Abstract
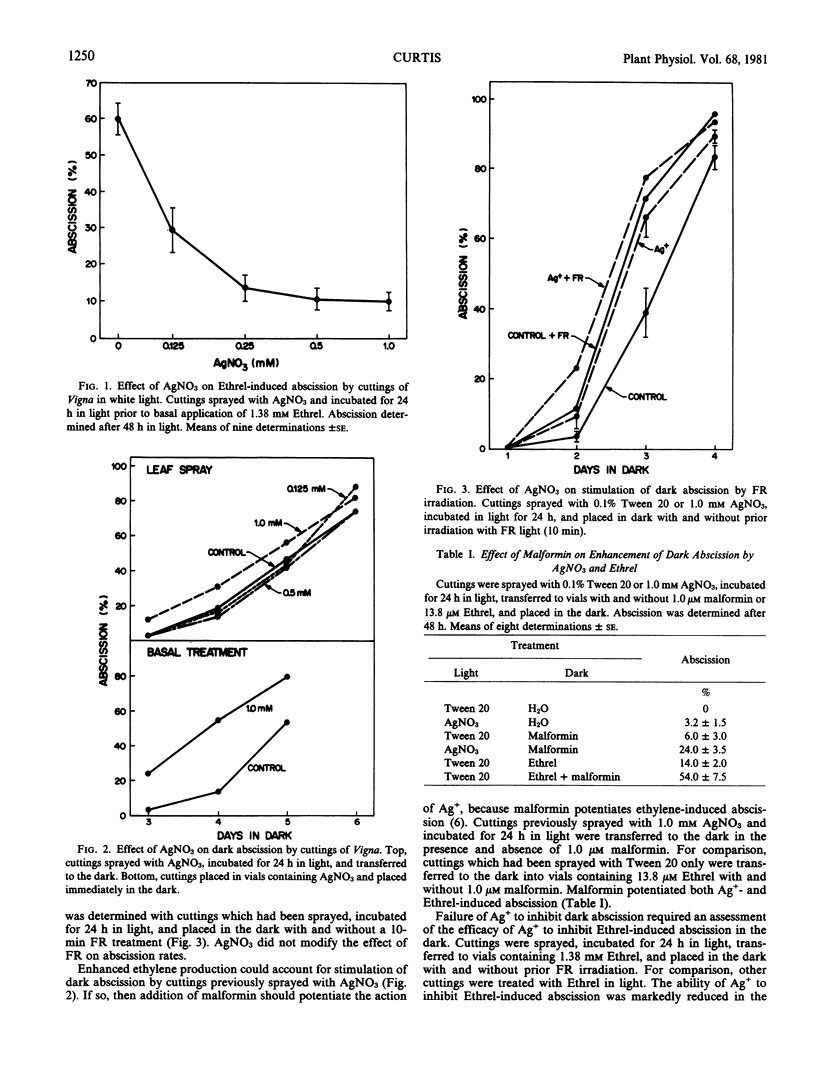
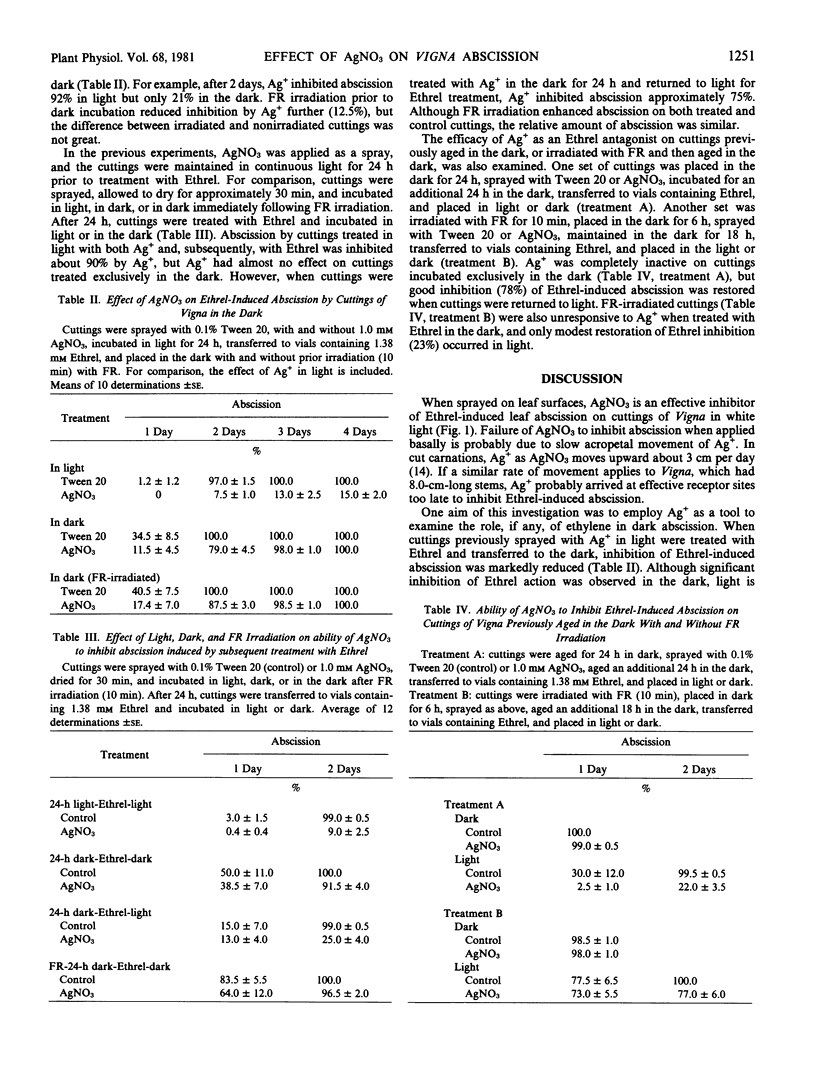
To obtain information regarding the antiethylene properties and binding site of Ag+, studies were initiated to define conditions under which Ag+ does or does not inhibit ethylene action. AgNO3, applied as a leaf spray, inhibited 2-chloroethylphosphonic acid (Ethrel)-induced leaf abscission from green cuttings of Vigna radiata in white light but lost considerable activity in the dark. In the absence of Ethrel, AgNO3 stimulated abscission in the dark. When cuttings were dark-aged for 24 hours prior to treatment with AgNO3 and aged for an additional 24 hours in the dark after treatment, good inhibition of subsequent Ethrel-induced abscission was restored by returning the cuttings to light. However, when dark aging was preceded by far-red irradiation, considerably less inhibition of Ethrel-induced abscission was restored in the light. AgNO3 was completely inactive on cuttings aged in the dark and treated with Ethrel in the dark. Light is required for the antiethylene activity of AgNO3 with regard to leaf abscission of Vigna.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N., Anderson J. D., Lieberman M. Production and action of ethylene in senescing leaf discs: effect of indoleacetic Acid, kinetin, silver ion, and carbon dioxide. Plant Physiol. 1979 Nov;64(5):805–809. doi: 10.1104/pp.64.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutelmann P., Kende H. Membrane Lipids in Senescing Flower Tissue of Ipomoea tricolor. Plant Physiol. 1977 May;59(5):888–893. doi: 10.1104/pp.59.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. A potent inhibitor of ethylene action in plants. Plant Physiol. 1976 Sep;58(3):268–271. doi: 10.1104/pp.58.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. C(2)H(4): Its Incorporation and Metabolism by Pea Seedlings under Aseptic Conditions. Plant Physiol. 1975 Aug;56(2):273–278. doi: 10.1104/pp.56.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. Effect of silver ion, carbon dioxide, and oxygen on ethylene action and metabolism. Plant Physiol. 1979 Jan;63(1):169–173. doi: 10.1104/pp.63.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R. W. Potentiation and inhibition of the effects of 2-chloroethylphosphonic Acid by malformin. Plant Physiol. 1971 Apr;47(4):478–482. doi: 10.1104/pp.47.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A. C. Sequence of Chloroplast Degreening in Calamondin Fruit as Influenced by Ethylene and AgNO(3). Plant Physiol. 1980 Oct;66(4):624–627. doi: 10.1104/pp.66.4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]


