Abstract
Tendons are able to transmit high loads efficiently due to their finely optimized hierarchical collagen structure. Two mechanisms by which tendons respond to load are collagen fibril sliding and deformation (stretch). While many studies have demonstrated that regional variations in tendon structure, composition, and organization contribute to the full tendon’s mechanical response, the location-dependent response to loading at the fibril level has not been investigated. In addition, the instantaneous response of fibrils to loading, which is clinically relevant for repetitive stretch or fatigue injuries, has also not been studied. Therefore, the purpose of this study was to quantify the instantaneous response of collagen fibrils throughout a mechanical loading protocol, both in the insertion site and in the midsubstance of the mouse supraspinatus tendon. Utilizing a novel atomic force microscopy-based imaging technique, tendons at various strain levels were directly visualized and analyzed for changes in fibril d-period with increasing tendon strain. At the insertion site, d-period significantly increased from 0% to 1% tendon strain, increased again from 3% to 5% strain, and decreased after 5% strain. At the midsubstance, d-period increased from 0% to 1% strain and then decreased after 7% strain. In addition, fibril d-period heterogeneity (fibril sliding) was present, primarily at 3% strain with a large majority occurring in the tendon midsubstance. This study builds upon previous work by adding information on the instantaneous and regional-dependent fibrillar response to mechanical loading and presents data proposing that collagen fibril sliding and stretch are directly related to tissue organization and function.
Keywords: collagen fibril, sliding, stretch, AFM, tendon, insertion site, midsubstance
1 Introduction
Tendon’s primary function is to transmit mechanical load and displacement from muscle to bone (Lichtwark and Barclay, 2010). It is able to perform this function due to its finely tuned hierarchical structure, composed of collagen fibrils organized into fibers or fascicles and further bundled to form tendon proper (Kastelic et al., 1978). Macroscopic structure-function studies of tendon have shown that mechanical changes occurring at lower scale levels are likely responsible for the complex non-linear and viscoelastic response of full tendon (Derwin and Soslowsky, 1999; Fessel and Snedeker, 2009; Lake et al., 2009; Lake et al., 2010; Rigozzi et al., 2009; Robinson et al., 2004). Recent evidence suggests that tendons are able to withstand high forces by employing a number of unique mechanisms occurring at many of the fibril and fiber length scales, including uncrimping, re-alignment, sliding, and deformation or stretch (Connizzo et al., 2013b; Gupta et al., 2010; Miller et al., 2012b; Screen et al., 2013). While collagen fiber uncrimping and re-alignment have been studied extensively in recent literature (Connizzo et al., 2013a; Connizzo et al., 2013b; Miller et al., 2012a; Miller et al., 2012b; Miller et al., 2012c; Miller et al., 2012d), the quantification of collagen fiber and fibril sliding and stretch has been studied less due to the experimental difficulties, particularly the inability to visualize individual collagen fibrils in vivo during mechanical loading.
Mechanical properties of single collagen fibrils have recently been investigated using several different technologies (Eppell et al., 2006; Graham et al., 2004; Tang et al., 2010; Yang et al., 2007). While these studies substantially improved our understanding of mechanics of individual collagen fibrils, they do not replicate the in vivo environment of collagen, where collagen fibrils are interacting with other collagen fibrils and with the surrounding extracellular matrix proteins. Furthermore, it has been reported that fiber-level elongation cannot be solely attributed to the deformation of the individual collagen fibrils, suggesting fibril-fibril and fibril-matrix interactions are likely responsible for this discrepancy (Fratzl et al., 1998; Puxkandl et al., 2002). Recent investigations utilizing atomic force microscopy have successfully measured d-period length changes as a quantitative measure of collagen fibril stretch in situ (Li et al., 2013; Rigozzi et al., 2011). This work introduces a significant advancement in the literature, allowing for the ability to study fibril stretch under various types of mechanical loading as well as with cases of altered structure, such as disease, aging, or injury (Li et al., 2013). However, these studies have primarily investigated fiber sliding during or following stress relaxation or creep events (Gupta et al., 2010; Li et al., 2013; Rigozzi et al., 2011; Screen et al., 2013). The strain rate dependence of tendon identifies that the timing and rate of loading, in addition to the magnitude, is extremely important to tendon’s response. Furthermore, since tendons have been known to rupture clinically due to a single traumatic event or impact (Moller et al., 1996), the instantaneous response to load, as well as the ability to repetitively undergo that impact stress, is critical to the overall function and has not been investigated.
In addition, fibril sliding and deformation have primarily been studied during the linear region of the mechanical test. Due to the prevalence of collagen uncrimping and re-alignment during the initial toe region (Miller et al., 2012b; Miller et al., 2012c), it is likely that these other fibrillar responses are also occurring. Finally, while many studies have demonstrated that the specific transition in composition, structure and collagen organization from the midsubstance to the insertion site contributes significantly to the full tendon’s mechanical response (Lake et al., 2009; Lake et al., 2010; Shaw and Benjamin, 2007), the location-dependent response to mechanical load at the fibril level has not been studied. Therefore, the purpose of this study was to quantify the instantaneous response of collagen fibrils throughout a mechanical loading protocol both in the insertion site and midsubstance of the mouse supraspinatus tendon. We hypothesized that more fibril stretch will occur at the insertion site than the midsubstance (higher strains) and that more fibril sliding will occur at the midsubstance than at the insertion site.
2 Methods
2.1 Sample Preparation
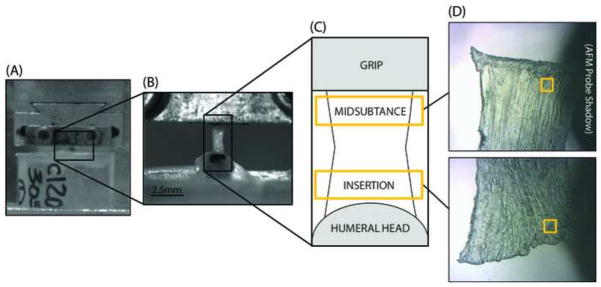
Fifteen C57BL/6 mice at 150 days of age were used in this study (IACUC approved). Supraspinatus tendons from both shoulders of each mouse were used for this study, but no two tendons from the same animal were used in the same testing group to ensure independence of samples. All soft tissues were removed from around the tendon, leaving the supraspinatus tendon attached to the humerus. Tendon cross-sectional area was then measured using a custom laser-based device (Peltz et al., 2009). The humerus was then embedded in an acrylic tube with PMMA. A second coating of PMMA was applied to prevent failure at the growth plate. The proximal end of the tendon was glued between two pieces of sandpaper with an initial gauge length of 2.5 mm and both the tendon and the acrylic pot were placed in custom grips for tensile testing (Fig. 1A, B), as described previously (Connizzo et al., 2013a).
Figure 1.
All samples were kept hydrated using phosphate buffered saline (PBS) and were then loaded in a tensile testing system (Instron, Norwood, MA) for mechanical testing. A 10 N load cell was used for all tests with a resolution of 0.01 N. All tendons underwent a preload to 0.02 N and ten cycles of preconditioning from 0.02 N to 0.04 N followed by a 60 second hold before the ramp to failure. Tendons were then divided into 6 groups and stretched to a randomly assigned grip-to-grip strain value (0, 1, 3, 5, 7, or 10%) at a rate of 0.1% strain per second. Tendons were then frozen using freezing spray (McMaster-Carr Electrical Cleaning and Maintenance Aerosol, Product #7437K43), removed from the mechanical testing setup, and placed in a specimen dish with tissue freezing medium. The sample was kept frozen during this process using freezing spray and the dish was then submerged in liquid nitrogen to complete the flash-freezing phase. Tendons were then stored at −20°C until they were sectioned in a cryostat microtome in the coronal anatomical plane at 20 microns and sections were then again kept frozen at −20°C until further processing. Frozen sections were then immersed in cold 10% neutral buffered formalin for 4 minutes for fixation and allowed to dry prior to imaging.
2.2 Atomic Force Microscopy (AFM)
Tendons were imaged in air using Peak Force Quantitative Nanomechanical Mapping mode using a Dimension Icon AFM (BrukerNano, Santa Barbara, CA). Imaging of 2 μm × 2 μm regions was performed using Bruker ScanAsyst Fluid+ probes (nominal spring constant k ≈ 0.70 N/m, radius R ≈ 2 nm). Tendons were scanned at 2–3 regions across the width of the insertion site and midsubstance of the tendon. The insertion and midsubstance locations were determined consistently by taking images within the bottom quarter (about 0.5 mm) of the specimen for the insertion site and the top quarter of the specimen for the midsubstance region (a single sample region shown in Figure 1C). Scans were also taken from 4–6 sections throughout the depth of the tendon.
2.3 Data Analysis
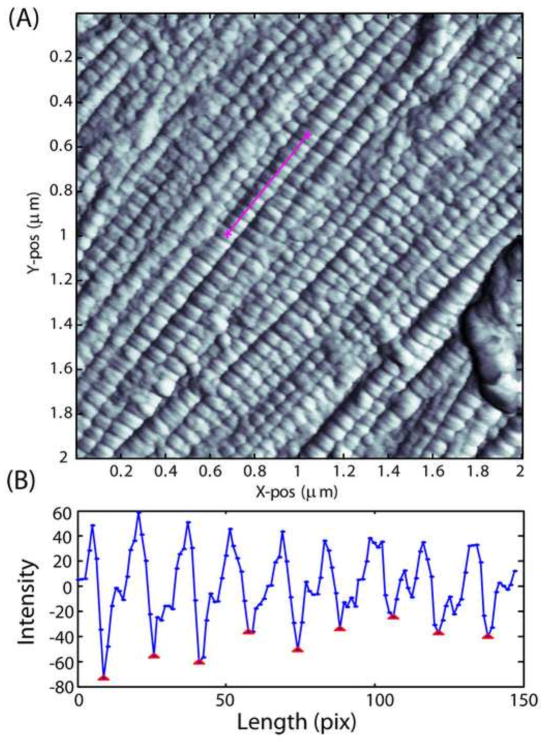
Several parameter maps were produced from imaging, including Height, Peak Force, Peak Force Error, Modulus, LogModulus, Dissipation, and Adhesion. Analysis on approximately two tendons (~20 images) showed no difference between the different parameter maps for measurement of d-period. Due to the strength of contrast from the LogModulus and Adhesion maps, these two maps were used to analyze all specimens. Custom MATLAB software (MathWorks, Natick, MA) was written to allow for the measurement of d-period length for many fibrils in a single image and for the ability to enhance the processing technique in the future. Images were first contrast enhanced by equalizing the histogram of intensities locally using a default MATLAB function (adapthisteq) with the default parameters. A line spanning at least 10 dark bands was then drawn manually over each fibril in the image by the user (Fig. 2A). Image intensity over the course of the line was then plotted and the distance between peaks or valleys in the image was calculated using a standard MATLAB peak-finding function, reducing user error from the analysis (Fig. 2B). The median distance between all peaks was then calculated and defined as the d-period length for that particular fibril. Fibrils with d-periods below 55 nm and above 80 nm were considered to be noise and were excluded from the analysis based on previous studies that cite high variability in collagen fibril populations using AFM (Choi et al., 2011; Fang et al., 2012; Wallace et al., 2010). D-period length was measured for fibrils in at least 5 images per specimen and 3–5 specimens per strain level. Fibril stretch/strain was calculated by dividing the d-period length at each applied strain level by the initial d-period length. Distributions of d-period lengths for each strain level in both the insertion site and midsubstance of the tissue were also produced and the variance of the distributions was calculated. A change in fibril d-period variance from one strain to the next is indicative of strain heterogeneity between fibrils, or fibril sliding.
Figure 2.
2.4 Statistical Analysis
Two tendons were excluded from this study due to failure prior to the allotted strain level and one tendon was excluded due to a tissue processing error leaving a sample size of n=3–5 tendons per strain level. It was determined via Mann-Whitney test that the variance within a single specimen was not different from the variance between specimens in the same strain level. Therefore, fibrils from all specimens in each strain level were pooled. Statistical comparisons of d-period length across different strains were then made using a non-parametric Kruskal-Wallis test at each location (insertion site and midsubstance), followed by post-hoc Dunn’s tests between the strain levels. Comparisons of variance between strain levels were performed using a Bartlett’s test for unequal variances at each location with post-hoc F tests between strain levels. A p-value of p < 0.05 was considered as statistically significant.
3 Results
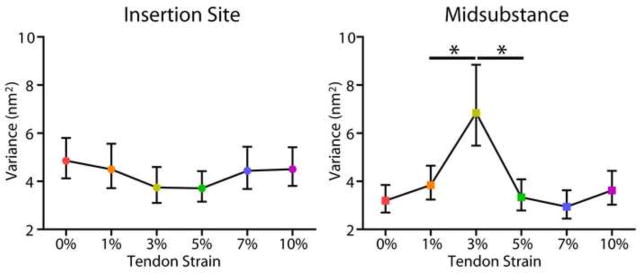
At both the insertion and midsubstance of the tendon, Kruskal-Wallis tests determined that there was a highly significant effect of increasing tendon strain on d-period length (p<0.001). D-period length at the insertion site significantly increased from 0% to 1% tendon strain, increased again from 3% to 5% strain, and decreased after 5% strain (Fig. 3). At the midsubstance, d-period length increased from 0% to 1% strain and then decreased after 7% strain. In addition, d-period distribution data displayed heterogeneity in d-period from one strain level to the next. Variance of the insertion site d-period distribution did not significantly change with increasing tendon strain (Fig. 4A). At the midsubstance, there was a highly significant increase in d-period variance with a peak at 3% strain and then a sharp decrease immediately following (Fig. 4B).
Figure 3.
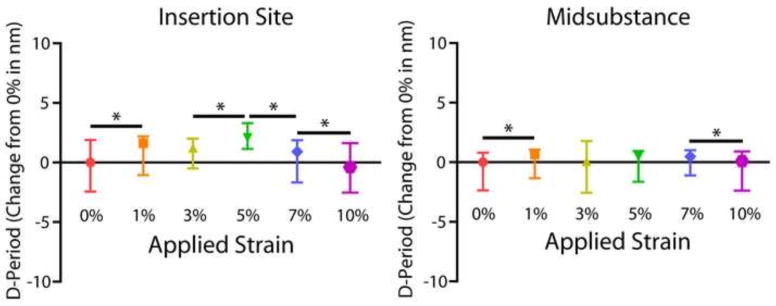
Figure 4.
4 Discussion
This is the first study to measure location-dependent collagen fibril stretch, or changes in collagen fibril d-period length with applied tendon strain. It has been well established that the insertion site and midsubstance of tendons often exhibit different mechanical, compositional, and structural properties, particularly in the supraspinatus tendon (Lake et al., 2010; Miller et al., 2012d; Shaw and Benjamin, 2007). In this study, the insertion site experienced higher fibril strains overall, reaching a peak of 3.66±4.84% fibril strain at 5% tendon strain compared to 1.10±2.67% fibril strain at 7% tendon strain in the midsubstance (Table 1). This is supported in the literature by full tendon studies that describe the insertion site as a high stress region (Shaw and Benjamin, 2007). Recent data have also shown that the processes of collagen fiber re-alignment and uncrimping occur differently at these two locations (Connizzo et al., 2013a; Miller et al., 2012d), so it is expected that collagen fibril stretch would also show a regional-dependence. This dependence could be due to organizational or compositional differences between the two regions such as an altered ratio or relationship between collagen fibril organization and extra-fibrillar matrix, which could lead to altered mechanical function (Thomopoulos et al., 2003; Thorpe et al., 2012). Due to the higher degree of disorganization at the insertion site, more re-alignment and uncrimping must occur here (Miller et al., 2012a; Miller et al., 2012b; Miller et al., 2012d). Given that these processes usually take place during the toe or transition region of the mechanical test, this could be one explanation for the pause in fibril stretch at the insertion site from 1 to 3% strain. Recent studies reported that the transition strain for the mouse supraspinatus at 90 and 300 days of age is at 2–3% strain (Connizzo et al., 2013a), supporting this theory.
Table 1.
Fibril strains are calculated by the change in fibril d-period from the initial (0%) d-period length at each level of full tendon strain.
| Applied Tendon Strain | Insertion Fibril Strain | Midsubstance Fibril Strain |
|---|---|---|
| 1% | 1.83±5.06% | 0.95±2.65% |
| 3% | 1.57±4.85% | 0.94±3.06% |
| 5% | 3.66±4.84% | 0.66±2.15% |
| 7% | 0.83±5.04% | 1.10±2.67% |
| 10% | −0.47±5.06% | 0.24±1.10% |
Data is presented as mean ± standard deviation.
In addition to regional dependence, this study is the first to quantify the instantaneous response of collagen fibrils to overall tendon strain during the mechanical test. The instantaneous response of collagen fibrils is an important measure of tendon biomechanical function, as many tendon injuries result from single, high impact loading trauma, particularly in athletes (Moller et al., 1996). However, previous investigations were unable to study this phenomenon due to fixation technique. Using the flash freezing method, we were able to preserve the instantaneous structural changes of tendon at varied strain levels. Our work demonstrated higher fibril strains than those previously reported (Rigozzi et al., 2011). Lower fibril strains in previous work could be attributed to the elongated holding period for chemical fixation, which would allow for creep and/or relaxation of the tissue to occur. Considering that a large amount of fiber/fibril sliding has been shown to occur during relaxation events (Gupta et al., 2010), it is possible that fibrils are relaxing to an altered state than the instantaneous positioning of fibrils. Future studies should focus on measuring both the instantaneous and relaxation response of collagen fibrils as both the elastic and viscoelastic components significantly contribute to the overall mechanical response (Dunn and Silver, 1983; Silver et al., 2002; Woo et al., 1993).
Results in this study are consistent with those in the literature demonstrating that strains of individual fibrils were much lower when compared to overall tendon tissue strain (Fratzl et al., 1998; Puxkandl et al., 2002), once again supporting the theory that inter- and intra-fibril mechanisms must be contributing to the overall mechanical response. The variance in d-period length distributions from one strain level to the next was also calculated in this study, where differences in d-period distribution variance across strain levels would indicate that fibril sliding is occurring. This analysis showed that most of the collagen fibril sliding, noted by either a positive or negative change in d-period variance, was occurring between 1 and 5% strain, which corresponds to the toe and transition periods of the tissue-level stress-strain curves. Simultaneously, fibril stretch was highest initially (from 0 to 1% strain) and then after 5% strain until gross tissue failure. This can be explained by a cooperative relationship between fibril stretch and fibril sliding, where these mechanisms occur in coordination. Furthermore, this study found a regional dependence on fibril sliding, with a much higher amount of fibril sliding occurring in the midsubstance, as indicated by the increased d-period variance. This provides another complexity to the relationship between fibril stretch and sliding, as organization seems to play a role in how and/or when these processes can occur. While we are not aware of any studies that have directly linked collagen organization with collagen fibril sliding, recent data suggests that tail tendon, which is highly organized like the supraspinatus tendon midsubstance, does exhibit collagen fibril and fiber sliding. In addition, studies investigating differences in functionally different tendons have shown structural differences as well as differences in the relative amounts of fiber sliding and stretch, also supporting the results here (Thorpe et al., 2013; Thorpe et al., 2012).
While this work provides a novel approach to answering complicated questions, this technique is not without limitations. First, this work employs a flash freezing and processing technique that is destructive to the tissue, requiring a large sample size to obtain a snapshot of each portion of the stress strain curve. However, with our ability to pool fibrils across several samples as described earlier, we can obtain enough fibrils to make powerful statistical comparisons in these experiments. In addition, our specific hypothesis was related to the instantaneous response of the tissue and as such, this technique does not allow for the real-time measurement of fibril stretch or sliding during loading of the tissue. Future efforts will focus on development of a technique to measure both the instantaneous and relaxation response in the same specimen since both responses contribute significantly to the overall function of the tissue. This study also did not measure the cross-sectional area of individual collagen fibrils. While evidence suggests that collagen fibril d-period length is independent of fibril diameter (Bozec et al., 2007; Reale et al., 1981), it is possible that some heterogeneity in fibril stretch could be due to fibril size and as such, future investigations will seek to normalize these measures. Finally, the heterogeneity between and within tendons is still a major area that needs to be explored since many analyses are performed on a single tendon type, making comparisons difficult to make. However, we believe the mouse supraspinatus tendon is an excellent model for this type of work due to the large changes in structure along the length of the tendon, which allow us to make generalizations about structure-function relationships that could be applicable to other tendons.
In conclusion, this work demonstrates a novel and innovative approach to measure the regional instantaneous response of collagen fibrils to overall tendon load without removing the fibrils from their native environment. Fibril stretch and sliding were found to be location-dependent and are speculated to have a strong relationship with collagen organization. This concept could lead to an explanation for differences that have been found recently in the mechanical responses of tendons with differing functional needs. Finally, this work could be a powerful tool utilized to study the altered fibrillar response to load in various animal models and could lead to a more complete visualization of the hierarchical complexity of tendon’s mechanical response.
Acknowledgments
This study was supported by NIH/NIAMS (T32-AR007132), the Penn Center for Musculoskeletal Disorders (NIH, P30 AR050950) and the Faculty Start-up Grant at Drexel University (LH).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors of this study have no personal or financial conflicts of interest with this work. All authors were fully involved in the study and preparation of this manuscript and the material within has not been and will not be submitted for publication elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bozec L, van der Heijden G, Horton M. Collagen fibrils: nanoscale ropes. Biophys J. 2007;92:70–75. doi: 10.1529/biophysj.106.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Cheong Y, Lee HJ, Lee SJ, Jin KH, Park HK. AFM study for morphological and mechanical properties of human scleral surface. J Nanosci Nanotechnol. 2011;11:6382–6388. doi: 10.1166/jnn.2011.4499. [DOI] [PubMed] [Google Scholar]
- Connizzo BK, Sarver JJ, Iozzo RV, Birk DE, Soslowsky LJ. Effect of age and proteoglycan deficiency on collagen fiber re-alignment and mechanical properties in mouse supraspinatus tendon. J Biomech Eng. 2013a;135:021019. doi: 10.1115/1.4023234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connizzo BK, Yannascoli SM, Soslowsky LJ. Structure-function relationships of postnatal tendon development: A parallel to healing. Matrix Biol. 2013b;32:106–116. doi: 10.1016/j.matbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwin KA, Soslowsky LJ. A quantitative investigation of structure-function relationships in a tendon fascicle model. J Biomech Eng. 1999;121:598–604. doi: 10.1115/1.2800859. [DOI] [PubMed] [Google Scholar]
- Dunn MG, Silver FH. Viscoelastic behavior of human connective tissues: relative contribution of viscous and elastic components. Connect Tissue Res. 1983;12:59–70. doi: 10.3109/03008208309005612. [DOI] [PubMed] [Google Scholar]
- Eppell SJ, Smith BN, Kahn H, Ballarini R. Nano measurements with micro-devices: mechanical properties of hydrated collagen fibrils. J R Soc Interface. 2006;3:117–121. doi: 10.1098/rsif.2005.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Goldstein EL, Turner AS, Les CM, Orr BG, Fisher GJ, Welch KB, Rothman ED, Banaszak Holl MM. Type I collagen D-spacing in fibril bundles of dermis, tendon, and bone: bridging between nano- and micro-level tissue hierarchy. ACS Nano. 2012;6:9503–9514. doi: 10.1021/nn302483x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessel G, Snedeker JG. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol. 2009;28:503–510. doi: 10.1016/j.matbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S. Fibrillar structure and mechanical properties of collagen. J Struct Biol. 1998;122:119–122. doi: 10.1006/jsbi.1998.3966. [DOI] [PubMed] [Google Scholar]
- Graham JS, Vomund AN, Phillips CL, Grandbois M. Structural changes in human type I collagen fibrils investigated by force spectroscopy. Exp Cell Res. 2004;299:335–342. doi: 10.1016/j.yexcr.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Gupta HS, Seto J, Krauss S, Boesecke P, Screen HR. In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. J Struct Biol. 2010;169:183–191. doi: 10.1016/j.jsb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. Journal of Orthopaedic Research. 2009;27:1596–1602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Tensile properties and fiber alignment of human supraspinatus tendon in the transverse direction demonstrate inhomogeneity, nonlinearity, and regional isotropy. J Biomech. 2010;43:727–732. doi: 10.1016/j.jbiomech.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fessel G, Georgiadis M, Snedeker JG. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol. 2013;32:169–177. doi: 10.1016/j.matbio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Barclay CJ. The influence of tendon compliance on muscle power output and efficiency during cyclic contractions. J Exp Biol. 2010;213:707–714. doi: 10.1242/jeb.038026. [DOI] [PubMed] [Google Scholar]
- Miller K, Connizzo B, Soslowsky L. Collagen Fiber Re-Alignment in a Neonatal Developmental Mouse Supraspinatus Tendon Model. Annals of Biomedical Engineering. 2012a;40:1102–1110. doi: 10.1007/s10439-011-0490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KS, Connizzo BK, Feeney E, Soslowsky LJ. Characterizing local collagen fiber realignment and crimp behavior throughout mechanical testing in a mature mouse supraspinatus tendon model. J Biomech Eng. 2012b;45:2061–2065. doi: 10.1016/j.jbiomech.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KS, Connizzo BK, Feeney E, Tucker JJ, Soslowsky LJ. Examining differences in local collagen fiber crimp frequency throughout mechanical testing in a developmental mouse supraspinatus tendon model. J Biomech Eng. 2012c;134:041004. doi: 10.1115/1.4006538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KS, Edelstein L, Connizzo BK, Soslowsky LJ. Effect of preconditioning and stress relaxation on local collagen fiber re-alignment: inhomogeneous properties of rat supraspinatus tendon. J Biomech Eng. 2012d;134:031007. doi: 10.1115/1.4006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller A, Astron M, Westlin N. Increasing incidence of Achilles tendon rupture. Acta Orthop Scand. 1996;67:479–481. doi: 10.3109/17453679608996672. [DOI] [PubMed] [Google Scholar]
- Peltz CD, Perry SM, Getz CL, Soslowsky LJ. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J Orthop Res. 2009;27:416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puxkandl R, Zizak I, Paris O, Keckes J, Tesch W, Bernstorff S, Purslow P, Fratzl P. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos Trans R Soc Lond B Biol Sci. 2002;357:191–197. doi: 10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale E, Benazzo F, Ruggeri A. Differences in the microfibrillar arrangement of collagen fibrils. Distribution and possible significance. J Submicrosc Cytol. 1981;13:135–143. [PubMed] [Google Scholar]
- Rigozzi S, Muller R, Snedeker JG. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. J Biomech. 2009;42:1547–1552. doi: 10.1016/j.jbiomech.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Rigozzi S, Stemmer A, Muller R, Snedeker JG. Mechanical response of individual collagen fibrils in loaded tendon as measured by atomic force microscopy. J Struct Biol. 2011;176:9–15. doi: 10.1016/j.jsb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Lin TW, Jawad AF, Iozzo RV, Soslowsky LJ. Investigating tendon fascicle structure-function relationships in a transgenic-age mouse model using multiple regression models. Ann Biomed Eng. 2004;32:924–931. doi: 10.1023/b:abme.0000032455.78459.56. [DOI] [PubMed] [Google Scholar]
- Screen HR, Toorani S, Shelton JC. Microstructural stress relaxation mechanics in functionally different tendons. Med Eng Phys. 2013;35:96–102. doi: 10.1016/j.medengphy.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Shaw HM, Benjamin M. Structure-function relationships of entheses in relation to mechanical load and exercise. Scand J Med Sci Sports. 2007;17:303–315. doi: 10.1111/j.1600-0838.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- Silver FH, Ebrahimi A, Snowhill PB. Viscoelastic properties of self-assembled type I collagen fibers: molecular basis of elastic and viscous behaviors. Connect Tissue Res. 2002;43:569–580. [PubMed] [Google Scholar]
- Tang Y, Ballarini R, Buehler MJ, Eppell SJ. Deformation micromechanisms of collagen fibrils under uniaxial tension. J R Soc Interface. 2010;7:839–850. doi: 10.1098/rsif.2009.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Klemt C, Riley GP, Birch HL, Clegg PD, Screen HR. Helical sub-structures in energy-storing tendons provide a possible mechanism for efficient energy storage and return. Acta Biomater. 2013;9:7948–7956. doi: 10.1016/j.actbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HR. Specialization of tendon mechanical properties results from interfascicular differences. J R Soc Interface. 2012;9:3108–3117. doi: 10.1098/rsif.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Chen Q, Fang M, Erickson B, Orr BG, Banaszak Holl MM. Type I collagen exists as a distribution of nanoscale morphologies in teeth, bones, and tendons. Langmuir. 2010;26:7349–7354. doi: 10.1021/la100006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SL, Johnson GA, Smith BA. Mathematical modeling of ligaments and tendons. J Biomech Eng. 1993;115:468–473. doi: 10.1115/1.2895526. [DOI] [PubMed] [Google Scholar]
- Yang L, van der Werf KO, Koopman BF, Subramaniam V, Bennink ML, Dijkstra PJ, Feijen J. Micromechanical bending of single collagen fibrils using atomic force microscopy. J Biomed Mater Res A. 2007;82:160–168. doi: 10.1002/jbm.a.31127. [DOI] [PubMed] [Google Scholar]