Abstract
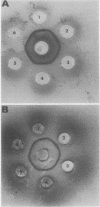
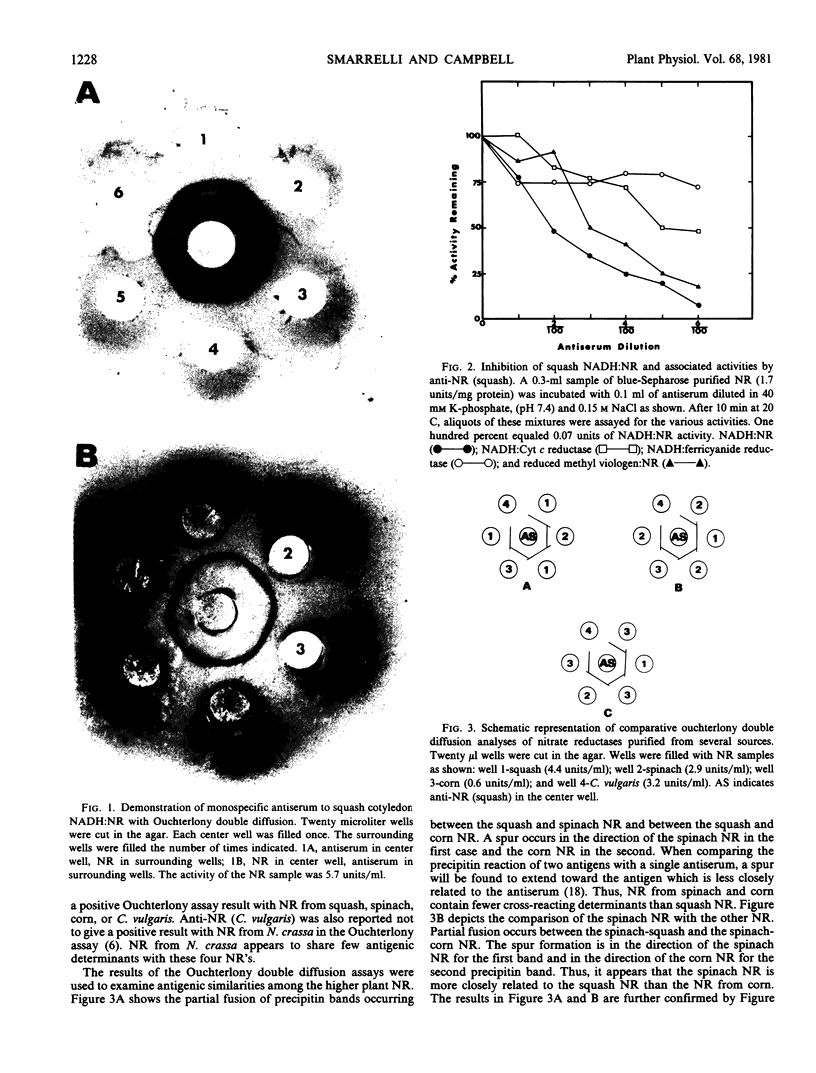
Homogeneous squash cotyledon reduced nicotinamide-adenine dinucleotide (NADH):nitrate reductase (NR) was isolated using blue-Sepharose and polyacrylamide gel electrophoresis. Gel slices containing NR were pulverized and injected into a previously unimmunized rabbit. This process was repeated weekly and antiserum to NR was obtained after four weeks. Analysis of the antiserum by Ouchterlony double diffusion using a blue-Sepharose preparation of NR resulted in a single precipitin band while immunoelectrophoresis revealed two minor contaminants. The antiserum was found to inhibit the NR reaction and the partial reactions to different degrees. When the NADH:NR and the reduced methyl viologen:NR activities were inhibited 90% by specifically diluted antiserum, the reduction of cytochrome c was inhibited 50%, and the reduction of ferricyanide was inhibited only 30%. Antiserum was also used to compare the cross reactivities of NR from squash cotyledons, spinach, corn, and soybean leaves, Chlorella vulgaris, and Neurospora crassa. These tests revealed a high degree of similarity between NADH:NR from the squash and spinach, while NADH:NR from corn and soybean and the NAD(P)H:NR from soybean were less closely related to the squash NADH:NR. The green algal (C. vulgaris) NADH:NR and the fungal (N. crassa) NADPH:NR were very low in cross reactivity and are apparently quite different from squash NADH:NR in antigenicity. Antiserum to N. crassa NADPH:NR failed to give a positive Ouchterlony result with higher plant or C. vulgaris NADH:NR, but this antiserum did inhibit the activity of squash NR. Thus, it can be concluded from these immunological comparisons that all seven forms of assimilatory NR studied here have antigenic determinants in common and are probably derived from a common ancestor. Although these assimilatory NR have similar catalytic characteristics, they appear to have diverged to a great degree in their structural features.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy N. K., Garrett R. H. Immunoelectrophoretic determination of nitrate reductase in Neurospora crassa. Anal Biochem. 1979 May;95(1):97–107. doi: 10.1016/0003-2697(79)90191-x. [DOI] [PubMed] [Google Scholar]
- Campbell W. H., Smarrelli J. Purification and Kinetics of Higher Plant NADH:Nitrate Reductase. Plant Physiol. 1978 Apr;61(4):611–616. doi: 10.1104/pp.61.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkhouser E. A. Synthesis of Nitrate Reductase in Chlorella: II. EVIDENCE FOR SYNTHESIS IN AMMONIA-GROWN CELLS. Plant Physiol. 1980 May;65(5):944–948. doi: 10.1104/pp.65.5.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri L., Ramadoss C. S. Physical studies on assimilatory nitrate reductase from Chlorella vulgaris. J Biol Chem. 1979 Nov 25;254(22):11703–11712. [PubMed] [Google Scholar]
- Gray J. C., Kerwick R. G. An immunological investigation of the structure and function of ribulose 1,5-bisphosphate carboxylase. Eur J Biochem. 1974 May 15;44(2):481–489. doi: 10.1111/j.1432-1033.1974.tb03506.x. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Cohen H. J., Rajagopalan K. V. Molecular basis of the biological function of molybdenum. Molybdenum-free sulfite oxidase from livers of tungsten-treated rats. J Biol Chem. 1974 Aug 25;249(16):5046–5055. [PubMed] [Google Scholar]
- Notton B. A., Graf L., Hewitt E. J., Povey R. C. The role of molybdenum in the synthesis of nitrate reductase in cauliflower (Brassica oleracea L. var Botrytis L.) and spinach (Spinacea oleracea L.). Biochim Biophys Acta. 1974 Sep 11;364(1):45–58. doi: 10.1016/0005-2744(74)90131-4. [DOI] [PubMed] [Google Scholar]
- PATEMAN J. A., COVE D. J., REVER B. M., ROBERTS D. B. A COMMON CO-FACTOR FOR NITRATE REDUCTASE AND XANTHINE DEHYDROGENASE WHICH ALSO REGULATES THE SYNTHESIS OF NITRATE REDUCTASE. Nature. 1964 Jan 4;201:58–60. doi: 10.1038/201058a0. [DOI] [PubMed] [Google Scholar]
- Pan S. S., Nason A. Purification and characterization of homogeneous assimilatory reduced nicotinamide adenine dinucleotide phosphate-nitrate reductase from Neurospora crassa. Biochim Biophys Acta. 1978 Apr 12;523(2):297–313. doi: 10.1016/0005-2744(78)90033-5. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P., Lorimer G. H., Hall R. L., Borchers R., Bailey J. L. Reduced nicotinamide adenine dinucleotide-nitrate reductase of Chlorella vulgaris. Purification, prosthetic groups, and molecular properties. J Biol Chem. 1975 Jun 10;250(11):4120–4127. [PubMed] [Google Scholar]
- White T. J., Ibrahimi I. M., Wilson A. C. Evolutionary substitutions and the antigenic structure of globular proteins. Nature. 1978 Jul 6;274(5666):92–94. doi: 10.1038/274092a0. [DOI] [PubMed] [Google Scholar]