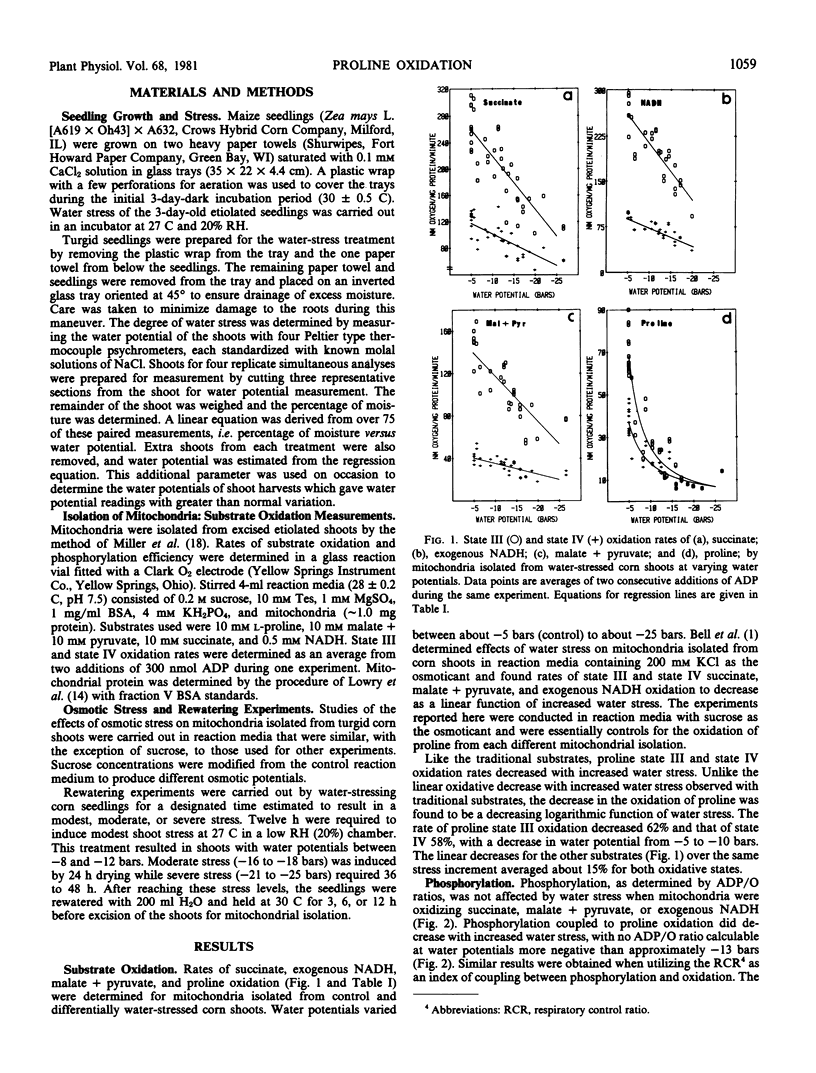
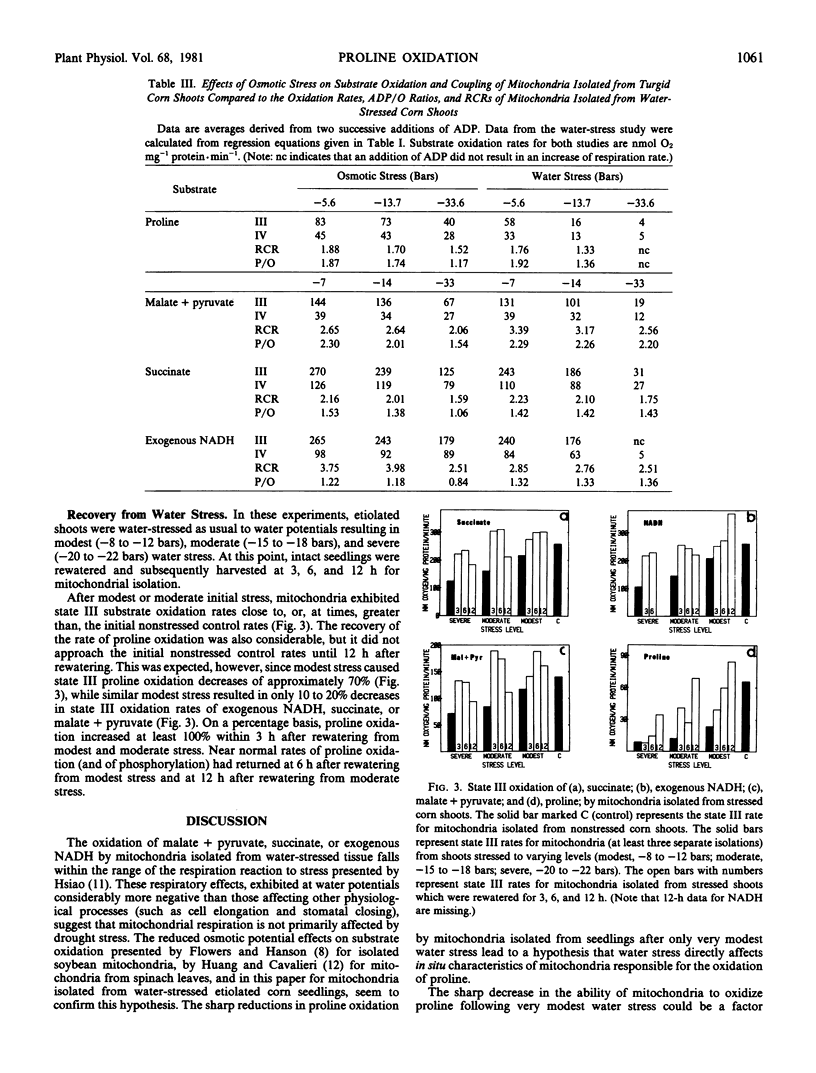
Proline oxidation and coupled phosphorylation were measured in mitochondria after isolation from shoots of water-stressed, etiolated maize (Zea mays L.) seedlings. Both state III and state IV rates of proline oxidation decreased as a logarithmic function of increased seedling water stress between −5 and −10 bars. Proline oxidation rates decreased 62% (state III) and 58% (state IV) as seedling water potentials were decreased from −5 to −10 bars. By comparison, oxidation of succinate, exogenous NADH, or malate + pyruvate decreased only 10 to 15% in this stress range. These decreases were a linear function of increased stress and were comparable to oxidation rates of mitochondria subjected to varying in vitro osmotic potentials. Osmotically induced in vitro stress reduced proline oxidation rates linearly with more negative osmotic potentials, a decrease that was similar to the responses of the other substrates to more negative osmotic potentials. Some decrease in coupling, with all substrates as determined by ADP/O ratios, was observed under osmotic stress. Mitochondria were also isolated from shoot tissue that had been stressed and then rewatered. On a percentage basis, the recovery of proline oxidation was greater than that of the other substrates.
The decreases in the proline oxidase activity of mitochondria after only slight stress indicate a mitochondrial sensitivity to water stress at significantly less negative water potentials than previously reported for measurements of maize membrane permeability and respiratory activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell D. T., Koeppe D. E., Miller R. J. The effects of drought stress on respiration of isolated corn mitochondria. Plant Physiol. 1971 Oct;48(4):413–415. doi: 10.1104/pp.48.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess S. F., Koeppe D. E. Oxidation of proline by plant mitochondria. Plant Physiol. 1978 Jul;62(1):22–25. doi: 10.1104/pp.62.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri A. J., Huang A. H. Carrier Protein-mediated Transport of Neutral Amino Acids into Mung Bean Mitochondria. Plant Physiol. 1980 Oct;66(4):588–591. doi: 10.1104/pp.66.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers T. J., Hanson J. B. The effect of reduced water potential on soybean mitochondria. Plant Physiol. 1969 Jul;44(7):939–945. doi: 10.1104/pp.44.7.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Cavalieri A. J. Proline Oxidase and Water Stress-induced Proline Accumulation in Spinach Leaves. Plant Physiol. 1979 Mar;63(3):531–535. doi: 10.1104/pp.63.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller R. J., Bell D. T., Koeppe D. E. The effects of water stress on some membrane characteristics of corn mitochondria. Plant Physiol. 1971 Aug;48(2):229–231. doi: 10.1104/pp.48.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J., Dumford S. W., Koeppe D. E., Hanson J. B. Divalent cation stimulation of substrate oxidation by corn mitochondria. Plant Physiol. 1970 Jun;45(6):649–653. doi: 10.1104/pp.45.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R. Inhibition of proline oxidation by water stress. Plant Physiol. 1977 May;59(5):930–932. doi: 10.1104/pp.59.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]


