Significance
Demands of modern society force many work operations into the night, when the intrinsic circadian timing system promotes sleep. Overnight shiftwork is associated with increased risk for adverse metabolic health and sleep disruption. Uncovering potential physiological mechanisms that contribute to metabolic dysregulation when work and eating occur at inappropriate circadian times is vital to the development of effective treatment strategies. In this study, healthy volunteers underwent a commonly used simulated shiftwork protocol to quantify changes in metabolic, sleep, and circadian physiology when working and eating during the night as compared with a traditional day work schedule. We demonstrate that nightshift work reduces total daily energy expenditure, representing a contributing mechanism for unwanted weight gain and obesity.
Keywords: insufficient sleep, melatonin, diet-induced thermogenesis, eating at night, appetite
Abstract
Eating at a time when the internal circadian clock promotes sleep is a novel risk factor for weight gain and obesity, yet little is known about mechanisms by which circadian misalignment leads to metabolic dysregulation in humans. We studied 14 adults in a 6-d inpatient simulated shiftwork protocol and quantified changes in energy expenditure, macronutrient utilization, appetitive hormones, sleep, and circadian phase during day versus nightshift work. We found that total daily energy expenditure increased by ∼4% on the transition day to the first nightshift, which consisted of an afternoon nap and extended wakefulness, whereas total daily energy expenditure decreased by ∼3% on each of the second and third nightshift days, which consisted of daytime sleep followed by afternoon and nighttime wakefulness. Contrary to expectations, energy expenditure decreased by ∼12–16% during scheduled daytime sleep opportunities despite disturbed sleep. The thermic effect of feeding also decreased in response to a late dinner on the first nightshift. Total daily fat utilization increased on the first and second nightshift days, contrary to expectations, and carbohydrate and protein utilization were reduced on the second nightshift day. Ratings of hunger were decreased during nightshift days despite decreases in 24-h levels of the satiety hormones leptin and peptide-YY. Findings suggest that reduced total daily energy expenditure during nightshift schedules and reduced energy expenditure in response to dinner represent contributing mechanisms by which humans working and eating during the biological night, when the circadian clock is promoting sleep, may increase the risk of weight gain and obesity.
Emerging evidence from nonhuman animal models indicates a fundamental interplay between circadian and metabolic physiology (1, 2) with implications for health and disease (3–5). Eating at inappropriate circadian times (e.g., at night) is considered a novel risk factor for weight gain and obesity, yet little research has been conducted in humans on this topic. The circadian time-keeping system in humans modulates energy metabolism so that wakefulness, activity, and food intake are promoted during the solar day and sleep, inactivity, and fasting occur during the solar night (2). With the widespread use of electrical lighting, however, work and social activities are capable of being extended further into the night (6, 7). Being awake during the biological night leads to disturbed physiology and behavior, because it creates a state of desynchrony between the circadian clock and wakefulness–sleep cycle known as circadian misalignment. Circadian misalignment is common in shiftwork. More than 20% of adults in the United States work nontraditional hours (8) and eat some of their meals during the biological night (9), which can increase blood glucose and triacylglycerol levels in response to a high-carbohydrate versus high-fat diet (10) and increase low-density lipoprotein cholesterol levels (11). Further, shiftwork is associated with increased risk for obesity (12), metabolic syndrome (13), and diabetes (14).
Evidence from nonhuman animal models reveals that consumption of calories at inappropriate circadian times contributes to weight gain (15, 16). Further, caloric intake during typical sleep time leads to greater weight gain than the same caloric intake during typical waketime (15, 16). Mechanisms by which eating during the biological night increases risk of weight gain in humans remain unknown. Therefore, the primary aim of this study was to examine the influence of circadian misalignment on total daily energy expenditure (EE), EE after food intake (referred to as the thermic effect of food; TEF), and total daily macronutrient utilization (use of energy from fats, carbohydrates, and proteins) in humans via whole-room indirect calorimetry. The 6-d inpatient nightshift work simulation protocol was also used to examine the influence of circadian misalignment on sleep staging, EE during scheduled sleep and for sleep stages, hunger ratings, and the appetitive hormone ghrelin and satiety hormones leptin and peptide-YY (PYY). We hypothesized that circadian misalignment would disrupt sleep, reduce fat utilization (because carbohydrate utilization is tightly controlled in humans) (17), reduce TEF following food intake during the biological night, and alter circulating concentrations of appetitive hormones associated with increased appetite [increase ghrelin and reduce leptin (5, 18) and PYY levels]. We also hypothesized that circadian misalignment would increase EE during scheduled daytime sleep opportunities due to increased arousals (19), but that a hypothesis can also be made for decreased total daily EE (9).
Results
Circadian Melatonin Rhythm and Sleep Staging.
Circadian misalignment occurred during simulated nightshift work (Fig. 1). Fig. S1 shows that the circadian melatonin rhythm did not adapt to the nightshift schedule, and thus sleep occurred during the biological day when melatonin levels were low and wakefulness occurred during the biological night when melatonin levels were high. Total sleep time, sleep efficiency, and percentage and minutes of stage 2 sleep were lower, minutes of wakefulness after sleep onset (WASO) was higher, and average duration of awakenings was increased during daytime sleep (Table S1). Further, daytime sleep opportunities showed shorter latencies to sleep onset (SOL), persistent sleep (LPS), and rapid eye movement sleep (REML). Last, percentage and minutes of REM sleep were lower during daytime sleep before nightshift 2 versus baseline.
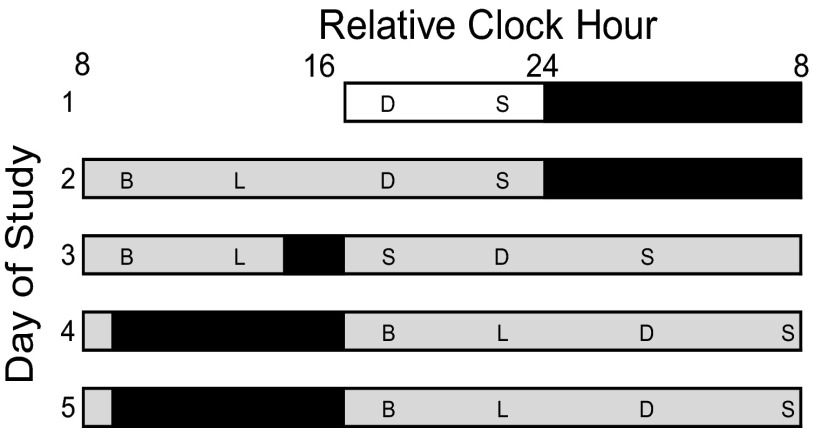
Fig. 1.
Study protocol. Open bars represent room light (<40 lx). Black bars represent scheduled polysomnography-recorded sleep. Time of day is plotted as relative clock hour with scheduled waketime arbitrarily assigned a value of 0800 hours and all other times referenced to this value. Shaded areas indicate scheduled wakefulness in dim light (<1 lx). B, breakfast; D, dinner; L, lunch; S, snack. Subjects were allowed three brief scheduled breaks per day to stand up and retrieve meals and use the toilet for bowel movements <3 m from the bed. Outside of scheduled breaks, subjects were given a urinal or bedpan when needed. Wakefulness and subject compliance during the constant posture protocol were verified via continuous monitoring by research staff and electroencephalography (EEG) recordings.
Energy Expenditure, Macronutrient Utilization and Balance, and Thermic Effect of Food.
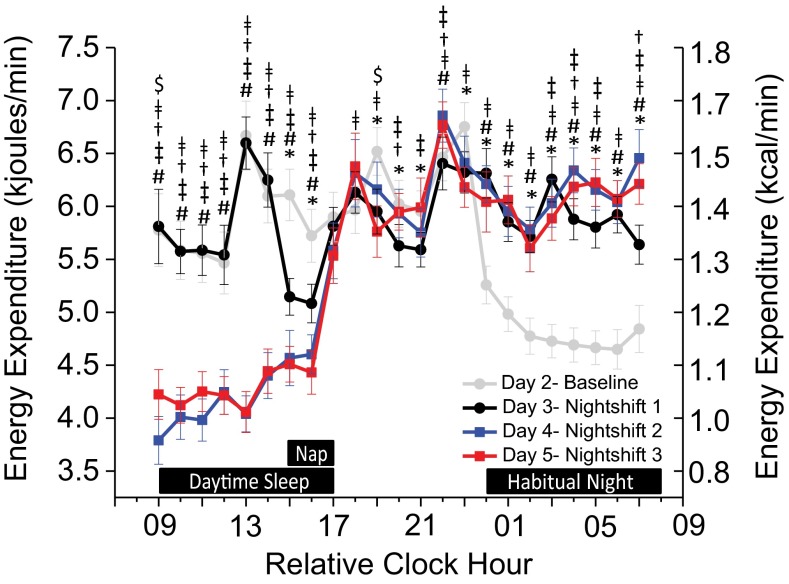
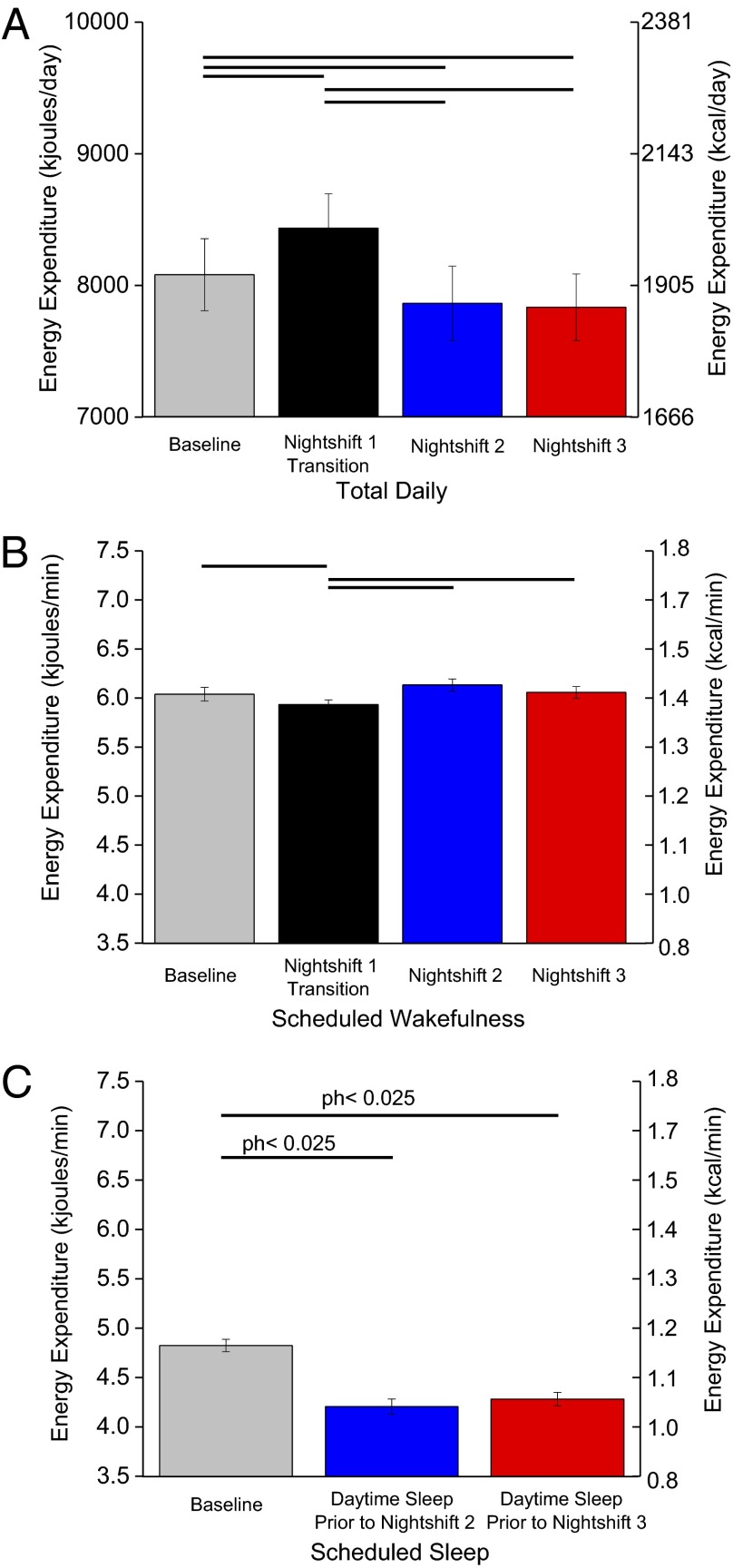
Regardless of when sleep occurred, average hourly EE was lower during scheduled sleep than wakefulness (Fig. 2). EE during the 2-h daytime nap (5.1 ± 0.7 kJ/min; 1.2 ± 0.02 kcal/min ± SEM) was reduced to similar levels seen in the first 2 h of the baseline nighttime sleep opportunity (Fig. 2). Compared with baseline (day 2), total daily EE was ∼353 ± 41.8 kJ (∼84 ±10 kcal) higher on nightshift 1 (day 3), which included the 2-h nap and extended wakefulness, and was ∼217 ± 69.9 kJ (∼52 ± 16.7 kcal) and ∼247 ± 66.2 kJ (∼59 ± 15.8 kcal) lower on nightshifts 2 and 3 (days 4 and 5), respectively (P < 0.025; Fig. 3A). Average per minute EE during scheduled wakefulness was ∼2.5% less on nightshift 1 (day 3) versus other days (P < 0.025; Fig. 3B) but was similar between baseline and nightshifts 2 and 3 (P > 0.33). Post hoc analyses showed that average per minute EE during scheduled daytime sleep was ∼12% less before nightshifts 2 and 3 versus baseline (Fig. 3C).
Fig. 2.
Hourly energy expenditure across the simulated shiftwork protocol (n = 14; P values, planned comparisons for day, two-tailed). The gray line represents day 2 (baseline), the black line is day 3 (nightshift 1), the blue line is day 4 (nightshift 2), and the red line is day 5 (nightshift 3). Black boxes along the x axis indicate scheduled sleep opportunities (day 2 nighttime, day 3 nap, days 4–5 daytime). *Differences between baseline and nightshift 1; #differences between baseline and nightshift 2; ǂdifferences between baseline and nightshift 3; †differences between nightshifts 1 and 2; ‡differences between nightshifts 1 and 3; $differences between nightshifts 2 and 3; P < 0.05. Error bars are SEM.
Fig. 3.
Total daily (24-h) (A) and scheduled wakefulness (B) and sleep (C) energy expenditure (n = 14; P values, planned comparisons for day, two-tailed). Solid lines represent significant differences at the end of each line (modified Bonferroni, P < 0.025). Exploratory post hoc (ph) two-tailed P values are shown for scheduled sleep EE, as findings were in the opposite direction of that hypothesized. Error bars are SEM.
Subjects were given meals designed to meet energy balance needs at baseline (7,643 ± 1,083 kJ/d; 1,827 ± 259 kcal/d). Table 1 shows that total daily fat utilization was ∼8% higher on nightshift 1 and ∼18% higher on nightshift 2 versus baseline (post hoc), resulting in negative fat balance for all subjects on the first two shiftwork days. Furthermore, total daily carbohydrate utilization was ∼20% lower on nightshift 2 versus baseline, resulting in greater positive carbohydrate balance. Also, protein utilization was ∼10% lower on nightshift 2 versus baseline. Macronutrient utilization on nightshift 3 was similar to baseline (Table 1). Postdinner TEF was lower on nightshift 1 (0.7 ± 1.3%; P < 0.025) versus baseline (4.8 ± 1.0%) and nightshift 3 (5.0 ± 0.9%). TEF postdinner on nightshift 2 was intermediate (2.8 ± 0.7%) and not significantly different from other days.
Table 1.
Total daily, macronutrient utilization and balance for baseline and nightshift days
| Measure, g/d | Day 2: baseline (n = 14) | Day 3: nightshift 1 (n = 14) | Day 4: nightshift 2 (n = 14) | Day 5: nightshift 3 (n = 13) |
| Macronutrient utilization | ||||
| Carbohydrate | 210.0 (8.1) | 212.1 (9.6) | 168.2 (6.3)*,† | 200.1 (9.5)‡ |
| Fat | 84.1 (6.6) | 91.0 (6.9)§ | 99.3 (6.9)¶ | 81.3 (6.8) |
| Protein | 58.8 (3.0) | 61.2 (4.4) | 53.0 (3.3)*,† | 59.8 (3.6)‡ |
| Macronutrient balance | ||||
| Carbohydrate | 26.5 (10.9) | 23.5 (9.7) | 67.3 (9.8)*,† | 29.9 (11.1)‡ |
| Fat | −21.6 (5.4) | −28.5 (5.1)§ | −37.5 (5.5)† | −20.6 (6.0)‡ |
| Protein | 11.5 (2.8) | 9.0 (4.9) | 16.5 (3.6)† | 8.6 (2.0)‡ |
Values in parentheses are SEM.
Differences between baseline and nightshift 2.
Differences between nightshift 1 and nightshift 2.
Differences between nightshift 2 and nightshift 3.
Differences between baseline and nightshift 1.
Exploratory post hoc differences for fat utilization (day 4 vs. day 2, P = 0.001; day 3, P = 0.01; day 5, P = 0.000005).
Sleep–Wakefulness Stage and Energy Expenditure.
EE did not significantly differ between stages of consolidated sleep within sleep opportunities (all P > 0.10; Fig. S2 and Table S2). During the baseline sleep opportunity, EE was higher during wakefulness prior to sleep onset (WPSO; from lights out to LPS) and WASO than during stage 2 and REM sleep. Further, EE was higher during WPSO than stage 1 and slow-wave sleep (SWS) (P < 0.05; Table S2). During daytime sleep opportunities, however, EE during WPSO was lower than that during consolidated sleep stages, whereas EE during WASO was higher than most consolidated sleep stages and WPSO (P < 0.05; Table S2).
EE was lower during WPSO, WASO, stage 2, and SWS for the daytime versus baseline nighttime sleep opportunity (P < 0.05; Fig. S2). Further, EE was lower during stage 1 sleep for daytime sleep before nightshift 2 and during REM sleep for daytime sleep before nightshift 3, both versus baseline. When examining differences in EE between baseline and daytime sleep opportunities averaging across all scored sleep epochs, subjects expended ∼16% and ∼12% less energy during sleep before nightshifts 2 (4.0 ± 0.02 kJ/min; ∼0.96 ± 0.005 kcal/min) and 3 (4.2 ± 0.02 kJ/min; ∼1 ± 0.005 kcal/min) versus baseline (4.8 ± 0.01 kJ/min; ∼1.14 ± 0.002 kcal/min P < 0.001), respectively.
Appetitive Hormones and Hunger Ratings.
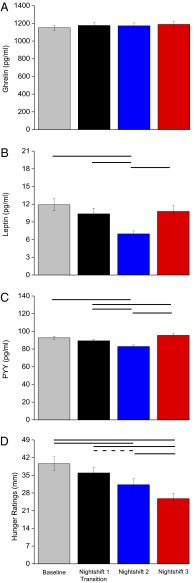
Average 24-h ghrelin levels were similar across days (Fig. 4A), whereas average 24-h leptin levels decreased by ∼41% on nightshift 2 versus baseline and by ∼33% and ∼35% versus nightshifts 1 and 3, respectively (Fig. 4B). Similarly, average 24-h PYY levels decreased by ∼11% on nightshift 2 versus baseline and by ∼7% and ∼13% versus nightshifts 1 and 3, respectively (Fig. 4C). In addition, average 24-h PYY levels increased by ∼6% on nightshift 3 versus nightshift 1. Fig. S3 shows that daily patterns of ghrelin, leptin, and PYY predominantly follow the sleep–wakefulness fasting–feeding cycle. Hunger ratings decreased across days and were ∼21% and 35% lower on nightshifts 2 and 3, respectively, versus baseline and were ∼28% and 18% lower on nightshift 3 versus nightshifts 1 and 2, respectively (Fig. 4D). A nonsignificant trend occurred for lower hunger ratings on nightshift 2 versus nightshift 1 (Fig. 4D).
Fig. 4.
Appetitive hormones (A–C) [n = 14; P values, planned comparisons for day (24 h), one-tailed] and hunger ratings (D) (n = 14; P values, planned comparisons for day, two-tailed). Solid lines represent significant differences at the end of each line (modified Bonferroni, P < 0.025), and the dashed line represents a nonsignificant trend (P = 0.03). Error bars are SEM.
Discussion
Findings from nonhuman animal models indicate that food intake during typical sleep time leads to metabolic dysregulation. We show that total daily EE was significantly increased on the first nightshift day due to extended wakefulness and was decreased on the second and third nightshift days compared with baseline. Additionally, decreases in total daily EE during the nightshift schedule were driven predominantly by decreases in sleeping EE and the EE of wakefulness during scheduled sleep opportunities. We also show that the TEF in response to dinner decreased on the first nightshift compared with baseline. Unexpectedly, fat utilization increased on the first and second nightshift days. Further, carbohydrate and protein utilization decreased on the second nightshift day. Last, subjects exhibited decreased hunger ratings despite similar levels of the hunger-stimulating hormone ghrelin and decreased levels of the satiety hormones leptin and PYY during the nightshift schedule. We studied lean, healthy subjects and strictly controlled for food intake, activity, posture, and light exposure so that we could examine the influence of circadian misalignment per se on energy metabolism. Our control over food intake and activity does not permit us to make strong comments about how total daily EE, energy balance, and hunger may change during uncontrolled conditions, something that should be examined in future studies. Also, we did not measure for possible changes in body composition nor did we have a control group without circadian misalignment; however, longer time in the laboratory with insufficient sleep induces consistent increases in 24-h EE (20). Future studies are needed to determine whether other subject populations and behavioral/environmental factors, including other models of circadian misalignment (e.g., rotating shift work, additional night shifts, circadian sleep–wake disorders), influence metabolic physiology. Some findings that were in opposite directions than expected require replication.
Total Daily Energy Expenditure.
Using a common nightshift schedule simulation, we found that acute circadian misalignment increased total daily EE on the transition day to nightshift 1 and decreased total daily EE on nightshifts 2 and 3. Increased total daily EE on the transition day was expected due to increased time awake and is consistent with prior findings from our laboratory that sleep deprivation increases energy expenditure (19, 20). Decreased EE per minute during scheduled wakefulness on nightshift 1, however, indicates that the duration of wakefulness, and not an increase in the metabolic cost of such wakefulness, is responsible for increased total daily EE. Our findings of decreased total daily EE during subsequent nightshift days may represent a contributing mechanism for unwanted weight gain observed in shiftworkers. Because shiftworkers report eating similar total 24-h calories as daytime workers (9, 11), the ∼217 to ∼247 kJ/d (52–59 kcal/d) reduction in total daily EE we observed could lead to positive energy balance and weight gain over time (21).
TEF accounts for ∼10% of total daily EE and reflects the energy needed for metabolism of consumed calories. A reduced TEF could contribute to reductions in 24-h EE and, if food intake remained stable, weight gain could ensue. We found that the TEF after a late dinner on nightshift 1 was lower compared with food consumed at a typical dinnertime. This decreased TEF may represent a contributing mechanism underlying the reported risk of weight gain associated with evening food intake. For example, food consumed past 2000 hours predicts higher body mass index (BMI) when controlling for sleep duration and timing (22), and night eating syndrome, a disorder in which individuals consume ≥25% of calories after their evening meal (23), is associated with a higher BMI (24). Reduced TEF after the late dinner is consistent with prior findings of an acute decrease in TEF after a meal consumed at 0100 hours compared with 0900 hours or 1700 hours (25). Our finding that TEF in response to dinner was intermediate on nightshift 2 and returned to levels similar to baseline on nightshift 3 was unexpected, but may suggest physiological adaptation in TEF during the acute challenge posed by such nightshift schedules.
Macronutrient Utilization and Balance.
Higher fat utilization (energy used from fat) on nightshifts 1 and 2 was not expected. This finding and the negative fat balance (fat loss), lower carbohydrate and protein utilization (energy used from carbohydrates and protein), and higher positive carbohydrate balance (carbohydrate storage) may represent acute physiological responses to higher EE during sleep deprivation, especially as the food intake provided to subjects was the amount predicted to maintain energy balance at baseline and thus subjects were in negative energy balance (weight loss) during sleep deprivation.
Sleep Staging and Energy Expenditure.
Consistent with prior findings, we observed that sleep during the daytime was disturbed (26, 27). Shorter sleep latencies during the nightshift schedule were expected due to increased sleep drive and the circadian promotion of sleep (28, 29). Reductions in total sleep time and stage 2 sleep and increases in duration of arousals were also expected due to the circadian promotion of arousal during the latter portion of the daytime sleep opportunity (28). The shorter REML during daytime sleep is consistent with the circadian rhythm in REM propensity, as these sleep opportunities were scheduled near the peak circadian time for REM (28).
Contrary to our hypothesis, we found decreased EE during scheduled daytime sleep opportunities. However, our findings of higher EE during awakenings from sleep compared with consolidated sleep and of similar EE among consolidated sleep stages within the sleep opportunity are consistent with prior findings (19, 30). The finding of lower WPSO EE than consolidated sleep stages for daytime sleep opportunities is contrary to the EE observed at baseline during the transition from wakefulness to sleep and is inconsistent with findings from prior research for nighttime sleep opportunities (19). Decreased EE during sleep and wakefulness of the daytime sleep opportunities may function to conserve energy in response to the increased metabolic cost of sleep deprivation on nightshift 1, as similar findings of reduced total daily EE are observed during recovery sleep after total sleep deprivation (19). Possible contributing mechanisms for decreased EE at the beginning of the daytime sleep opportunity include reduced resting metabolic rate (31) and sleep-induced decreases in the metabolic hormones cortisol, norepinephrine, and epinephrine that typically increase EE (32, 33) and are highest at this circadian time (5). During sleep, circulating cortisol, norepinephrine, and epinephrine levels are reduced and morning sleep after the nightshift could decrease these hormones (34, 35), leading to decreased EE. Our finding of shorter REM latencies during daytime sleep may be associated with reduced catecholamine levels, thereby potentially decreasing EE (34). These possible mechanisms, however, cannot explain why EE is lower before daytime sleep. We did not measure cortisol and catecholamines, and further studies are needed to study these and other potential mechanisms that decrease EE when sleep occurs at inappropriate circadian times.
Satiety and Appetitive Hormones.
Appetitive hormones are altered during circadian misalignment (reduced leptin) (5, 18) and insufficient sleep (reduced leptin and increased ghrelin) (2, 36) when subjects are given meals designed to meet energy balance at baseline. Appetite increases have been reported in response to insufficient sleep (2, 36). Our findings of decreased leptin and PYY levels, with no change in ghrelin, would be hypothesized to increase hunger ratings as seen with insufficient sleep (2, 36), yet we observed decreased hunger ratings during the nightshift schedule. The decrease in leptin levels may be associated with the negative fat balance observed. Also, percent change in leptin was larger than the change reported after 3 d of 30% food restriction in healthy subjects (37), suggesting that the leptin change we observed is physiologically meaningful. Our findings suggest a disassociation between the peripheral appetitive hormones examined and subjective hunger during acute circadian misalignment. Why hunger was not increased may be related to circadian mechanisms promoting satiety at night (38), lack of a change in ghrelin, or changes in other peripheral satiety hormones not measured such as cholecystokinin and insulin. Our findings also provide further evidence that leptin and ghrelin are primarily modulated by wakefulness–sleep, feeding–fasting cycles compared with circadian phase (5, 18). Further, our findings demonstrate that the daily pattern in PYY levels is also primarily wakefulness–sleep– versus circadian-driven.
How Circadian Misalignment May Contribute to Weight Gain.
Knowledge about potential mechanisms for increased risk of weight gain and obesity in nightshift workers is important for developing evidence-based treatment strategies. Self-reported total daily food intake is similar in day and shiftworkers (9). Thus, other mechanisms may be needed to help explain weight gain in shiftworkers. Our findings suggest that circadian misalignment during nightshift schedules may disturb metabolic physiology and contribute to adverse metabolic health outcomes by reducing total daily EE. Specifically, the ∼217 to ∼247 kJ (52–59 kcal) lower total daily EE on nightshifts 2 and 3, if recurrent without a reduction in food intake, would contribute to weight gain, because as little as ∼209 kJ (∼50 kcal) excess calorie storage per day can increase weight over time (21). Further, if increased exhaustion and fatigue levels (27) associated with shiftwork results in reduced physical activity levels, this would promote positive energy balance and weight gain. The decrease in TEF observed after a late meal applies to shiftworkers and also to day workers who eat later at night and have a higher risk of obesity (22). Further research is needed to extend current findings to include all components of energy balance, in particular the effects of dietary choices and timing of meals on risk of obesity in shiftworkers.
Methods
Subjects.
Fourteen healthy subjects (eight women; aged 26.4 ± 1.2 y, mean ± SD, with BMI 22.7 ± 0.5 kg/m2 and percent body fat 27.4 ± 2.2% as determined by dual-energy X-ray absorptiometry; Prodigy Advance; GE Lunar) completed the protocol. Data from two additional subjects who did not complete the protocol are not included, one due to claustrophobia in the whole-room calorimeter and the other to gastrointestinal discomfort that prevented meal consumption. Procedures were approved by the scientific and advisory review committee of the Colorado Clinical and Translational Sciences Institute, the Colorado Multiple Institutional Review Board (IRB), and the University of Colorado Boulder IRB. Subjects gave written informed consent and then underwent health screening at the Sleep and Chronobiology Laboratory and the University Colorado Boulder Clinical Translational Research Center (CTRC). Exclusion criteria consisted of diagnoses with any known medical, psychiatric, or sleep disorder, current smoker, pregnancy, or a habitual sleep duration of <7 h or >9.25 h. Subjects were deemed healthy based on clinical history, physical examination, psychological tests, a psychological interview with a trained clinician, blood chemistries (complete blood cell count and comprehensive metabolic panel), clinical electrocardiogram, and medication-free status. No subjects reported regular night work in the preceding year or crossing more than one time zone in the previous 3 wk. Subjects were minimally physically active before the CTRC procedures to minimize the detraining effects of the posture controlled study. Women were not scheduled to start the study according to menstrual phase.
Study Protocol.
For 1 wk before admission to the University of Colorado Hospital (UCH) CTRC, subjects maintained a self-selected sleep schedule of ∼8 h per night and caffeine, alcohol, nicotine, and over-the-counter medication use was proscribed. The sleep schedule was verified via continuous wrist actigraphy with light-exposure monitoring (Actiwatch-L; Mini-Mitter/Respironics), sleep–wakefulness logs, and call-in bed and waketimes to a time-stamped voice recorder. Drug use was determined by self-report and verified by urine toxicology at screening and by urine toxicology and a breath alcohol tester (Lifeloc Technologies; model FC10) upon CTRC admission. Three days before CTRC admission, exercise was proscribed and subjects were provided a 3-d outpatient diet designed to meet their individual daily caloric needs as determined from resting metabolic rate with a 1.5 activity factor (9). Subjects were instructed to consume meals provided and nothing else but water to ensure energy balance at the start of the CTRC protocol.
Subjects lived at the UCH CTRC for ∼6 d to simulate a daytime work followed by a 3-d nightshift schedule (Fig. 1). A polysomnography (PSG)-recorded 8-h nighttime sleep opportunity at habitual bedtime verified that subjects were free from sleep disorders. Following the sleep disorders screening night, subjects were awakened at habitual waketime and maintained a constant posture protocol with brief breaks: seated semirecumbent posture in a hospital bed with the head raised to ∼35°, room temperature maintained at 22–24 °C, and dim lighting (<1 lx in the angle of gaze, <5 lx max) during scheduled wakefulness and 0 lx during scheduled sleep. Day 2 served as a baseline day shift, with 16 h of daytime wakefulness and an 8-h nighttime sleep opportunity. This procedure permitted subjects to sleep at their habitual circadian phase at baseline. Day 3 served as the transition to working nightshift 1. Subjects were awakened at habitual waketime and scheduled to a 2-h afternoon nap opportunity before nightshift 1. After nightshift 1, subjects were provided an 8-h daytime sleep opportunity that began 1 h after their habitual baseline waketime. This was followed by nightshifts 2 and 3 (Fig. 1). After nightshift 3, subjects were provided a non–PSG-recorded 8-h sleep opportunity and discharged from the CTRC.
Subjects received scheduled meals (percent daily caloric intake: 30% breakfast, 30% lunch, 30% dinner, 10% snack) at ∼1.5, 5.5, 10.5, and 14.5 h postawakening on day 2 (relative clock hour 0930, 1330, 1830, and 2230 hours for a subject with scheduled waketime arbitrarily assigned a value of 0800 hours and all other times referenced to this value; actual clock hour was determined by the participant’s habitual wakefulness–sleep schedule assessed during ambulatory baseline) and on days 4 and 5 (relative clock hour 1730, 2130, 0330, and 0730 hours). Meals were similar across days (e.g., the food served for breakfast was the same each day) and subjects were instructed to consume all food provided. Because day 3 simulated the transition day to nightshift 1 with a 2-h afternoon nap opportunity, the timing and percent of daily caloric intake (30% breakfast, 25% lunch, 10% snack, 25% dinner, 10% snack) were spread across the day; these meals were given at 1.5, 5.5, 10.5, 15.5, and 20.5 hours (relative clock hour 0930, 1330, 1830, 2230, and 2330 hours) after scheduled awakening. Dinner was a cold meal kept in a refrigerator next to the bed so subjects did not change posture, permitting TEF analysis.
Measures.
EE and macronutrient utilization were determined via whole-room indirect calorimetry during days 2–5 (20, 39). Subjects’ carbon dioxide (CO2) production and oxygen (O2) consumption were assessed to calculate EE and respiratory quotient (RQ) by measuring gas concentration differences entering and exiting the calorimeter with fuel cell-based dual-channel O2 analyzers (FC-2 Oxzilla; Sable Systems International) and two infrared CO2 analyzers (CA-10 CO2 analyzers; Sable Systems International) (39). Monthly propane combustion tests validated the precision and accuracy of the gas concentration measurements with average recoveries of ∼99%. Nonprotein carbohydrate and fat utilization were determined from O2 consumption and RQ (40), and protein utilization was calculated using 24-h urine total nitrogen (41).
Leptin, ghrelin, and PYY levels were assessed from blood samples drawn every 2 h on days 2–5. Melatonin was assessed every 2 h during the daytime and hourly at night starting at 13 h after habitual waketime (relative clock hour 1900 hours) as a marker of internal circadian time. Blood was drawn using a 12-foot extension tubing connected to an indwelling venous catheter and kept patent with a heparinized saline drip (20). Extension tubing through a porthole in the calorimeter allowed for blood sampling without entering the room or disrupting scheduled sleep. Hunger ratings were determined via visual analog scales collected every 2 h starting at 2 h postwaketime and ending 2 h before sleep.
Sleep and wakefulness recordings, including sleep disorders screen night 1, were obtained using Siesta digital recorders (Compumedics). Sleep latencies were defined as follows: SOL, time from lights out to the onset of three continuous epochs of PSG-defined sleep; LPS, time from lights out to the onset of 10 continuous minutes of PSG-defined sleep; latency to SWS (SWSL), time from LPS to SWS; and REML, time from LPS to REM sleep.
Data Analysis.
EE and macronutrient utilization were averaged hourly during scheduled wakefulness and sleep and averaged for the 24-h day. Total daily carbohydrate balance was calculated accounting for fiber intake. EE was also calculated for consolidated sleep and wakefulness stages during scheduled sleep opportunities as in ref. 19 and for all scored sleep epochs. Specifically, sleep and wakefulness stages (WPSO, WASO, stage 1 sleep, stage 2 sleep, SWS, and REM) were matched with EE values that were offset by 2 min to account for the lag-time response of the whole-room calorimeter relative to the timing of the sleep recording (19, 42). Also see Table S2. Blood data were aligned and plotted beginning from scheduled wakefulness. TEF was measured as a deviation from the EE 1 h before dinner (premeal baseline, subjects seated for ∼4 h before baseline measurement) for the 3.5-h postdinner consumption and analyzed as the percentage of the caloric content of the dinner. Appetitive hormones were analyzed for 24-h and 2-h averages. Protein utilization was unavailable for one subject on nightshift 3 and thus excluded from the macronutrient utilization analysis for that day. Three subjects on nightshift 2 and two subjects on nightshift 3 did not have documented completion times of dinner and were therefore excluded from the TEF analysis for those days. The sleep recording for one subject was missing for the sleep opportunity before nightshift 2 and therefore was excluded from the sleep analyses for that day.
Data were analyzed via mixed-model ANOVAs with day, relative clock time, and/or sleep stage as fixed factors and subject as a random factor using STATISTICA version 10.0 (StatSoft). One-tailed a priori directional planned comparison dependent t tests, with modified Bonferroni correction for multiple planned comparisons to reduce type 1 error (43), examined the hypothesized reduction in fat utilization, increase in sleep opportunity EE, and changes in 24-h appetitive hormones. Two-tailed planned comparisons for other measures were also computed. Exploratory post hoc analyses were performed for fat utilization and sleep opportunity EE after the failure to confirm hypotheses. Data in the text are presented as mean ± SEM.
Supplementary Material
Acknowledgments
We thank the participants, the University Colorado Boulder Clinical Translational Research Center staff, and B. Birks, B. Smith, B. Brainard, B. Griffin, T. Dear, and G. Wright, for study assistance. This research was supported by NIH R21 DK092624, NIH 1UL1 RR025780, P30 DK048520, and the Undergraduate Research Opportunities Program in collaboration with the Howard Hughes Medical Institute and Biological Sciences Initiative.
Footnotes
Conflict of interest statement: There are no conflicts of interest directly related to this project. E.L.M. and K.P.W. have current funding through a Philips research grant.
This article is a PNAS Direct Submission.
1Division of Sleep and Circadian Disorders, Department of Medicine, Brigham and Women's Hospital and Division of Sleep Medicine, Harvard Medical School, Boston, MA 02115.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412021111/-/DCSupplemental.
References
- 1.Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: Key components in the regulation of energy metabolism. FEBS Lett. 2008;582(1):142–151. doi: 10.1016/j.febslet.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 2.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA. 2011;108(4):1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright KP, Jr, et al. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23(16):1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czeisler CA. Perspective: Casting light on sleep deficiency. Nature. 2013;497(7450):S13. doi: 10.1038/497S13a. [DOI] [PubMed] [Google Scholar]
- 8.McMenamin TM. Time to work: Recent trends in shift work and flexible schedules. Mon Labor Rev. 2007;130(12):9–11. [Google Scholar]
- 9.Markwald RR, Wright KP., Jr . Circadian misalignment and sleep disruption in shift work: Implications for fatigue and risk of weight gain and obesity. In: Shiromani P, Horvath T, Redline S, Van Cauter E, editors. Sleep Loss and Obesity: Intersecting Epidemics. Springer; New York: 2012. pp. 101–118. [Google Scholar]
- 10.Holmbäck U, et al. Metabolic responses to nocturnal eating in men are affected by sources of dietary energy. J Nutr. 2002;132(7):1892–1899. doi: 10.1093/jn/132.7.1892. [DOI] [PubMed] [Google Scholar]
- 11.Lennernäs M, Åkerstedt T, Hambraeus L. Nocturnal eating and serum cholesterol of three-shift workers. Scand J Work Environ Health. 1994;20(6):401–406. doi: 10.5271/sjweh.1381. [DOI] [PubMed] [Google Scholar]
- 12.Di Lorenzo L, et al. Effect of shift work on body mass index: Results of a study performed in 319 glucose-tolerant men working in a southern Italian industry. Int J Obes Relat Metab Disord. 2003;27(11):1353–1358. doi: 10.1038/sj.ijo.0802419. [DOI] [PubMed] [Google Scholar]
- 13.Canuto R, Garcez AS, Olinto MT. Metabolic syndrome and shift work: A systematic review. Sleep Med Rev. 2013;17(6):425–431. doi: 10.1016/j.smrv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y, et al. The effects of shift work on sleeping quality, hypertension and diabetes in retired workers. PLoS ONE. 2013;8(8):e71107. doi: 10.1371/journal.pone.0071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17(11):2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonken LK, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107(43):18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurni M, Burnand B, Pittet P, Jequier E. Metabolic effects of a mixed and a high-carbohydrate low-fat diet in man, measured over 24 h in a respiration chamber. Br J Nutr. 1982;47(1):33–43. doi: 10.1079/bjn19820006. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen J, Wright KP., Jr Influence of weeks of circadian misalignment on leptin levels. Nat Sci Sleep. 2009;2010(2):9–18. doi: 10.2147/NSS.S7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung CM, et al. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589(Pt 1):235–244. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markwald RR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA. 2013;110(14):5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: Where do we go from here? Science. 2003;299(5608):853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 22.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19(7):1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 23.Allison KC, et al. Proposed diagnostic criteria for night eating syndrome. Int J Eat Disord. 2010;43(3):241–247. doi: 10.1002/eat.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colles SL, Dixon JB, O’Brien PE. Night eating syndrome and nocturnal snacking: Association with obesity, binge eating and psychological distress. Int J Obes (Lond) 2007;31(11):1722–1730. doi: 10.1038/sj.ijo.0803664. [DOI] [PubMed] [Google Scholar]
- 25.Romon M, Edme JL, Boulenguez C, Lescroart JL, Frimat P. Circadian variation of diet-induced thermogenesis. Am J Clin Nutr. 1993;57(4):476–480. doi: 10.1093/ajcn/57.4.476. [DOI] [PubMed] [Google Scholar]
- 26.McHill AW, Smith BJ, Wright KP., Jr Effects of caffeine on skin and core temperatures, alertness, and recovery sleep during circadian misalignment. J Biol Rhythms. 2014;29(2):131–143. doi: 10.1177/0748730414523078. [DOI] [PubMed] [Google Scholar]
- 27.Åkerstedt T, Wright KP., Jr Sleep loss and fatigue in shift work and shift work disorder. Sleep Med Clin. 2009;4(2):257–271. doi: 10.1016/j.jsmc.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15(5 Pt 1):3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14(6):557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 30.Gonnissen HK, Drummen M, Rosique Esteban N, Schoffelen PF, Westerterp-Plantenga MS. Overnight energy expenditure determined by whole-body indirect calorimetry does not differ during different sleep stages. Am J Clin Nutr. 2013;98(4):867–871. doi: 10.3945/ajcn.113.067884. [DOI] [PubMed] [Google Scholar]
- 31.Buxton OM, et al. Metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brillon DJ, Zheng B, Campbell RG, Matthews DE. Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am J Physiol. 1995;268(3 Pt 1):E501–E513. doi: 10.1152/ajpendo.1995.268.3.E501. [DOI] [PubMed] [Google Scholar]
- 33.Blaak EE, van Baak MA, Kempen KP, Saris WH. Role of alpha- and beta-adrenoceptors in sympathetically mediated thermogenesis. Am J Physiol. 1993;264(1 Pt 1):E11–E17. doi: 10.1152/ajpendo.1993.264.1.E11. [DOI] [PubMed] [Google Scholar]
- 34.Dodt C, Breckling U, Derad I, Fehm HL, Born J. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. 1997;30(1 Pt 1):71–76. doi: 10.1161/01.hyp.30.1.71. [DOI] [PubMed] [Google Scholar]
- 35.Rehman JU, Brismar K, Holmbäck U, Åkerstedt T, Axelsson J. Sleeping during the day: Effects on the 24-h patterns of IGF-binding protein 1, insulin, glucose, cortisol, and growth hormone. Eur J Endocrinol. 2010;163(3):383–390. doi: 10.1530/EJE-10-0297. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 37.Chin-Chance C, Polonsky KS, Schoeller DA. Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab. 2000;85(8):2685–2691. doi: 10.1210/jcem.85.8.6755. [DOI] [PubMed] [Google Scholar]
- 38.Scheer FA, Morris CJ, Shea SA. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring) 2013;21(3):421–423. doi: 10.1002/oby.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melanson EL, et al. A new approach for flow-through respirometry measurements in humans. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1571–R1579. doi: 10.1152/ajpregu.00055.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jéquier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr. 1987;7(1):187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 41.Skogerboe KJ, Labbé RF, Rettmer RL, Sundquist JP, Gargett AM. Chemiluminescent measurement of total urinary nitrogen for accurate calculation of nitrogen balance. Clin Chem. 1990;36(5):752–755. [PubMed] [Google Scholar]
- 42.Fontvieille AM, Rising R, Spraul M, Larson DE, Ravussin E. Relationship between sleep stages and metabolic rate in humans. Am J Physiol. 1994;267(5 Pt 1):E732–E737. doi: 10.1152/ajpendo.1994.267.5.E732. [DOI] [PubMed] [Google Scholar]
- 43.Keppel G. Design and Analysis: A Researcher’s Handbook. Prentice Hall; Englewood Cliffs, NJ: 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.