Abstract
HER2-positive (HER2+) breast cancer accounts for 18%–20% of all breast cancer cases and has the second poorest prognosis among breast cancer subtypes. Trastuzumab, the first Food and Drug Administration-approved targeted therapy for breast cancer, established the era of personalized treatment for HER2+ metastatic disease. It is well tolerated and improves overall survival and time-to-disease progression; with chemotherapy, it is part of the standard of care for patients with HER2+ metastatic disease. However, many patients do not benefit from it because of resistance. Substantial research has been performed to understand the mechanism of trastuzumab resistance and develop combination strategies to overcome the resistance. In this review, we provide insight into the current pipeline of drugs used in combination with trastuzumab and the degree to which these combinations have been evaluated, especially in patients who have experienced disease progression on trastuzumab. We conclude with a discussion of the current challenges and future therapeutic approaches to trastuzumab-based combination therapy.
Keywords: Targeted therapy, combination approaches, HER2-positive breast cancer, trastuzumab resistance
1. Overview of trastuzumab's effects and clinical challenges in HER2-positive breast cancer
HER2 is a member of the epidermal growth factor receptor (EGFR) family, which comprises the 4 tyrosine kinase proteins HER1 (or EGFR), HER2, HER3, and HER4, that regulates cell proliferation, survival, and differentiation[1]. HER2 is overexpressed or amplified in 18%–20% of all patients with HER2 positive (HER2+) BC [1,2,3]. When highly expressed at the membrane, HER2 undergoes hyperdimerization with itself or with other receptors in the family. This activates various mitogenic pathways, specifically phosphatidylinositide 3-kinase (PI3K)/AKT and mitogen-activated protein kinase (MAPK) [4]. These pathways drive continuous cell proliferation and new vasculature formation, which leads to an aggressive phenotype and poor prognosis. For more details on the process, readers are recommended to an expert review by Hynes et al (2005) [1].
Given that HER2 is overexpressed in BC cells and drives malignancy, HER2-targeted therapy represents an attractive therapeutic approach. In fact, considerable research is being conducted to identify targets of HER2 and its family members. Trastuzumab, the first humanized monoclonal antibody to be developed against HER2, is the most successful targeted therapy for HER2+ BC [5]. The results of several large randomized trials (NCCTG N9831, NSABP B-31, BCIRG 006, and HERA) strongly demonstrate that adding trastuzumab to standard chemotherapy reduces disease recurrence compared to chemotherapy alone in patients with surgically resectable tumors (table 1) [5,6]. On the basis of these data, the addition of trastuzumab to chemotherapy has become the standard of care in women with early HER2+ BC or metastatic breast cancer (MBC). Its significant clinical benefit results from multiple mechanisms, including inhibition of the HER2 downstream pathway, prevention of HER2 shedding, and induction of antibody-dependent cell-mediated cytotoxicity [7,8,9]. For comprehensive information on trastuzumab's mechanism of action and the results of clinical trials, please see the excellent review by Spector et al (2009) [8].
Table 1.
Clinical trials mentioned in this review
| Trial name | Official title | ClinicalTrials.gov identifier |
|---|---|---|
| Pertuzumab (or 2C4; commercial name, Perjeta) – Genentech/Roche Monoclonal antibody disrupting HER2-HER3 heterodimerization | ||
| CLEOPATRA | A phase III, randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of pertuzumab + trastuzumab + docetaxel vs. placebo + trastuzumab + docetaxel in previously untreated HER2+ MBC | NCT00567190 |
| NeoSphere | A randomized, open label study to compare the complete pathological response rate achieved with 4 combinations of herceptin, docetaxel and pertuzumab in patients with locally advanced, inflammatory or early stage HER2+ BC | NCT00545688 |
| Lapatinib (or GW572016; commercial name, Tykerb/Tyverb) – GlaxoSmithKline Small molecule inhibitor blocking tyrosine kinase activities of HER1, HER2, and p95-HER2 | ||
| Neo ALTTO | A randomised, multicenter open-label phase III study of neoadjuvant lapatinib, trastuzumab and their combination plus paclitaxel in women with HER2/ErbB2 positive primary BC | NCT00553358 |
| EGF104900 | A randomized, multicenter, open-label, phase III study of lapatinib in combination with trastuzumab versus lapatinib in subjects with HER2+ MBC whose disease has progressed on trastuzumab-containing regimens | NCT00320385 |
| Afatinib or BIBW 2992 (commercial name, Tomtovok) – Boehringer Ingelheim Small molecule inhibitor blocking tyrosine kinase activities of HER1 and HER2 | ||
| LUX-Breast 1 | A open label, randomised phase III trial of BIBW 2992 and vinorelbine versus trastuzumab and vinorelbine in patients with metastatic HER2-overexpressing BC failing one prior trastuzumab treatment | NCT01125566 |
| Neratinib (commercial name, HKI-272) – Wyeth Small molecule inhibitor blocking tyrosine kinase activities of HER1, HER2, and HER4 | ||
| A phase II trial of hki-272 (neratinib) for patients with human epidermal growth factor receptor 2 (HER2)-positive BC and brain metastases | NCT01494662 | |
| MM-111 (or SAR256212) – Merrimack A bi-specific antibody targeting HER2 and HER3 | ||
| A phase 1 study of MM-111 in combination with herceptin in patients with advanced, refractory HER2 amplified, heregulin positive BC | NCT01097460 | |
| T-DM1 or trastuzumab emtansine (commercial name, Kadcyla) – Genentech/Roche Drug-conjugated HER2 | ||
| TDM4450g | A randomized, multicenter, phase II study of the efficacy and safety of trastuzumab-MCC-DMl vs. trastuzumab (herceptin®) and docetaxel (taxotere®) in patients with metastatic HER2+ BC who have not received prior chemotherapy for metastatic disease | NCT00679341 |
| EMILIA | A randomized, multicenter, phase III open-label study of the efficacy and safety of trastuzumab emtansine vs. capecitabine + lapatinib in patients with HER2+ locally advanced or MBC who have received prior trastuzumab-based therapy | NCT00829166 |
| MARIANNE | A study of trastuzumab-DMl plus pertuzumab versus trastuzumab [herceptin] plus a taxane in patients with MBC | NCT01120184 |
| TH3RESA | A phase III randomized, multicenter, two-arm, open-label trial to evaluate the efficacy of trastuzumab-emtansine compared with treatment of physician's choice in patients with HER2+ MBC who have received at least two prior regimens of HER2 directed therapy | NCT01419197 |
| GDC-0941 – Genentech/Roche PI3K inhibitor | ||
| A phase IB open-label, dose-escalation study of the safety and pharmacology of PI3-kinase inhibitor GDC-0941 in combination with paclitaxel, with and without bevacizumab or trastuzumab, in patients with locally recurrent or MBC | NCT00960960 | |
| NVP-BKM-120 – Novartis Selective PI3K inhibitor | ||
| A phase Ib/II, open label, multi-center study evaluating the safety and efficacy of BKM120 in combination with trastuzumab in patients with relapsing HER2 overexpressing BC who have previously failed trastuzumab | NCT01132664 | |
| Everolimus (orRAD-001; commercial name, Afinitor) – Novartis mTOR inhibitor | ||
| B0LER0-3 | A randomized phase III, double-blind, placebo-controlled multicenter trial of daily everolimus in combination with trastuzumab and vinorelbine, in pretreated women with HER2/neu over-expressing locally advanced or MBC | NCT01007942 |
| Ridaforolimus (or AP23573 or MK-8669; commercial name, ARIAD) – Merck mTOR inhibitor | ||
| 8669-009 | A phase II trial of oral deforolimus (AP23573; MK-8669), an mTOR inhibitor, in combination with trastuzumab for patients with HER2+ trastuzumab-refractory MBC | NCT00736970 |
| NVP-BEZ235 – Norvatis Dual mTOR-PI3K inhibitor | ||
| A phase I/II, multi-center, open-label study of BEZ235, administered orally on a continuous daily dosing schedule in adult patients with advanced solid malignancies including patients with advanced BC | NCT00620594 | |
| MK2206 - National Cancer Institute (NCI) – Merck Akt inhibitor | ||
| A phase I study of MK-2206 in combination with trastuzumab and lapatinib in HER2+ breast and gastric cancer | NCT01705340 | |
| Panobinostat (or LBH-589) – Norvatis HDAC inhibitor | ||
| A phase Ib/IIa trial of panobinostat in combination with trastuzumab in adult female patients with HER2 positive MBC whose disease has progressed during or following therapy with trastuzumab | NCT00567879 | |
| BMS-754807 – Bristol-Myers Squibb IGF-1R inhibitor | ||
| A phase I/II trial of BMS-754807 in combination with trastuzumab (Herceptin) in subjects with advanced or metastatic HER2 positive breast cancer | NCT00788333 | |
| Cixutumumab (or IMC-A12) – ImClone Monoclonal antibody against IGF-1R | ||
| Randomized phase II trial of capecitabine and lapatinib with or without EVIC-A12 in patients with HER2 positive breast cancer previously treated with trastuzumab and an anthracycline and/or a taxane | NCT00684983 | |
| Bevacizumab (commercial name, Avastin) – Genentech/Roche Monoclonal antibody against VEGF | ||
| BETH | A multicenter phase III randomized trial of adjuvant therapy for patients with HER2-positive node-positive or high risk node-negative BC comparing chemotherapy plus trastuzumab with chemotherapy plus trastuzumab plus bevacizumab | NCT00625898 |
| AVEREL | A randomized, open-label study to compare the effect of first-line treatment with avastin in combination with herceptin/docetaxel and herceptin/docetaxel alone on progression-free survival in patients with HER2+ locally recurrent or MBC | NCT00391092 |
| IPI-504 or retaspimycin hydrochloride – Infinity HSP90 inhibitor | ||
| Efficacy and safety of IPI-504 with trastuzumab pretreated, locally advanced or metastatic HER2+ BC | NCT00817362 | |
| Chemotherapy | ||
| NCCTCN9831 | Phase III trial of doxorubicin and cyclophosphamide (AC) followed by weekly paclitaxel with or without trastuzumab as adjuvant treatment for women with HER-2 overexpressing node positive or high-risk node negative BC | NCT00005970 |
| NSABPB-31 | A randomized trial comparing the safety and efficacy of adriamycin and cyclophosphamide followed by taxol (AC-T) to that of adriamycin and cyclophosphamide followed by taxol plus herceptin (AC-T+H) in node-positive BC patients who have tumors that overexpress HER2 | NCT00004067 |
| BCIRG 006 | Multicenter phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC-T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (herceptin)(AC-TH) and with docetaxel, carboplatin and trastuzumab (TCH) in the adjuvant treatment of node positive and high risk node negative patients with operable BC containing the HER2 alteration | NCT00021255 |
| HERA | HERA: A randomised three-arm multi-centre comparison of 1 year and 2 years of herceptin versus no herceptin in women with HER2+ primary BC who have completed adjuvant chemotherapy | NCT00045032 |
Despite trastuzumab's significant effect on patient outcomes, studies by Vogel et al (2002) and Slamon et al (2001) showed that 74% of patients with HER2+ MBC did not experience a response to first-line trastuzumab monotherapy and about 50% did not experience a response to trastuzumab with anthracycline and cyclophosphamide. This is referred to as primary resistance [10,11]. Moreover, even though trastuzumab with chemotherapy has significantly improved disease-free survival (DFS) and overall survival (OS) in patients with early-stage HER2+ BC, relapses eventually occur after a year of initial treatment; this is categorized as acquired resistance [6]. These observations raise 3 questions: 1) Why do patients with HER2 overexpression not experience a response to trastuzumab? 2) Should trastuzumab continue to be used in patients who have experienced progression? and 3) What should trastuzumab be combined with to overcome resistance?
The first question is controversial and is discussed at the end of this review. The second concern was identified in a clinical trial called EGF104900 that was performed by Blackwell et al (2009) (table 1). In the study, patients whose disease was refractory to trastuzumab were treated with a small molecular inhibitor called lapatinib, alone or in combination with trastuzumab [12,13]. The combined therapy significantly improved the progression-free survival (PFS), clinical benefit rate, and OS compared with single-agent therapy [12,13]. Another important study that addressed the same question, was conducted by von Minckwitz et al (2009) on 156 HER2+ MBC patients who had experienced progression while undergoing first-line trastuzumab-based chemotherapy [14]. The patients were randomly assigned to receive capecitabine, either alone or in combination with trastuzumab. Although the difference in OS between the 2 treatment groups was not statistically significant, there was a major improvement in overall response and time-to-disease progression when trastuzumab was added to capecitabine [14,15]. These data strongly demonstrate that trastuzumab-resistant tumors continue to be dependent on HER2 signaling and that combining trastuzumab with other targeted therapies is beneficial, even in patients who have experienced progression on trastuzumab.
In this review, we mainly focus on the third question: we review current combination therapies that have efficacy in patients whose disease is refractory to trastuzumab treatment. Generally, the term “combination therapy” refers to the “art” of combining different drugs to enhance the anti-proliferative effect of treatment or to overcome resistance [16,17]. Combinatorial strategies can target multiple nodes within the same oncogenic pathway (“vertical” combination) or multiple sites across a signaling network (“horizontal” combination) [16]. In reality, when 2 or more drugs are combined, they can have additive, synergistic, or antagonistic effects or may produce more distinct complex outcomes [17]. Moreover, the key factors in successful combinations are tolerability and avoidance of possible pharmacokinetic interactions [18]. For expert opinions on potential drug interactions, practical issues with combinatorial options, and proposed guidelines for the clinical development of combinations based on patients' genetic profiles, please read the comprehensive reviews by Rodon et al., 2010; Al-Lazikani et al., 2012; and Yap et al., 2013 [16,17,18].
In the following sections, we review the 2 approaches used in trastuzumab-based combination therapy: the introduction of compounds with complement mechanisms of action to trastuzumab and the combination of trastuzumab with agents that have similar targets but produce synergistic efficacy. These strategies include inhibitors of multiple growth factor receptors (GFRs) and heat shock protein (Fig 1), blockage of HER2 downstream effectors and histone deacetylase (Fig 2), and agents against type I insulin-like growth factor receptor (IGF-1R) and angiogenesis (Fig 3). Each combination approach is analyzed, from hallmark laboratory studies to the most current clinical trials.
Figure 1.
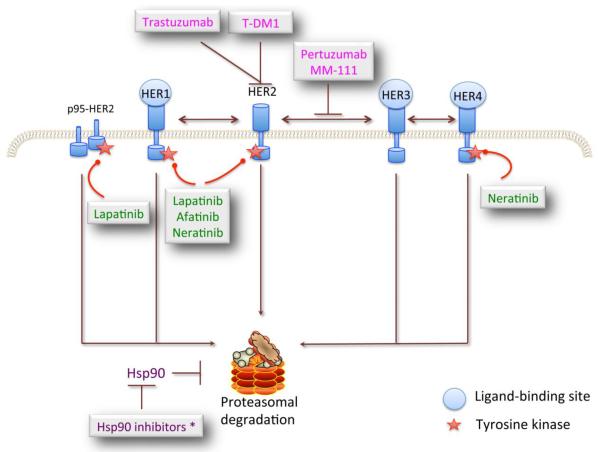
Inhibitors of epidermal growth factor receptors (HER) combined with trastuzumab in active clinical practice. There are 4 members of the HER family, HER1–4. Each is composed of 3 domains: an extracellular domain that is responsible for ligand binding, a transmembrane domain, and the intracellular tyrosine kinase domain, which interacts with intracellular signaling molecules. Currently, there are 4 approaches to inhibiting HER: i) Monoclonal antibodies, including trastuzumab, pertuzumab, and MM111, to inhibit its dimerization; ii) small molecule inhibitors (lapatinib, neratinib, and afatinib) to inhibit its kinase activation; iii) toxin-conjugated antibodies to selectively target toxic agents to HER2-overexpressing BC cells; and iv) HSP90 inhibitors to rapidly degrade HER2, 3, and 4. However, this strategy (iv) has not resulted in encouraging clinical benefits in HER2+ BC patients.
Figure 2.
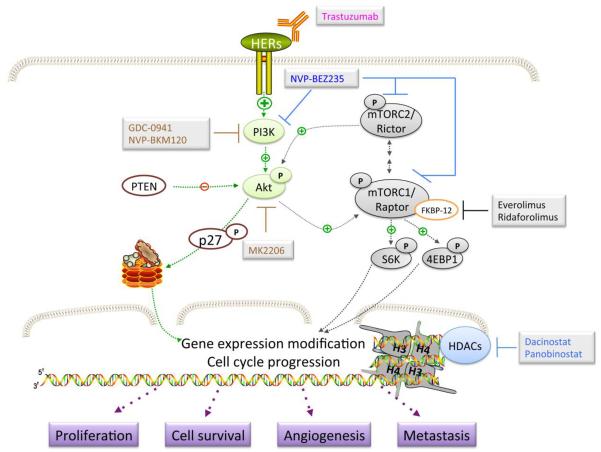
Schematic representation of drugs used with trastuzumab to target HER2-mediated signaling. This includes dual blocking of PI3K and mTOR activity (NVP-BEZ235), mTORC1 inhibitors (everolimus and ridaforolimus), PI3K inhibitors (GDC-0941 and NVP-BKM120), Akt inhibitor (MK2206), and histone deacetylase inhibitors (dacinostat and panobinostat).
Figure 3.
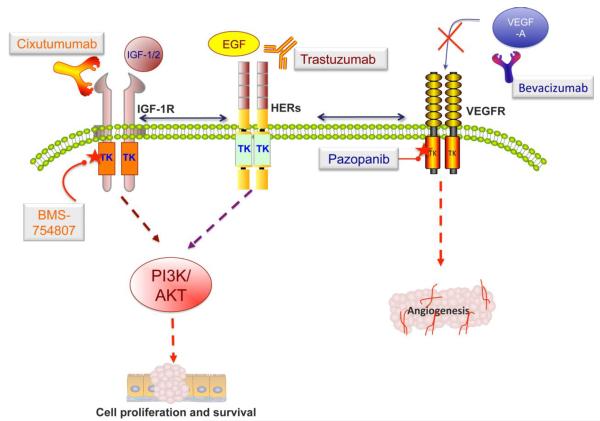
Therapeutic strategies targeting VEGF and IGF signaling that are undergoing active clinical development for patients whose disease is resistant to trastuzumab. For VEGF signaling, these include using bevacizumab to block secreted VEGF to bind to its receptors, and the small molecule, pazopanib, which inhibits the kinase activation of VEGFR-1, -2, and -3. The monoclonal antibody cixutumumab, which specifically targets the extracellular domain of IGF-1R, and the small molecular tyrosine kinase inhibitor BMS-754807 are promising treatments for trastuzumab-resistant patients.
2. Inhibition of multiple growth factor receptors
Previous studies revealed that the overexpression of tyrosine kinase receptors such as HER1 or HER3 can compensate for HER2 signaling and promote cell proliferation, even in the presence of trastuzumab [5,8,19]. Moreover, the expression of a constitutively active HER2 that lacks an extracellular domain, known as p95-HER2, also contributes to resistance to therapy and is correlated with poor outcome in patients [20,21,22]. Therefore, targeting these escape mechanisms should provide complete blockage of redundant pathways and improve patients' responses to therapy. This approach includes humanized monoclonal antibodies or small molecule inhibitors (SMIs), bi-specific antibodies, and drug-conjugated antibodies (Fig 1).
2.1 Dual monoclonal antibody blockade
Dual monoclonal antibody blockade is designed to prevent HER2 activation through heterodimerization with other receptor tyrosine kinases, such as HER1 and HER3; this should block ligand-activated downstream signaling more completely than should targeting HER2 alone [23].
One successful example of the dual blockade approach is pertuzumab (2C4 or Perjeta), another humanized monoclonal antibody that targets HER2 [23]. The difference between pertuzumab and trastuzumab is that pertuzumab specifically binds to HER2, close to the center of domain II, and prevents its heterodimerization with HER3 [24]. Given that overexpression of HER3 is required for HER2-driven cell proliferation, the combination of the 2 antibodies should completely block their activation and signaling, thus inhibiting the proliferation of resistant cells [25]. In fact, the results from in vitro and in vivo models demonstrated that pertuzumab was effective at disrupting HER2-HER3 heterodimers, leading to inhibition of PI3K signaling and apoptosis [23,26]. The synergistic effect of trastuzumab and pertuzumab was fully supported by xenograft models, in which enhanced tumor regression was observed for combination therapy but not monotherapy [25,27].
Data from phase II clinical trials suggested that trastuzumab and pertuzumab were well tolerated and was beneficial after disease progression on trastuzumab therapy in MBC [28,29]. Later, CLEOPATRA, a large phase III study, was conducted to compare the efficacy and safety of trastuzumab and docetaxel, with and without pertuzumab (table 1). An analysis demonstrated that the PFS and OS durations were significantly extended with the addition of pertuzumab [30,31]. In another clinical study in early BC, NeoSphere, researchers found that the combination was much more effective at improving the rate of tumor disappearance (pathological complete response rate) than was the individual treatment (table1) [32]. On the basis of the outstanding clinical benefits of pertuzumab, the drug was approved by the FDA, in combination with trastuzumab, for the treatment of HER2+ BC in both the neoadjuvant and metastatic setting.
One concern about this approach is the risk of additive side effects because both agents target HER2. However, no significant difference was found in cardiac dysfunction in patients who enrolled in the CLEOPATRA study (table 1) [33].
2.2. Combination of trastuzumab and small molecule tyrosine kinase inhibitors (SMIs)
SMIs are designed to bind to the ATP-binding pocket of kinase receptors, inhibiting their catalytic activity [1]. Even though both monoclonal antibodies and SMIs ultimately lead to downstream signaling inhibition, they differ in their mechanisms of action and pharmacological properties [34]. Antibodies are administered intravenously and target the extracellular domains of growth factor receptors [34]. Tyrosine kinase inhibitors are small orally available, membrane-permeable compounds that act inside cells [34]. In addition, because of their large size, monoclonal antibodies do not efficiently cross the blood-brain barrier; SMIs may have this ability, but it has not been clinically confirmed [34]. The half-life of many tyrosine kinase inhibitors, such as lapatinib and gefitinib, is approximately 24–48 hours, whereas the half-life of monoclonal antibodies such as trastuzumab is much longer—about 3–4 weeks [35]. However, small molecules are generally thought to be less specific than therapeutic antibodies and may be associated with a higher risk of toxicity [34]. For a comprehensive comparison of antibodies and SMIs, please refer to the excellent reviews by Imai and Takaoka (2006) and Lin and Winer (2007) [34,36].
One of the first SMIs approved by the FDA for treating HER2+ MBC was lapatinib, a pyrido- [3,4-d]-pyrimidine derivative [37]. Lapatinib potently inhibits the kinase activity of both HER1 and HER2, thus terminating mitogenic signaling in vitro and in vivo [38]. In addition, although PTEN loss confers trastuzumab resistance, lapatinib retains anti-tumor activity in PTEN-null, HER2-overexpressing cell lines [39]. Furthermore, trastuzumab-resistant, p95HER2-expressing cancer cells are sensitive to lapatinib [22]. Importantly, patients with p95HER2 expression responded similarly to lapatinib, as did patients with full-length HER2 [40,41]. Together, these findings suggest that lapatinib benefits patients with trastuzumab-refractory BC. The drug was approved by the FDA in 2007, in combination with capecitabine, for the treatment of advanced HER2-overexpressing BC [42]. Lapatinib was beneficial in patients who experienced progression on trastuzumab, as confirmed in several large randomized trials, such as EGF104900 and NeoALTTO (table 1). The preliminary analysis from these studies demonstrated a significant improvement in pathological complete response and PFS in patients treated with lapatinib and trastuzumab versus individual therapy alone [12,43]. Importantly, no major cardiac dysfunction due to lapatinib treatment has been reported [43,44].
Besides lapatinib, afatinib (BIBW-2992) and vinorelbine are being compared to trastuzumab plus vinorelbine for HER2+ MBC patients in a phase III trial known as LUX-Breast 1 (table 1). Afatinib is an anilino–quinazoline-derived, irreversible, oral SMI of HER1, mutated HER1, and HER2 that was shown to possess potent anti-tumor activities in tumor cell lines[45]. In addition, a phase II trial of afatinib demonstrated the drug's promising activity in pretreated HER2+ BC patients who had experienced progression after trastuzumab treatment [46].
Another potent SMI against HER1, HER2, and HER4, known as neratinib (HKI-272), is currently being evaluated in phase II trials in HER2+ MBC patients with brain metastases. Preliminary data showed that oral neratinib had encouraging anti-tumor activity and was well tolerated in heavily pretreated and trastuzumab treatment-naive patients with advanced HER2+ BC [47]. For more information on afatinib and neratinib, please see the reviews by Minkovsky et al (2008) and López-Tarruella et al (2012), respectively [45,48].
2.3. Bi-specific antibodies against HER2 and its partners
Bi-specific antibodies are designed to be directed against 2 different antigens; thus, they are expected to have higher therapeutic efficacy than agents that inhibit a single target [49]. There are at least 2 different methods of generating bi-specific antibodies. First, the variable domains of 2 well-characterized monoclonal antibodies can be combined [49]. Second, an additional paratope is attached to the variable domain of an existing antibody [49,50].
One advanced example of bi-specific antibodies being used as solid tumor treatment is MM-111, which was developed to target both HER2 and HER3 [51]. MM-111 consists of fully human anti-HER2 and anti-HER3 single chain antibody moieties that are linked by modified human serum albumin. The Nielsen group, who investigated MM-111, found that trastuzumab, lapatinib, and pertuzumab were not effective at inhibiting heregulin-induced HER3 activation in HER2+ BC cells [51]. In contrast, MM-111 could prevent the HER2-HER3 heterodimer and promote the formation of inactive trimeric complexes [51]. Moreover, they found that the addition of MM-111 to trastuzumab significantly suppressed in vivo tumor growth, even in the presence of heregulin. Moreover, the combination could inhibit the proliferation of BT474-M3 and NCI-N87 more effectively than could pertuzumab and trastuzumab [51]. Therefore, MM-111 and trastuzumab are currently being evaluated in a phase I trial that includes about 21 trastuzumab-resistant HER2+ BC patients (table 1).
2.4. Drug-conjugated antibodies against HER2: the first targeted chemotherapy for solid tumors
One attractive strategy for enhancing drug potency is to improve its specificity using antibody-drug conjugates. This strategy takes advantage of antibodies to directly and specifically deliver potent therapeutic agents to tumor sites. It also allows the drug to reserve its toxicity for the target, minimize damage to normal cells, and accumulate at a much higher concentration in cancer cells [49,52]. Generally, 3 classes of cytotoxic agents are commonly used to attach to antibodies: calicheamicin based, maytansinoid based, and auristatin based [52]. Calicheamicin, the subject of extensive research in drug delivery, is a natural product that can bind to DNA in the minor groove, resulting in DNA cleavage. Maytansinoid and auristatin derivatives act by binding to tubulin to inhibit tubular polymerization [52]. Readers interested in conjugated antibodies should refer to the comprehensive reviews by Senter's research group [52].
Particularly in HER2+ BC, the first drug-conjugated antibody approved by the FDA was ado-trastuzumab emtansine (T-DM1) in early 2013. T-DM1 consists of 1 molecule of trastuzumab that is covalently bonded with 3 or 4 molecules of emtansine (DM1), a derivative of maytansine, via a stable thioether linker [53]. When the parent compound, maytansine, is used alone, it is non-specific and thus too toxic to be used in patients [54]. However, T-DM1 targets DM1 specifically to HER2-overexpressing cells and thus spares the cells with low HER2 overexpression. Additionally, T-DM1 binds to HER2 with a similar affinity to that of trastuzumab [53,55]. Therefore, the cytotoxicity of T-DM1 at the tumor sites can be enhanced to around 100 to 10,000 times that of standard chemotherapy [53]. Interestingly, in vitro results indicated that T-DM1 retained all known mechanisms of trastuzumab, such as antibody-dependent cell-mediated cytotoxicity, PI3K/AKT signaling inhibition, and suppression of p95-HER2 formation [54]. Moreover, as DM1 works independently of growth factor signaling inhibition, it was anticipated that its conjugate would be effective in trastuzumab-resistant cases. In fact, T-DM1 had potent cytotoxicity in a panel of sensitive and resistant HER2+ BC cells [55]. Furthermore, in vivo data indicated that the drug significantly inhibited tumor growth and caused cancer regression in mouse xenograft models [54,55].
In the phase II trial TDM4450g, T-DM1 was compared to trastuzumab and docetaxel for the first-line treatment of HER2+ MBC; the PFS duration was significantly extended in patients treated with T-DM1(table 1) [56]. Of note, T-DM1 also had a more favorable toxicity profile than did trastuzumab with docetaxel, including a lower incidence of grade 3 adverse effects and a lower risk of cardiotoxicity [53,56]. These findings were further confirmed by the results of a large phase III study, EMILIA, in which T-DM1 significantly increased PFS and OS, with less toxicity than capecitabine and lapatinib, in patients with HER2+ advanced BC (table 1) [53,57]. In addition, data from another large phase III international study called TH3RESA also showed the important clinical effect of T-DM1 on patients with HER2+ advanced BC (table 1). The TH3RESA trial, which enrolled more than 600 patients who had experienced progression on both trastuzumab and lapatinib, was conducted to compare the efficacy of T-DM1 to a treatment of the physician's choice. The preliminary data was reported by Dr. Wildiers at the European Cancer Congress 2013 (abstract #LBA15). T-DM1 was demonstrated to reduce the risk of disease progression by 47% compared to the standard therapy. Specifically, the duration of PFS was 2 times longer in patients receiving T-DM1 than in those receiving a trastuzumab-based regimen. Another phase III study, MARIANNE, is ongoing to compare the efficacy of T-DM1, with or without pertuzumab with trastuzumab and taxane, as first-line targeted therapy for women with advanced HER2+ BC (table1). In summary, the data from these trials should provide physicians and patients with comprehensive toxicity and efficacy profiles of T-DM1. For more detailed information on T-DM1, including its activity, safety, and current and past clinical trials, please see LoRusso's and Boyraz's reviews (2011 and 2013, respectively) [53,57].
3. Inhibition of HER2 downstream signaling components in combination with trastuzumab
Deregulation of the PI3K/AKT pathway is proposed to be the most common contributors to trastuzumab resistance [58]. Several research groups have demonstrated that continuous AKT activation due to PTEN loss or activating mutations of PI3K maintains continuous cell proliferation, even in the presence of trastuzumab [7,59,60]. In addition, HER2+ cancers with PI3KCA mutations or PTEN downregulation showed inferior prognoses after trastuzumab treatment [60]. Interestingly, in vitro and in vivo results revealed that BCs with HER2 overexpression were significantly and selectively sensitive to AKT1/2 inhibitors[61]. These findings suggest that combining the inhibitors of the PI3K/AKT pathway with anti-HER2 therapies can restore trastuzumab sensitivity. This strategy, moreover, may overcome the resistance as a result of the formation of the truncated, constitutively active mutant p95-HER2. Here, we discussed the combination of trastuzumab with the inhibitors of PI3K, mTOR and AKT (Fig 2).
3.1. PI3K inhibitors
Most of the specific PI3K inhibitors inhibit the class I PI3K (Fig 2). One of the first PI3K inhibitors, a quercetin derivative known as LY294002, inhibited tumor growth in PTEN-loss cell lines and xenograft models [62]. However, because of its unfavorable pharmacokinetic properties and toxicity, LY294002 has not been studied clinically [63]. Another promising class I PI3K inhibitor with selective activity against class 1A isoforms is GDC-0941, a thieno [3,2-d] pyrimidine derivative that is being studied in phase I trials that are nearing completion [64,65]. Preclinical studies demonstrated that GDC-0941 in combination with trastuzumab was efficacious in trastuzumab-refractory cells with a PI3K-activating mutation or PTEN loss [7,66,67]. Another potent and reversible PI3K inhibitor, NVP-BKM120, is being evaluated with trastuzumab in a phase Ib/II trial in HER2+ MBC patients who had been pre-treated with trastuzumab (table 1) [63,68]. In general, even though there has been extensive research indicating that PI3K activation plays a crucial role in trastuzumab resistance, most PI3K inhibitors are still being evaluated in early clinical trials for HER2+ MBC.
3.2. mTOR inhibitors
The mammalian target of rapamycin (mTOR), a serine-threonine protein kinase, is a major downstream effector of the PI3K/AKT pathway [69]. mTOR, which comprises the mTORC1 and mTORC2 complexes, plays a master role in regulating protein synthesis and cell metabolism and is constitutively active in cancer and malignancies [69]. Recently, activated mTOR, characterized by increased levels of phosphorylated initiation factor 4E-binding protein 1, was found to be involved in resistance to trastuzumab in primary HER2+ BC [70]. This finding indicates that blocking activated mTOR will restore trastuzumab sensitivity.
One of the first mTORC1 inhibitors to be identified was rapamycin (sirolimus), an antibiotic produced by Streptomyces hygroscopicus [69,71]. The compound forms a complex with FK506 binding protein-12, which is then recognized by mTOR. The complex prevents mTOR activity and inhibits its downstream cascade [69]. In vitro data demonstrated that inhibiting mTOR resulted in increased levels of the cell cycle inhibitor p27CIP/KIP, which led to cell cycle arrest and inhibition of cell proliferation [69]. Furthermore, mTOR inhibitors were found to have anti-angiogenic effects by suppressing VEGF production in HER2+ BC cells [72]. These anti-proliferative effects make rapamycin an attractive therapeutic. However, because of the pharmaceutical limitations of rapamycin, several rapalogs (synthetic derivatives with improved properties) have been developed, including temsirolimus (CCI-779) and everolimus (RAD001) (Fig 2). Everolimus is the most advanced mTOR inhibitor in clinical development. In 2009, Arteaga's group demonstrated that everolimus combined with trastuzumab induced tumor regression in mouse models. The authors also found that the feedback loop induced by RAD001 on PI3K/AKT, which resulted in increased AKT phosphorylation, was blocked by trastuzumab in vitro. These findings indicate that mTOR inhibitor and trastuzumab show a synergistic effect. In fact, the preliminary results of a phase I study of everolimus were encouraging: the drugs had an anticancer effect in patients whose disease was resistant to trastuzumab, and the combinations of trastuzumab and everolimus were well tolerated [73,74]. Everolimus is being investigated, in combination with the antimitotic agent vinorelbine and trastuzumab, in a phase III trial (BOLERO-3) in trastuzumab-resistant MBC patients (table 1) [75]. The combination of ridaforolimus (AP23573 or MK-8669, formerly known as deforolimus) and trastuzumab is being evaluated in a phase II trial in trastuzumab-refractory MBC patients (table 1). Preliminary data from the study suggest that the combination is feasible and well tolerated, with early evidence of anti-tumor activity in patients whose disease is resistant to trastuzumab [76]. For comprehensive discussion on mTOR activity and updated data on clinical trials, please see the recent review by Nahta and O'Regan (2010) [76].
3.3. Dual mTOR-PI3K inhibitors
It is hypothesized that the simultaneous inhibition of mTOR and PI3K results in enhanced anticancer effects, which provides the rationale for developing dual PI3K-mTOR blockage [63]. In addition, suppression of mTORC1 unexpectedly activates the PI3K pathway, promoting cell survival and conferring resistance to mTOR inhibitors. Dual PI3K-mTOR inhibitors are therefore, expected to address these issues. Interestingly, these dual inhibitors also bind both the mTOTC1 and mTORC2 complexes; thus, they may be able to suppress the PI3K/AKT/mTOR pathway more completely than can rapalogs (Fig 2) [63].
A promising dual inhibitor that is under investigation is an imidazoquinoline, NVP-BEZ235 (BEZ) [77]. BEZ, which acts in an ATP-competitive manner, showed potent in vitro anti-proliferative activity against HER2-overexpressing BC cells that were resistant to trastuzumab and lapatinib [77,78]. In vitro and in vivo data demonstrated that BEZ suppressed tumor growth in a panel of BC cell lines and xenograft models with activating PI3K mutation [79]. Mechanistically, BEZ abrogated the phosphorylation of AKT and p70S6 kinase in both lapatinib- and trastuzumab-resistant BC cells as a result of PTEN knockdown or PI3K-, E545K-, and H1047R-activating mutations [78,79]. BEZ is now being evaluated in phase I and II trials in advanced cancer patients with activating PI3K mutants or PTEN loss (table 1) [63].
3.4. AKT inhibitors
AKT, a serine and threonine kinase, acts as a central downstream target of PI3K and upstream target of mTORC1 [58]. Activated AKT triggers numerous cellular processes, including cell proliferation and survival. Mechanistically, it negatively regulates cell cycle inhibitor p27KIP/CIP, tumor suppressor protein p53, and pro-apoptotic protein BAD or activates the cell-cycle progression proteins, c-Myc and cyclin D [80]. Recently, Chandarlapaty et al (2011) found that AKT inhibition caused negative feedback on receptor tyrosine kinase phosphorylation and expression [81]. As AKT was inhibited, there was a marked increase in HER3 phosphorylation in BT474 BC cells [81]. Importantly, HER1/2 kinase inhibitor, lapatinib, could reverse the feedback and that the combination of lapatinib and AKT inhibitors significantly reduced tumor growth in a BT474 xenograft model [81].
These findings indicate that AKT inhibitors can be combined clinically combined with trastuzumab. Unfortunately, only a few AKT inhibitors have been evaluated in clinical trials. One of the most advanced AKT inhibitors in clinical trials is MK-2206, an oral allosteric AKT inhibitor with nano-molar potency against purified recombinant human AKT1 and AKT2 but much lower potency against human AKT3 [82]. In vitro and in vivo data identify that MK-2206 inhibits cell proliferation, tumor growth and induces apoptosis by specifically blocking AKT phosphorylation at both Thr308 and Ser473 (Fig 2) [82,83]. Importantly, MK-2206 demonstrates more than 100-fold selectivity for AKT against 256 other kinases, and its anti-proliferative activity is significantly enhanced in BC cells with PTEN loss or PI3K mutations [83,84]. The specificity of MK-2206 for AKT and its efficacy towards the cancers with alteration in PI3K/ PTEN would allow us to select patients who will get most benefit from this therapeutic approach. Several phase I studies are investigating the maximum tolerated dose, pharmacokinetics, and pharmacodynamics of MK2206, alone or in combination with trastuzumab and lapatinib, in patients with HER2+ BC and other solid tumors (table 1). Preliminary data reported by Yap et al (2011) showed that MK-2206 could be administered safely at doses that inhibited AKT phosphorylation [84]. The results encourage future trials to rationally and effectively develop therapeutic approaches that incorporate AKT inhibitors.
4. Other combination approaches in combination with trastuzumab
4.1. Histone deacetylase inhibitors
Histone proteins organize DNA into nucleosomes, which are repeating structures of chromatin [85]. The acetylation and deacetylation status of histones regulates gene expression and chromatin remodeling. These processes are mediated by 2 classes of enzymes, histone deacetylases (HDACs) and histone acetyltransferases [85]. Histone acetyltransferase catalyzes the acetylation of histones H3 and H4 at specific lysine residues; this neutralizes the positive charge on the histone, resulting in transcriptional activation (Fig 2). Therefore, the histones associated with active genes are highly acetylated [85]. In contrast, HDACs remove acetyl groups from lysines, allowing interactions to occur between negatively charged DNA and positively charged histone proteins, which leads to transcriptional suppression [85]. Treatment with HDAC inhibitors (HDIs) prevents HDAC activity, resulting in chromatin relaxation and gene expression (Fig 2). HDIs have been found to transcriptionally up-regulate critical tumor suppressor genes such as p27 and p21, which are associated with cell cycle arrest and apoptosis in cancer cells [86]. A cinnamic acid hydroxamate, LAQ824 (or Dacinostat), acts as a potent HDI that exerts its anti-proliferative effects at a nanomolar concentration [87]. In vitro data demonstrated that HDIs significantly decreased the mRNA and protein levels of HER2, resulting in the attenuation of AKT activity [87]. LAQ824 also promoted the proteasomal degradation of HER2 and its dissociation from HER3. Co-treatment of trastuzumab with HDIs led to significant increase in apoptosis in BT474 and SKBR-3 cells [87]. However, although HDIs have had encouraging preclinical results, the clinical use of these agents in solid malignancies has been disappointing. Panobinostat is one of the few HDIs that has been thoroughly studied and had promising results in phase I clinical studies when used in combination with trastuzumab in resistant disease (table 1) [88,89]. A preliminary analysis also demonstrated that the combination of panobinostat and trastuzumab was well tolerated, although additional data on patient safety, efficacy, and pharmacokinetic are not yet available [89]. For further opinions on the development and current clinical trial status of panobinostat and other HDIs, please see the review by Wagner et al (2010) [88].
4.2. HSP90 inhibitors
HSP90 is a ubiquitous chaperone protein that assists in folding and protecting client proteins from degradation and environmental stress, including heat, hypoxia, free radicals, radiation, and chemotherapy. Mechanistically, HSP90 regulates the stabilization, activity, and turnover of numerous critical proteins, such as AKT, HER2, focal adhesion kinase, and serine and threonine kinases such as Raf-1, Cdk4/Cdk6, and casein kinase II (Fig 1) [90]. Elevated expression of HSP90, which is frequently observed in cancer, leads to the accumulation of these proteins and ultimately results in continuous cell proliferation [90]. Thus, blocking the function of HSP90 can cause degradation of these onco-proteins and inhibit tumor growth. Specifically, HSP90 inhibitors lead to rapid HER2 degradation, concomitant inhibition of PI3K/AKT signaling, and in vivo growth suppression in both xenograft and transgenic HER2+ BC models [91,92]. In addition, Chandarlapaty et al (2010) demonstrated that p95-HER2 was an HSP90 client protein and that the p95-HER2-dependent in vivo tumor model was sensitive to HSP90 inhibitors (Fig 1) [93]. These findings indicate that HSP90 inhibitor in combination with trastuzumab will benefit patients with refractory disease, especially those with p95-HER2 circulation.
The first HSP90 inhibitor, geldanamycin, is a benzoquinone ansamycin antibiotic that was isolated from Streptomyces hygroscopicus. It was found to possess anticancer properties; however, it is too toxic to be used clinically [94]. Geldanamycin was anticipated to bind to the ATP-binding pocket of HSP90 and inhibit its function [95]. Its analog, tanespimycin, or 17-allylamino-17-desmethoxy geldanamycin, is less toxic and more stable than is the original agent and works in a similar manner [96]. However, it was not developed clinically because of poor aqueous solubility and pharmacokinetic properties [97]. A new formulation of tanespimycin, KOS-953, which contains Cremophory EL (polyethoxylated castor oil), has been developed. Pharmacological research has demonstrated that both formulations achieve similar pharmacokinetics of 17-allylamino-17-desmethoxy geldanamycin [97]. The results of phase I and II trials of KOS-953 and trastuzumab for locally advanced BC or MBC that has progressed on trastuzumab-based therapy revealed that the combination was more effective than was either therapy alone [98,99]. However, the sponsor suspended the clinical development of tanespimycin, and all trials of this drug have been closed [97]. A second generation of KOS-953, retaspimycin hydrochloride (IPI-504), showed antitumor activity in preclinical and phase I studies in combination with trastuzumab. Yet despite these earlier encouraging results, a phase II trial of retaspimycin hydrochloride and trastuzumab in patients with HER2+BC did not meet the pre-specified expansion criteria [90]. In conclusion, the strategy that targets HSP90 has not resulted in significant clinical benefits in HER2+ BC patients.
4.3. Insulin-like growth factor I receptor (IGF-1R) inhibitors
The insulin-like growth factor (IGF) signaling pathway is a complex system composed of the circulating ligands IGF-I, IGF-II, and insulin; the cell membrane receptors type 1 and 2 IGF receptors (IGF-1R and IGF-2R) and the insulin receptor (IR); the IGF binding proteins (IGFBP 1–7); and several associated proteins (Fig 3) [100,101]. Among these, IGF signaling through IGF-IR has been shown to activate the MAPK and PI3K/AKT pathways and thus protect tumor cells from damage due to cytotoxic chemotherapeutic agents and ionizing radiation (table 1) [100,102]. Therefore, IGF-1R has been the key target of therapy against IGF signaling to suppress tumor growth and increase the efficacy of other anticancer therapeutics. For a comprehensive discussion about IGF signaling, its roles in cancer and angiogenesis, and its cross-talk with other pathways, readers should read the excellent reviews by Pollak and Buck and Mulvihill (2011) [100,103,104]
In HER2+ BC, IGF-1R upregulation was proposed to act as an adaptive mechanism to trastuzumab's inhibitory effects. In a neoadjuvant trial of trastuzumab with vinorelbine, IGF-1R membrane expression was associated with a lower response rate than was in IGF-1R negatice staining [105]. Moreover, preclinical studies showed that upregulation of IGF-1R promoted cell cycle progression, cell growth, and cell proliferation, which resulted in trastuzumab resistance [106,107,108]. Interestingly, this resistance was reversed by blocking IGF-1R signaling with recombinant IGF-binding protein-3 or IGF-1R antagonist NVP-AEW541 [108,109]. These findings led to clinical studies to evaluate the efficacy of IGF-1R inhibitors in patients who had experienced disease progression on trastuzumab-based therapy. Currently, more than 25 agents disrupt IGF signaling at different stages of development, including monoclonal antibodies against IGF-1R, SMIs targeting kinase domains of the IGF receptor, antisense of IGF-1R, and the neutralizing antibody to IGF-I and –II [110]. These approaches, including their efficacies and toxicities and the detailed results of current clinical trials, have been extensively reviewed by Rodon et al. (2008), Buck and Mulvihill (2011), and King and Wong (2012) [101,104,110].
SMIs and monoclonal antibodies against IGF-1R are the most investigated approaches to targeting IGF signaling. BMS-754807 is a selective, non ATP-competitive IGF-1R SMI [111]. The drug showed anti-proliferative synergy when combined with trastuzumab in HER2+ BC cell lines [111]. BMS-754807 in combination with trastuzumab was evaluated in a phase I/II study in patients with advanced or HER2+ MBC (NCT00788333, table 1). Since the trial has been completed, an analysis of the primary data will provide valuable guidelines for the combination of the drug with trastuzumab for further studies [101,104].
A promising antibody against IGF-1R, cixutumumab (IMC-A12), is being evaluated in a phase II study with capecitabine and lapatinib in HER2+ MBC patients who had been previously treated with trastuzumab and an anthracycline or taxane (NCT00684983, table 1). IMC-A12 is a fully human monoclonal antibody that selectively binds to the IGF-1R with high affinity and causes subsequent internalization and degradation of the receptor [112]. IMC-A12 treatment has been demonstrated in vitro and in vivo to inhibit IGF ligand-induced growth of various tumor types, including BC, by suppressing the ERK-MAPK and PI3K/AKT pathways [112,113]. Since IMC-A12 does not bind to or recognize the IR, the drug is expected to confer higher therapeutic indices in the clinic than is the non-specific small molecule strategy. Rowinsky et al. (2007) and McKian and Haluska (2010) have reviewed the development of IMC-A12, including preclinical to early and current clinical studies and all reported toxicities associated with IMC-A12 treatment of patients [112,113].
IGF-1R inhibitors are undergoing active clinical evaluation. However, as IGF is ubiquitously expressed in cells, careful attention needs to be paid to these inhibitors' potential adverse effects and to their doses and schedules.
4.4. Angiogenesis inhibitors
Angiogenesis describes the formation of new blood vessels (neovascularization) from the existing vasculature, a complex process that is orchestrated by vascular endothelial growth factors (VEGFs), VEGF receptors (VEGFRs), and VEGF antagonists (Fig 3) [114]. Angiogenesis is essential for normal development but is dysfunctional in malignancies, resulting in tumor invasion and metastasis [114]. Clinical data have revealed that serum VEGF levels were significantly elevated in BC patients and that VEGF inhibition can slow disease progression [115,116]. An analysis of primary breast tumor tissues from 611 patients demonstrated a positive correlation between HER2 and VEGF and an association between elevated HER2, VEGF levels, and poor outcome [117]. For more information about studies of VEGF as a prognostic marker of BC progression, please see the excellent review by Banerjee et al (2007) [116].
In a BC xenograft model, VEGF expression was found to be elevated in the trastuzumab-resistant group, and sensitivity to trastuzumab was restored upon treatment with bevacizumab, a monoclonal antibody against VEGF [118]. The results of this study provide a rationale for using HER2 and VEGF inhibitors in clinical practice. Bevacizumab can block VEGF binding to its receptor, inhibiting tumor-related angiogenesis (Fig 3) [118]. Bevacizumab was granted accelerated approval by the FDA for the first-line treatment of HER2-negative MBC, in combination with paclitaxel, in 2008 [5]. However, data from phase III trials demonstrated that it only had a small effect on tumor growth, with no significant evidence of a longer survival duration or better quality of life than for standard chemotherapy alone. Therefore, in 2011, the FDA withdrew approval of bevacizumab for the treatment of HER2-negative MBC, although it remains on the market as an approved treatment for colon, lung, kidney, and brain cancer [5]. Currently, 2 large phase III trials are evaluating the efficacy of bevacizumab and trastuzumab with chemotherapy in HER2+ MBC (BETH and AVEREL, table 1). Preliminary data from AVAREL demonstrated that the PFS duration was longer in patients receiving bevacizumab, although the data did not reach statistical significance [119]. Another anti-VEGF approach is to use small molecule inhibitors that target the tyrosine kinase domain of VEGFR-1, -2, and -3, particularly pazopanib is currently being assessed in a phase III trial in combination with lapatinib in HER2+ inflammatory BC (Fig 3) [5].
5. Ongoing issues and challenges
Significant advances have been made in discovering and validating combination treatments that overcome trastuzumab resistance in HER2+ BC. However, there are still critical issues that need to be addressed in future studies.
5.1. Re-assessing HER2 status
HER2 status is currently assessed using fluorescent in situ hybridization and immunohistochemical analysis [120]. However, according to Wolff et al. (2007), about 20% of HER2 tests may be inaccurate [121]. For example, a re-analysis of data in NSABP B-31 demonstrated that 9.7% of the samples analyzed in local laboratories did not meet the criteria for HER2 amplification by fluorescent in situ hybridization or immunohistochemical analysis [120,122,123]. Unexpectedly, those patients had a longer DFS duration than did the control group [120]. In contrast, not all patients responded equally to trastuzumab, despite having HER2+ BC [75]. These findings indicate that (1) additional research is necessary to standardize the criteria for HER2 positivity and (2) HER2 overexpression does not always predict patients' responses to therapy. To address the latter, it is important to understand the mechanism behind patients' responses and establish additional responsive markers. In general, given the high costs of targeted therapies and the potential toxicities, it is important to develop a more effective system for defining patient populations that will most benefit from therapy.
5.2. Clinical validation of resistant mechanisms: from bench to patient-side
Given that most resistant mechanisms to trastuzumab were identified in laboratory models, it is important to validate that targeting the proposed mechanisms from preclinical studies will result in clinically meaningful benefits in selected patients. Moreover, all signaling networks are interconnected, and there are many alternative pathways through the resistance process. Therefore, it is important to distinguish the bypass mechanism from the mechanism that plays a dominant role in driving resistance. With the rapid development of system biology, a clear solution will be available in the near future.
5.3. Development of biomarker for trastuzumab-based combination therapy
In HER2-targeted treatment, there are no reliable biomarkers that can be used to make individual therapeutic decisions [124]. In addition, many drugs (such as PI3K/AKT pathway inhibitors and anti-angiogenesis) can be combined with trastuzumab, but no formal process exists for identifying predictive and responsive biomarkers of these combinations. Therefore, to provide better drug combinations and minimize patient expense, we must determine (1) how to efficiently and appropriately combine and administer different therapeutic agents to minimize toxicity while maximizing the effectiveness of the combinations and (2) how to select patients who will most benefit from these strategies.
Furthermore, there is currently no standard protocol or guidelines for evaluating antibodies that can be used to identify potential markers [120]. For example, although PTEN loss can be used to predict the outcome of some targeted therapies, neither the antibody used to stain samples nor the definitive cut-off to define PTEN loss has been standardized among laboratories.
Generally, the results of large ongoing clinical studies, together with a focus on the development of appropriate biomarkers, may answer these questions and reduce costs for patients.
5.5. Development of drug-conjugated antibodies
Although a huge research effort has been made in the field of drug-conjugated antibodies in solid tumors, few of these conjugates are being developed for clinical use, for several reasons. First, the selected drugs must have sufficient potency because only about 2 or 3 drug molecules can be directly attached to an IgG molecule without damaging its function [120]. Second, the number of monoclonal antibodies that can reach a tumor site is extremely low. Third, the conjugated drugs must be non-immunogenic and non-active while circulating in the blood. Fourth, linkers between toxic agents and a monoclonal antibody must be stable enough to remain intact until the antibody reaches target cells. Finally, once the conjugated complex is efficiently internalized, it must be properly released. Continued efforts from different scientific disciplines, including chemistry, bio-engineering, pharmacology, and molecular biology, are needed to develop better antibody-drug conjugates, especially for use against solid tumors.
6. Concluding remarks
It is clear that HER2-targeted therapy for BC has been clinically established and that the drug combinations are rationally designed for more effective therapy. We now need to better understand how to combine different drugs and to determine the extent to which they can be combined successfully, with or without chemotherapy. Recently developed technologies in multi-omics analysis, together with a growing understanding of prognosis and predictive biomarkers, will shape the future of successful personalized, precision medicine for HER2+ BC patients and all cancer patients.
Acknowledgements
This project was sponsored by grants from the NIH (R01-CA90853) and Cancer Prevention Research Institute of Texas (RP120451) (to F.X. Claret); by the Vietnam Education Foundation (to T. Vu); and by MD Anderson's Cancer Center Support Grant (CA016672). We thank Ann M. Sutton for editing the manuscript.
We have made every attempt to discuss all trials published so far; we apologize if we have omitted any trials that have been presented, whether in abstract or published form.
Abbreviations
- BC
breast cancer
- BEZ
BEZ235
- DFS
disease-free survival
- EGFR
epidermal growth factor receptor
- HDACs
histone deacetylases
- HDIs
HDAC inhibitors
- HER2+
HER2-positive
- IGF
insulin-like growth factor
- IGF-1R
type I insulin-like growth factor receptor
- IR
insulin receptor
- MBC
metastatic breast cancer
- mTOR
mammalian target of rapamycin
- OS
overall survival
- PFS
progression-free survival
- PI3K
phosphatidylinositide 3-kinase
- SMIs
small molecule inhibitors
- T-DM1
trastuzumab-DM1
- VEGFs
vascular endothelial growth factors
- VEGFRs
vascular endothelial growth factor receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors' Disclosures of Potential Conflicts of Interests M.X.S. is an employee of Genentech, a member of the Roche Group, and a shareholder of Roche. The other authors have no conflicts of interest to declare.
References
- [1].Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- [2].Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- [3].Eroles P, Bosch A, Perez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38:698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- [4].Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- [5].Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- [6].Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J, G. Breast Cancer International Research Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- [8].Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–5847. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- [9].Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- [10].Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- [11].Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- [12].Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O'Shaughnessy J. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- [13].Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, Ellis C, Florance A, Vukelja S, Bischoff J, Baselga J, O'Shaughnessy J. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol. 2012;30:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- [14].von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann M, Bauer W, Baumann KH, Clemens MR, Duerr R, Uleer C, Andersson M, Stein RC, Nekljudova V, Loibl S. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- [15].von Minckwitz G, Schwedler K, Schmidt M, Barinoff J, Mundhenke C, Cufer T, Maartense E, de Jongh FE, Baumann KH, Bischoff J, Harbeck N, Luck HJ, Maass N, Zielinski C, Andersson M, Stein RC, Nekljudova V, Loibl S, G.B.s. group, i. participating Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3–05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011;47:2273–2281. doi: 10.1016/j.ejca.2011.06.021. [DOI] [PubMed] [Google Scholar]
- [16].Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30:679–692. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- [17].Rodon J, Perez J, Kurzrock R. Combining targeted therapies: practical issues to consider at the bench and bedside. Oncologist. 2010;15:37–50. doi: 10.1634/theoncologist.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31:1592–1605. doi: 10.1200/JCO.2011.37.6418. [DOI] [PubMed] [Google Scholar]
- [19].Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- [20].Christianson TA, Doherty JK, Lin YJ, Ramsey EE, Holmes R, Keenan EJ, Clinton GM. NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998;58:5123–5129. [PubMed] [Google Scholar]
- [21].Saez R, Molina MA, Ramsey EE, Rojo F, Keenan EJ, Albanell J, Lluch A, Garcia-Conde J, Baselga J, Clinton GM. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res. 2006;12:424–431. doi: 10.1158/1078-0432.CCR-05-1807. [DOI] [PubMed] [Google Scholar]
- [22].Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, Di Cosimo S, Matias-Guiu X, Ramon y Cajal S, Arribas J, Baselga J. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- [23].Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, Scher HI, Sliwkowski MX. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- [24].Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- [25].Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- [26].Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- [27].Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- [28].Baselga J, Gelmon KA, Verma S, Wardley A, Conte P, Miles D, Bianchi G, Cortes J, McNally VA, Ross GA, Fumoleau P, Gianni L. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192–1198. doi: 10.1126/science.1241145. [DOI] [PubMed] [Google Scholar]
- [30].Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM, Group CS. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A, Clark E, Ross G, Benyunes MC, Baselga J. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gradishar WJ. HER2 therapy--an abundance of riches. N Engl J Med. 2012;366:176–178. doi: 10.1056/NEJMe1113641. [DOI] [PubMed] [Google Scholar]
- [33].Swain SM, Ewer MS, Cortes J, Amadori D, Miles D, Knott A, Clark E, Benyunes MC, Ross G, Baselga J. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist. 2013;18:257–264. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6:714–727. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- [35].Nielsen DL, Andersson M, Kamby C. HER2-targeted therapy in breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Cancer Treat Rev. 2009;35:121–136. doi: 10.1016/j.ctrv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- [36].Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- [37].Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, Keith BR, Murray DM, Knight WB, Mullin RJ, Gilmer TM. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- [38].Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- [39].Xia W, Husain I, Liu L, Bacus S, Saini S, Spohn J, Pry K, Westlund R, Stein SH, Spector NL. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67:1170–1175. doi: 10.1158/0008-5472.CAN-06-2101. [DOI] [PubMed] [Google Scholar]
- [40].Scaltriti M, Chandarlapaty S, Prudkin L, Aura C, Jimenez J, Angelini PD, Sanchez G, Guzman M, Parra JL, Ellis C, Gagnon R, Koehler M, Gomez H, Geyer C, Cameron D, Arribas J, Rosen N, Baselga J. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res. 2010;16:2688–2695. doi: 10.1158/1078-0432.CCR-09-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hutchinson L. Targeted therapies: Lapatinib is effective in patients with p95HER2-positive tumors. Nat Rev Clin Oncol. 2010;7:358. doi: 10.1038/nrclinonc.2010.93. [DOI] [PubMed] [Google Scholar]
- [42].Rusnak D, Gilmer TM. The discovery of lapatinib (GW572016) Mol Cancer Ther. 2011;10:2019. doi: 10.1158/1535-7163.MCT-11-0697. [DOI] [PubMed] [Google Scholar]
- [43].Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gomez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horvath Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M, Neo AST. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008;83:679–686. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- [45].Minkovsky N, Berezov A. BIBW-2992, a dual receptor tyrosine kinase inhibitor for the treatment of solid tumors. Curr Opin Investig Drugs. 2008;9:1336–1346. [PubMed] [Google Scholar]
- [46].Lin NU, Winer EP, Wheatley D, Carey LA, Houston S, Mendelson D, Munster P, Frakes L, Kelly S, Garcia AA, Cleator S, Uttenreuther-Fischer M, Jones H, Wind S, Vinisko R, Hickish T. A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab. Breast Cancer Res Treat. 2012;133:1057–1065. doi: 10.1007/s10549-012-2003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, Awada A, Ranade A, Jiao S, Schwartz G, Abbas R, Powell C, Turnbull K, Vermette J, Zacharchuk C, Badwe R. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- [48].Lopez-Tarruella S, Jerez Y, Marquez-Rodas I, Martin M. Neratinib (HKI-272) in the treatment of breast cancer. Future Oncol. 2012;8:671–681. doi: 10.2217/fon.12.66. [DOI] [PubMed] [Google Scholar]
- [49].Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10:345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- [50].Bostrom J, Yu SF, Kan D, Appleton BA, Lee CV, Billeci K, Man W, Peale F, Ross S, Wiesmann C, Fuh G. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science. 2009;323:1610–1614. doi: 10.1126/science.1165480. [DOI] [PubMed] [Google Scholar]
- [51].McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, Zhang B, Luus L, Overland R, Nguyen S, Gu J, Kohli N, Wallace M, Feldhaus MJ, Kudla AJ, Schoeberl B, Nielsen UB. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther. 2012;11:582–593. doi: 10.1158/1535-7163.MCT-11-0820. [DOI] [PubMed] [Google Scholar]
- [52].Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- [53].LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res. 2011;17:6437–6447. doi: 10.1158/1078-0432.CCR-11-0762. [DOI] [PubMed] [Google Scholar]
- [54].Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128:347–356. doi: 10.1007/s10549-010-1090-x. [DOI] [PubMed] [Google Scholar]
- [55].Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blattler WA, Lambert JM, Chari RV, Lutz RJ, Wong WL, Jacobson FS, Koeppen H, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- [56].Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, Guardino E, Song C, Tong B, Ng V, Chu YW, Perez EA. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–1163. doi: 10.1200/JCO.2012.44.9694. [DOI] [PubMed] [Google Scholar]
- [57].Boyraz B, Sendur MA, Aksoy S, Babacan T, Roach EC, Kizilarslanoglu MC, Petekkaya I, Altundag K. Trastuzumab emtansine (T-DM1) for HER2-positive breast cancer. Curr Med Res Opin. 2013;29:405–414. doi: 10.1185/03007995.2013.775113. [DOI] [PubMed] [Google Scholar]
- [58].Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- [59].Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN, Hung MC, Yu D. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- [60].Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- [61].She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, DeFeo-Jones D, Huber HE, Rosen N. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS One. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].DeGraffenried LA, Fulcher L, Friedrichs WE, Grunwald V, Ray RB, Hidalgo M. Reduced PTEN expression in breast cancer cells confers susceptibility to inhibitors of the PI3 kinase/Akt pathway. Ann Oncol. 2004;15:1510–1516. doi: 10.1093/annonc/mdh388. [DOI] [PubMed] [Google Scholar]
- [63].Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Raynaud FI, Eccles SA, Patel S, Alix S, Box G, Chuckowree I, Folkes A, Gowan S, De Haven Brandon A, Di Stefano F, Hayes A, Henley AT, Lensun L, Pergl-Wilson G, Robson A, Saghir N, Zhyvoloup A, McDonald E, Sheldrake P, Shuttleworth S, Valenti M, Wan NC, Clarke PA, Workman P. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther. 2009;8:1725–1738. doi: 10.1158/1535-7163.MCT-08-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Raynaud FI, Workman P. Discovering and developing PI3 kinase inhibitors for cancer: rapid progress through academic-biotech-pharma interactions. Mol Cancer Ther. 2011;10:2017–2018. doi: 10.1158/1535-7163.MCT-11-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hynes NE, Dey JH. PI3K inhibition overcomes trastuzumab resistance: blockade of ErbB2/ErbB3 is not always enough. Cancer Cell. 2009;15:353–355. doi: 10.1016/j.ccr.2009.04.004. [DOI] [PubMed] [Google Scholar]
- [67].Yao E, Zhou W, Lee-Hoeflich ST, Truong T, Haverty PM, Eastham-Anderson J, Lewin-Koh N, Gunter B, Belvin M, Murray LJ, Friedman LS, Sliwkowski MX, Hoeflich KP. Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clin Cancer Res. 2009;15:4147–4156. doi: 10.1158/1078-0432.CCR-08-2814. [DOI] [PubMed] [Google Scholar]
- [68].Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, Brachmann S, Fritsch C, Dorsch M, Chene P, Shoemaker K, De Pover A, Menezes D, Martiny-Baron G, Fabbro D, Wilson CJ, Schlegel R, Hofmann F, Garcia-Echeverria C, Sellers WR, Voliva CF. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- [69].Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7:209–219. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- [70].Miller TW, Forbes JT, Shah C, Wyatt SK, Manning HC, Olivares MG, Sanchez V, Dugger TC, de Matos Granja N, Narasanna A, Cook RS, Kennedy JP, Lindsley CW, Arteaga CL. Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin Cancer Res. 2009;15:7266–7276. doi: 10.1158/1078-0432.CCR-09-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- [72].Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- [73].O'Donnell A, Faivre S, Burris HA, 3rd, Rea D, Papadimitrakopoulou V, Shand N, Lane HA, Hazell K, Zoellner U, Kovarik JM, Brock C, Jones S, Raymond E, Judson I. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- [74].Morrow PK, Wulf GM, Ensor J, Booser DJ, Moore JA, Flores PR, Xiong Y, Zhang S, Krop IE, Winer EP, Kindelberger DW, Coviello J, Sahin AA, Nunez R, Hortobagyi GN, Yu D, Esteva FJ. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Stern HM. Improving treatment of HER2-positive cancers: opportunities and challenges. Sci Transl Med. 2012;4:127rv122. doi: 10.1126/scitranslmed.3001539. [DOI] [PubMed] [Google Scholar]
- [76].Nahta R, O'Regan RM. Evolving strategies for overcoming resistance to HER2-directed therapy: targeting the PI3K/Akt/mTOR pathway. Clin Breast Cancer. 2010;10(Suppl 3):S72–78. doi: 10.3816/CBC.2010.s.015. [DOI] [PubMed] [Google Scholar]
- [77].Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, Garcia-Echeverria C. Identification and characterization of NVP BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- [78].Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, Beijersbergen RL, Valero V, Seoane J, Bernards R, Baselga J. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, Maira M, Garcia-Echeverria C, Parra JL, Arribas J, Baselga J. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- [80].Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- [81].Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- [83].Sangai T, Akcakanat A, Chen H, Tarco E, Wu Y, Do KA, Miller TW, Arteaga CL, Mills GB, Gonzalez-Angulo AM, Meric-Bernstam F. Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. Clin Cancer Res. 2012;18:5816–5828. doi: 10.1158/1078-0432.CCR-12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, Baird RD, Delgado L, Taylor A, Lupinacci L, Riisnaes R, Pope LL, Heaton SP, Thomas G, Garrett MD, Sullivan DM, de Bono JS, Tolcher AW. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- [85].Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- [86].Grant S, Dai Y. Histone deacetylase inhibitors and rational combination therapies. Adv Cancer Res. 2012;116:199–237. doi: 10.1016/B978-0-12-394387-3.00006-9. [DOI] [PubMed] [Google Scholar]
- [87].Fuino L, Bali P, Wittmann S, Donapaty S, Guo F, Yamaguchi H, Wang HG, Atadja P, Bhalla K. Histone deacetylase inhibitor LAQ824 down-regulates Her-2 and sensitizes human breast cancer cells to trastuzumab, taxotere, gemcitabine, and epothilone B. Mol Cancer Ther. 2003;2:971–984. [PubMed] [Google Scholar]
- [88].Wagner JM, Hackanson B, Lubbert M, Jung M. Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin Epigenetics. 2010;1:117–136. doi: 10.1007/s13148-010-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Campone PCM, Amadori D, Pronzato P, Wardley A, McBride K, Fandi A. Phase I Trial of Panobinostat (LBH589) in Combination with Trastuzumab in Pretreated HER2-Positive Metastatic Breast Cancer (mBC): Preliminary Safety, Efficacy and Pharmacokinetic Results. Cancer Res. 2009;69:6101. [Google Scholar]
- [90].Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Leow CC, Chesebrough J, Coffman KT, Fazenbaker CA, Gooya J, Weng D, Coats S, Jackson D, Jallal B, Chang Y. Antitumor efficacy of IPI-504, a selective heat shock protein 90 inhibitor against human epidermal growth factor receptor 2-positive human xenograft models as a single agent and in combination with trastuzumab or lapatinib. Mol Cancer Ther. 2009;8:2131–2141. doi: 10.1158/1535-7163.MCT-08-1038. [DOI] [PubMed] [Google Scholar]
- [92].Scaltriti M, Serra V, Normant E, Guzman M, Rodriguez O, Lim AR, Slocum KL, West KA, Rodriguez V, Prudkin L, Jimenez J, Aura C, Baselga J. Antitumor activity of the Hsp90 inhibitor IPI-504 in HER2-positive trastuzumab-resistant breast cancer. Mol Cancer Ther. 2011;10:817–824. doi: 10.1158/1535-7163.MCT-10-0966. [DOI] [PubMed] [Google Scholar]
- [93].Chandarlapaty S, Scaltriti M, Angelini P, Ye Q, Guzman M, Hudis CA, Norton L, Solit DB, Arribas J, Baselga J, Rosen N. Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene. 2010;29:325–334. doi: 10.1038/onc.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Grenert JP, Sullivan WP, Fadden P, Haystead TA, Clark J, Mimnaugh E, Krutzsch H, Ochel HJ, Schulte TW, Sausville E, Neckers LM, Toft DO. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- [96].Schulte TW, Neckers LM. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother Pharmacol. 1998;42:273–279. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]
- [97].Arteaga CL. Why is this effective HSP90 inhibitor not being developed in HER2+ breast cancer? Clin Cancer Res. 2011;17:4919–4921. doi: 10.1158/1078-0432.CCR-11-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, Ma W, Wheler J, Rosen N, Norton L, Cropp GF, Johnson RG, Hannah AL, Hudis CA. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- [99].Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, D'Andrea G, Dickler M, Moynahan ME, Sugarman S, Ma W, Patil S, Norton L, Hannah AL, Hudis C. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17:5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- [100].Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- [101].King ER, Wong KK. Insulin-like growth factor: current concepts and new developments in cancer therapy. Recent Pat Anticancer Drug Discov. 2012;7:14–30. doi: 10.2174/157489212798357930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gooch JL, Van Den Berg CL, Yee D. Insulin-like growth factor (IGF)-I rescues breast cancer cells from chemotherapy-induced cell death--proliferative and anti-apoptotic effects. Breast Cancer Res Treat. 1999;56:1–10. doi: 10.1023/a:1006208721167. [DOI] [PubMed] [Google Scholar]
- [103].Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- [104].Buck E, Mulvihill M. Small molecule inhibitors of the IGF-1R/IR axis for the treatment of cancer. Expert Opin Investig Drugs. 2011;20:605–621. doi: 10.1517/13543784.2011.558501. [DOI] [PubMed] [Google Scholar]
- [105].Harris LN, You F, Schnitt SJ, Witkiewicz A, Lu X, Sgroi D, Ryan PD, Come SE, Burstein HJ, Lesnikoski BA, Kamma M, Friedman PN, Gelman R, Iglehart JD, Winer EP. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res. 2007;13:1198–1207. doi: 10.1158/1078-0432.CCR-06-1304. [DOI] [PubMed] [Google Scholar]
- [106].Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- [107].Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- [108].Lu Y, Zi X, Pollak M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int J Cancer. 2004;108:334–341. doi: 10.1002/ijc.11445. [DOI] [PubMed] [Google Scholar]
- [109].Esparis-Ogando A, Ocana A, Rodriguez-Barrueco R, Ferreira L, Borges J, Pandiella A. Synergic antitumoral effect of an IGF-IR inhibitor and trastuzumab on HER2-overexpressing breast cancer cells. Ann Oncol. 2008;19:1860–1869. doi: 10.1093/annonc/mdn406. [DOI] [PubMed] [Google Scholar]
- [110].Rodon J, DeSantos V, Ferry RJ, Jr., Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther. 2008;7:2575–2588. doi: 10.1158/1535-7163.MCT-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Carboni JM, Wittman M, Yang Z, Lee F, Greer A, Hurlburt W, Hillerman S, Cao C, Cantor GH, Dell-John J, Chen C, Discenza L, Menard K, Li A, Trainor G, Vyas D, Kramer R, Attar RM, Gottardis MM. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther. 2009;8:3341–3349. doi: 10.1158/1535-7163.MCT-09-0499. [DOI] [PubMed] [Google Scholar]
- [112].McKian KP, Haluska P. Cixutumumab. Expert Opin Investig Drugs. 2009;18:1025–1033. doi: 10.1517/13543780903055049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549s–5555s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- [114].Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- [115].Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- [116].Banerjee S, Dowsett M, Ashworth A, Martin LA. Mechanisms of disease: angiogenesis and the management of breast cancer. Nat Clin Pract Oncol. 2007;4:536–550. doi: 10.1038/ncponc0905. [DOI] [PubMed] [Google Scholar]
- [117].Konecny GE, Meng YG, Untch M, Wang HJ, Bauerfeind I, Epstein M, Stieber P, Vernes JM, Gutierrez J, Hong K, Beryt M, Hepp H, Slamon DJ, Pegram MD. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004;10:1706–1716. doi: 10.1158/1078-0432.ccr-0951-3. [DOI] [PubMed] [Google Scholar]
- [118].Rugo HS. Bevacizumab in the treatment of breast cancer: rationale and current data. Oncologist. 2004;9(Suppl 1):43–49. doi: 10.1634/theoncologist.9-suppl_1-43. [DOI] [PubMed] [Google Scholar]
- [119].Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, Chan S, Wardley A, Greil R, Moore N, Prot S, Pallaud C, Semiglazov V. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31:1719–1725. doi: 10.1200/JCO.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- [120].Abramson V, Arteaga CL. New strategies in HER2-overexpressing breast cancer: many combinations of targeted drugs available. Clin Cancer Res. 2011;17:952–958. doi: 10.1158/1078-0432.CCR-09-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. O. American Society of Clinical, P. College of American, American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- [122].Paik S, Bryant J, Tan-Chiu E, Romond E, Hiller W, Park K, Brown A, Yothers G, Anderson S, Smith R, Wickerham DL, Wolmark N. Real-world performance of HER2 testing--National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002;94:852–854. doi: 10.1093/jnci/94.11.852. [DOI] [PubMed] [Google Scholar]
- [123].Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409–1411. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- [124].Rexer BN, Engelman JA, Arteaga CL. Overcoming resistance to tyrosine kinase inhibitors: lessons learned from cancer cells treated with EGFR antagonists. Cell Cycle. 2009;8:18–22. doi: 10.4161/cc.8.1.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]