Abstract
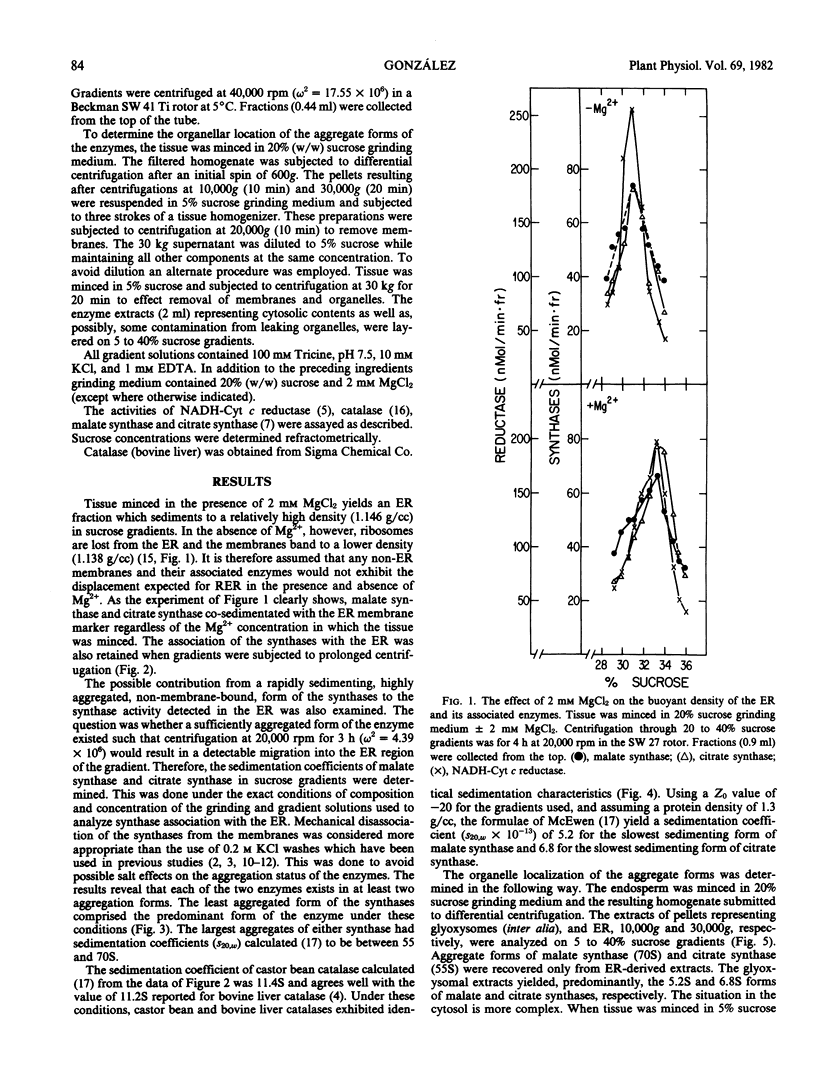
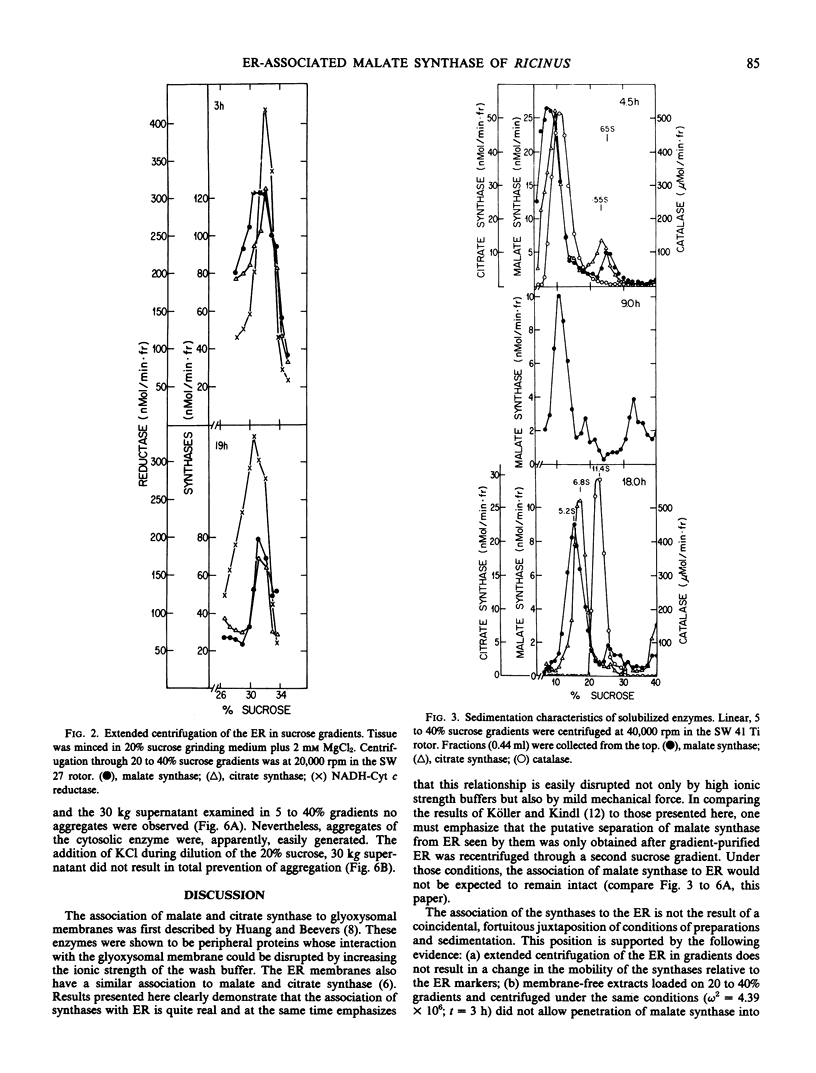
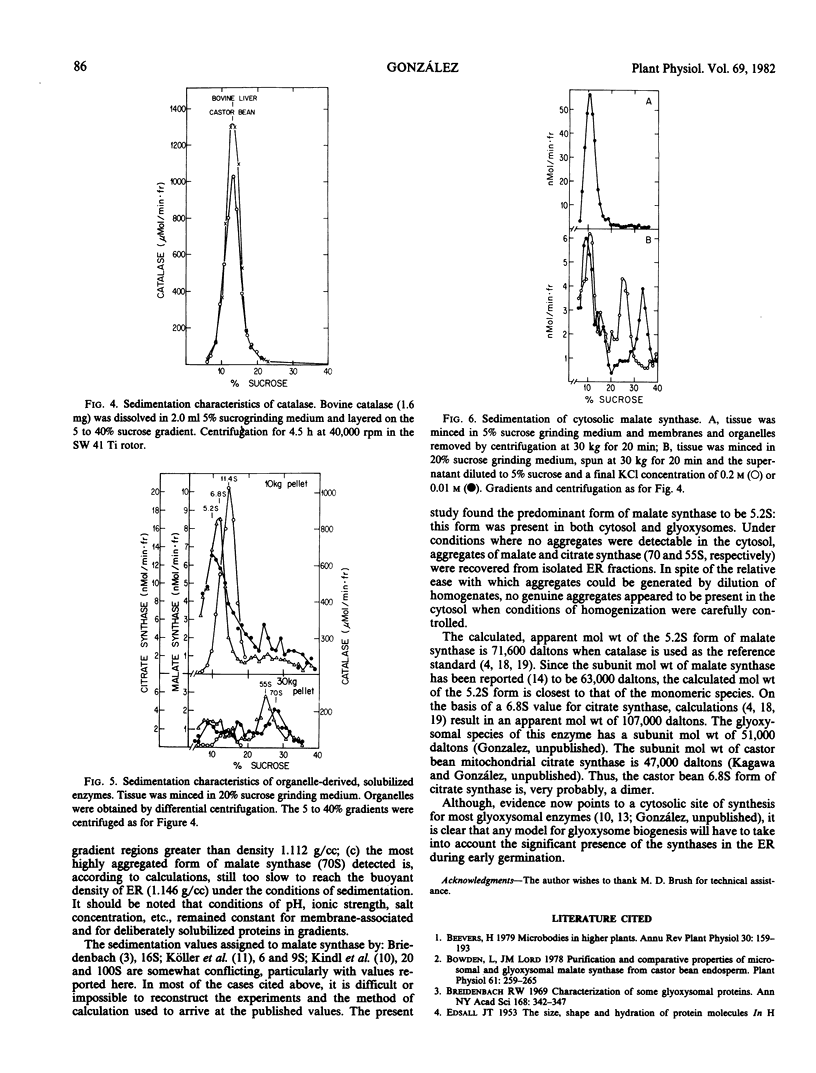
The endosperm of 3-day germinated seedlings of Ricinus communis was homogenized in the presence or absence of Mg2+. When the Mg2+ -containing homogenate was fractionated on linear, 20 to 40% sucrose gradients, the endoplasmic reticulum (ER) reached equilibrium at a density of 1.146 grams per cubic centimeter. Absence of Mg2+ in the grinding medium resulted in displacement of the ER in the gradient from a density of 1.146 to 1.138 grams per cubic centimeter. At either density, the activities of both malate and citrate synthase were found to overlap the activity of NADH-cytochrome c reductase (an ER marker) in the gradient. Furthermore, this overlap of activities was observed whether the gradients were centrifuged for 3 or 19 hours. An analysis of sedimentation characteristics of the solubilized enzymes revealed that they exist, predominantly, as a 5.2S (s20,w × 10−13) form (malate synthase) and a 6.8S form (citrate synthase) in the glyoxysomes and cytosol. When the two enzymes were released from the ER, they appeared as aggregate forms of 70S and 55S, respectively. These results support the conclusion that the synthases are associated with the ER.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden L., Lord J. M. Purification and comparative properties of microsomal and glyoxysomal malate synthase from castor bean endosperm. Plant Physiol. 1978 Feb;61(2):259–265. doi: 10.1104/pp.61.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W. Characterization of some glyoxysomal proteins. Ann N Y Acad Sci. 1969 Dec 19;168(2):342–347. doi: 10.1111/j.1749-6632.1969.tb43120.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez E., Beevers H. Role of the endoplasmic reticulum in glyoxysome formation in castor bean endosperm. Plant Physiol. 1976 Mar;57(3):406–409. doi: 10.1104/pp.57.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González E. Effect of gibberellin a(3) on the endoplasmic reticulum and on the formation of glyoxysomes in the endosperm of germinating castor bean. Plant Physiol. 1978 Sep;62(3):449–453. doi: 10.1104/pp.62.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T. Organelle-specific isozymes of citrate synthase in the endosperm of developing ricinus seedlings. Plant Physiol. 1981 Oct;68(4):845–850. doi: 10.1104/pp.68.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindl H., Köller W., Frevert J. Cytosolic precursor pools during glyoxysome biosynthesis. Hoppe Seylers Z Physiol Chem. 1980;361(3):465–467. [PubMed] [Google Scholar]
- Köller W., Frevert J., Kindl H. Albumins, glyoxysomal enzymes and globulins in dry seeds of cucumis sativus: qualitative and quantitative analysis. Hoppe Seylers Z Physiol Chem. 1979 Feb;360(2):167–176. doi: 10.1515/bchm2.1979.360.1.167. [DOI] [PubMed] [Google Scholar]
- Köller W., Kindl H. 19S cytosolic malate synthase. A small pool characterized by rapid turnover. Hoppe Seylers Z Physiol Chem. 1980 Sep;361(9):1437–1444. doi: 10.1515/bchm2.1980.361.2.1437. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Bowden L. Evidence that glyoxysomal malate synthase is segregated by the endoplasmic reticulum. Plant Physiol. 1978 Feb;61(2):266–270. doi: 10.1104/pp.61.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. R., Shelton J. B., Robberson B., Apell G. The amino acid sequence of bovine liver catalase: a preliminary report. Arch Biochem Biophys. 1969 May;131(2):653–655. doi: 10.1016/0003-9861(69)90441-x. [DOI] [PubMed] [Google Scholar]


