Abstract
In exploring the role of the chloroplast in the multiplication of turnip yellow mosaic virus, the biosyntheses of the major viral polyamine, spermidine, as well as that of the tetramine, spermine were studied. The synthesis of these polyamines from [2-14C]methionine in protoplasts of Chinese cabbage leaf cells derived from healthy plants or those infected by turnip yellow mosaic virus were examined. Populations of protoplasts of infected leaves are homogeneous with respect to containing chloroplast aggregates in contrast to those of healthy leaves. Protoplast preparations have been shown to incorporate methionine into protein, spermidine, and spermine more rapidly than do fresh leaf discs, which also show a very slow utilization of labeled arginine and ornithine into polyamine.
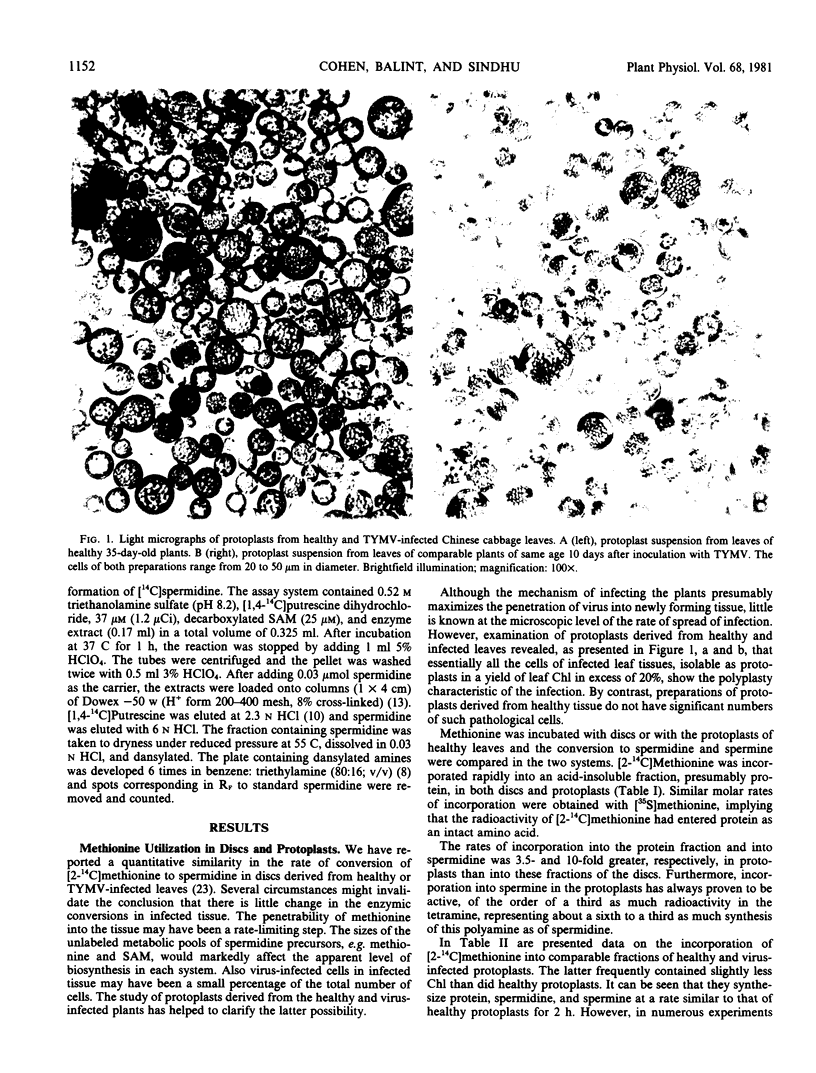
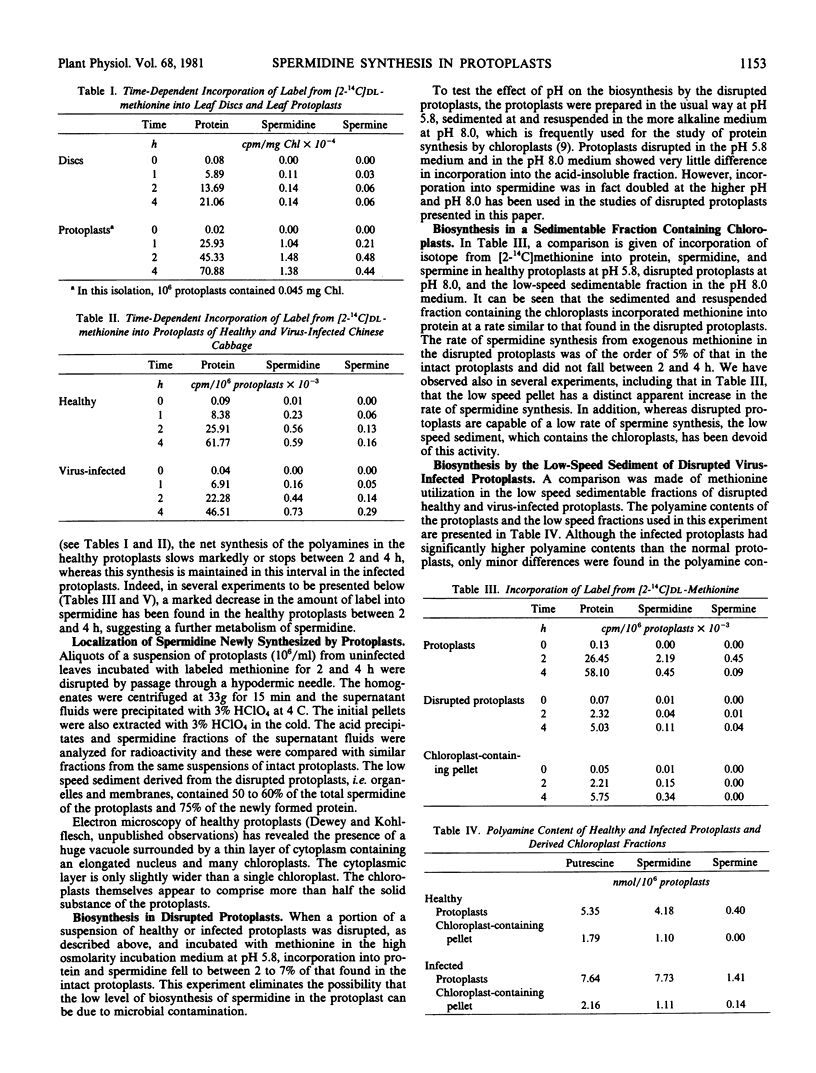
Protein synthesis is similar for 4 hours in both healthy and infected protoplasts. Accumulation of labeled spermidine stops after 2 hours in healthy protoplasts but continues in the infected protoplasts. Much of the newly synthesized protein and spermidine is present in the easily sedimentable fraction of the readily disrupted protoplasts.
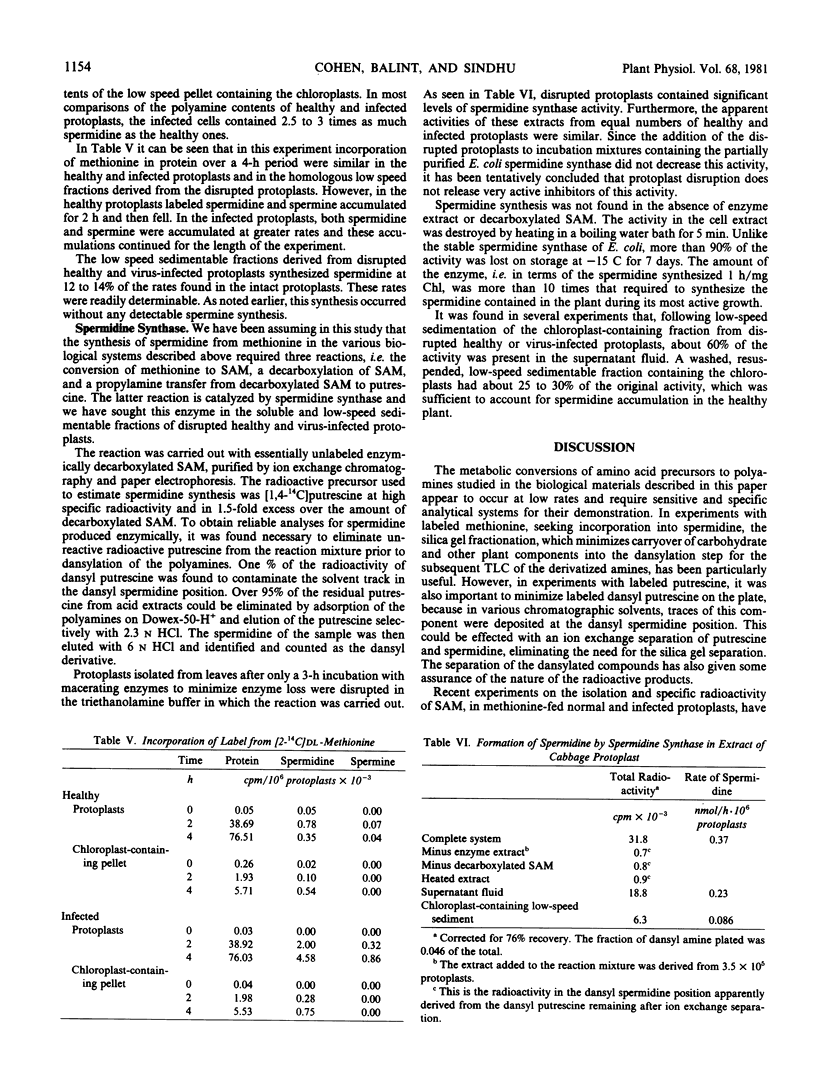
Disrupted and diluted protoplasts have a decreased ability to metabolize methionine to protein and spermidine. The residual synthetic activity is essentially entirely in the easily sedimentable fraction. However, this fraction is unable to synthesize spermine, an activity found in protoplasts and disrupted protoplasts. Disrupted protoplasts contain spermidine synthase (EC 2.5.1.16) and about a quarter of this activity is present in a low-speed sedimentable fraction containing the chloroplasts. The protoplast system is suitable for an analysis of polyamine synthesis in turnip yellow mosaic virus infection and appears particularly suitable for study of the distribution of the enzymes involved.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N., Anderson J. D., Lieberman M. Production and action of ethylene in senescing leaf discs: effect of indoleacetic Acid, kinetin, silver ion, and carbon dioxide. Plant Physiol. 1979 Nov;64(5):805–809. doi: 10.1104/pp.64.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer S. V., Kosuge T. Spermidine and spermine--polyamine components of turnip yellow mosaic virus. Virology. 1970 Apr;40(4):930–938. doi: 10.1016/0042-6822(70)90139-x. [DOI] [PubMed] [Google Scholar]
- Cohen A. S., Popovic R. B., Zalik S. Effects of Polyamines on Chlorophyll and Protein Content, Photochemical Activity, and Chloroplast Ultrastructure of Barley Leaf Discs during Senescence. Plant Physiol. 1979 Nov;64(5):717–720. doi: 10.1104/pp.64.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Morgan S., Streibel E. The polyamine content of the tRNA of E. coli. Proc Natl Acad Sci U S A. 1969 Oct;64(2):669–676. doi: 10.1073/pnas.64.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamines in the synthesis of bacteriophage deoxyribonucleic acid. II. Requirement for polyamines in T4 infection of a polyamine auxotroph. J Virol. 1972 Mar;9(3):423–430. doi: 10.1128/jvi.9.3.423-430.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty A., Gray J. C. Synthesis of cytochrome f by isolated pea chloroplasts. Eur J Biochem. 1979 Jul;98(1):87–92. doi: 10.1111/j.1432-1033.1979.tb13164.x. [DOI] [PubMed] [Google Scholar]
- Gahl W. A., Vale A. M., Pitot H. C. Separation of putrescine oxidase and spermidine oxidase in foetal bovine serum with the aid of a specific radioactive assay of spermidine oxidase. Biochem J. 1980 Apr 1;187(1):197–204. doi: 10.1042/bj1870197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta T., Matthews R. E. The sequence of early cytological changes in Chinese cabbage leaf cells following systemic infection with turnip yellow mosaic virus. Virology. 1974 Jun;59(2):383–396. doi: 10.1016/0042-6822(74)90452-8. [DOI] [PubMed] [Google Scholar]
- Inoue H., Mizutani A. A new method for isolation of polyamines from animal tissues. Anal Biochem. 1973 Dec;56(2):408–416. doi: 10.1016/0003-2697(73)90206-6. [DOI] [PubMed] [Google Scholar]
- JOHNSON M. W., MARKHAM R. Nature of the polyamine in plant viruses. Virology. 1962 Jun;17:276–281. doi: 10.1016/0042-6822(62)90117-4. [DOI] [PubMed] [Google Scholar]
- Mouches C., Bove C., Bove J. M. Turnip yellow mosaic virus-RNA replicase: partial purification of the enzyme from the solubilized enzyme-template complex. Virology. 1974 Apr;58(2):409–423. doi: 10.1016/0042-6822(74)90076-2. [DOI] [PubMed] [Google Scholar]
- Takebe I., Otsuki Y. Infection of tobacco mesophyll protoplasts by tobacco mosaic virus. Proc Natl Acad Sci U S A. 1969 Nov;64(3):843–848. doi: 10.1073/pnas.64.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torget R., Lapi L., Cohen S. S. Synthesis and accumulation of polyamines and S-adenosylmethionine in Chinese cabbage infected by turnip yellow mosaic virus. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1132–1139. doi: 10.1016/s0006-291x(79)80025-x. [DOI] [PubMed] [Google Scholar]



