Abstract
Objective
Approximately 50% of human malignancies present with mutations in p53, which is the most common tumor suppressor gene involved with human malignancies. Bcl-2 is a protooncogene, and expression of its protein product is associated with a better prognosis in several malignancies. Ki-67 is a marker of cellular proliferation. The purpose of this study was to determine whether simultaneous detection of p53, bcl-2 and Ki-67 using immunohistochemical staining can be used as a diagnostic factor in the assessment of human ovarian epithelial tumors.
Materials and Methods
The study was performed on formalin-fixed, paraffin-embedded tissue samples from 75 epithelial ovarian tumors, 15 serous cystadenomas, 15 mucinous cystadenomas, 5 borderline serous cystadenomas, 5 borderline mucinous cystadenomas, 15 serous cystadenocarcinomas, 15 mucinous cystadenocarcinomas and 5 endometrioid carcinomas. Immunohistochemical staining was performed using monoclonal antibodies against p53, bcl-2, and Ki-67(MIB1).
Results
Anti-p53 reactivity was observed in 14 tumors, all of which were malignant tumors, and no reactivity was observed in borderline or benign tumors. Overexpression of bcl-2 was observed in 12 benign neoplasms (40%), 5 of which were borderline (50%), but was not observed in any of the malignant tumors. There was a statistically significantly higher level of Ki-67 LI positivity in the malignant tumors than in the benign and borderline tumors (p<0.005).
Conclusion
These data show significant differences in the expression of these markers in ovarian tumors and suggest a possible role for these tumor-associated genes as supplemental tools in diagnostic pathology. Furthermore, our findings support the redesignation of low malignant potential tumors (current nomenclature) to benign ovarian carcinoma.
Keywords: Ovarian cancer, p53, bcl-2, Ki-67
Özet
Amaç
p53 insan malignesilerinde mutasyonu en sık izlenen tümör baskılayıcı gendir. Bütün kanserlerin %50 sinde izlenir. Bcl-2 ise çeşitli kanserlerde görülen, protein ürünü iyi prognozla ilişkili olan bir protoonkogendir. Ki-67 ise hücre proliferasyon markırıdır. Bu çalışmanın amacı overin epitelyal tümörlerinde immunohistokimyasal çalışma yaparak p53, bcl-2 ve Ki-67’nin tanıda yerini değerlendirmektir.
Gereç ve Yöntem
Bu çalışma; 15 seröz ve müsinöz kistadenom, 15 seröz ve müsinöz kistadenokarsinom, 5 borderline seröz ve müsinöz kistadenom ve 5 endometroid karsinomalı vakalara ait formalin ile fikse parafin bloklarda yapıldı. İmmunohistokimyasal olarak anti-p53, the anti-bcl-2, the anti-Ki-67(MIB1) uygulandı.
Bulgular
Anti-p53 aktivitesi bütün malign tümörlerin 14’ünde pozitif olup borderline ve benign tümörlerde boyanma izlenmedi. Bcl-2 overekspresyonu 12 (%40) benign tümörde, 5 (%50) borderline tümörde izlendi fakat malign tümörlerde gözlenmedi. Ki-67 LI ise malign tümörlerde benign ve borderline tümörlere göre istatistik olarak anlamlı daha yüksekti (p<0.005).
Sonuç
Bu veriler over tümörlerinde bu markırların tanıda kullanılabileceğini, özellikle düşük malign potansiyelli tümörler ile benign vakaların ayırımında faydalı olabileceğini göstermektedir.
Introduction
Ovarian surface epithelial tumors represent the most common lethal gynecologic neoplasms for women of reproductive age and older [1–5] and continue to present a challenge despite advances in our knowledge of the disease over the past 20 years [6]. These tumors display biological behaviors that follow their histopathological grading of malignant, borderline or low malignant potential (LMP), or benign. Of particular interest are those classified as borderline or LMP because the pathologist must rely on somewhat vague and poorly reproducible morphological criteria. These include architectural criteria, such as the increased complexity of papillary excrescences with the stratification of epithelial nuclei and epithelial budding or tufting in the absence of stromal invasion, and cytological criteria, such as nuclear atypia and mitosis. Clinically, these LMP lesions display a more indolent behavior, with an overall 10-year survival rate of 80–90% [7].
Previous studies have shown that the p53 gene is mutated in 30-80% of ovarian carcinomas [1,8]. The role of p53 in ovarian cancer is contentious, as there are a number of contradictory studies. Several studies have identified p53 protein expression, detected by immunohistochemistry, as an adverse prognostic factor for survival in human ovarian cancer [9–11]. Other studies have suggested that alterations in p53 expression in ovarian cancer affect sensitivity to chemotherapy [12]. In contrast, there are a number of studies that suggest that p53 expression has no prognostic value in epithelial ovarian cancer [5,13–15].
The role of bcl-2 in gynecological malignancies has been investigated [5]. Expression of bcl-2 has been correlated with improved survival in ovarian cancer [16,17].
The proliferation index has been correlated with prognosis and other clinicopathological features in a number of human malignancies [18–20]. Expression of the Ki-67 proliferation marker, which detects all phases of the cell cycle except G0, is known to predict disease outcome in many human malignancies [20]. The tumor proliferative fraction of ovarian carcinomas has been investigated immunohistochemically by means of antibodies that recognize the nuclear antigen Ki-67 expressed in proliferating cells. A number of studies on ovarian cancer have reported an association between high proliferation indexes and reduced overall survival or reduced disease-free survival [21,22]. Multivariate analysis revealed a significant relationship between high Ki-67 immunostaining in ovarian neoplasms and disease-free survival and also found that high proliferation was associated with poor prognosis for ovarian cancer in both univariate and multivariate analyses [23,24].
The aim of this study was to better define the biology of surface ovarian neoplasms using monoclonal antibodies (MoAbs) that recognize P-53, Ki-67 and Bcl-2.
Patients and Methods
Case Selection
A total of 75 cases of ovarian surface epithelial neoplasms, including 15 serous cystadenomas, 15 mucinous cystadenomas, 5 borderline serous cystadenomas, 5 borderline mucinous cystadenomas, 15 serous cystadenocarcinomas, 15 mucinous cystadenocarcinomas and 5 endometrioid carcinomas, were retrieved from the Surgical Pathology Archives, Medical Faculty Atatürk University between 1996–2001. The diagnoses were reviewed, and a representative block was selected from each case for immunohistochemical analysis.
Immunohistochemistry
Parallel 4-mm serial sections cut from paraffin-embedded tumor blocks were mounted on Superfrost glass slides, dewaxed and rehydrated in a graded alcohol series, followed by microwave antigen target retrieval. Immunostaining was performed using the rabbit or mouse DAKO EnVision(TM)+System, Peroxidase (DAB) Kit (DAKO). The sections were incubated for 35 min with the following primary antibodies at the dilutions specified: mouse monoclonal anti-bcl-2 antibody (Clone 124) (DAKO) diluted 1:40; mouse monoclonal anti-p53 antibody (DO-1) (Santa Cruz Biotechnology) diluted 1:200; and monoclonal mouse anti-Ki-67 antibody (Clone 7B11) diluted 1:100.
Each stained section and the controls were examined via light microscopy by two independent observers who were blinded to both the clinicopathological data and the results from the scoring for each section with each of the other primary antibodies. Tissues with defined expression or absence of expression of a particular antigen were used as positive and negative controls, respectively. Each section was examined for positive staining in the cytoplasm and nuclei of the malignant cells, the absolute numbers of malignant cells stained, as well as the pattern and `intensity’ of the staining pattern in the sections.
Statistical analysis
The Chi-square test for contingency tables or the Fisher’s exact test were performed to determine if there was a difference in the Ki-67 staining between proliferative and malignant lesions, with the hypothesis that P-53 and bcl-2 are expressed to a significantly greater degree in malignant compared to proliferative tumors. All statistical analyses were performed using the SAS statistical software package.
Results
Tumor suppressor protein (p53)
None of the benign tumors and ten of the proliferative neoplasms were observed to have immunoreactivity with anti-p53. However, expression of p53 protein, which was found exclusively in the nucleus of the epithelial cells, was frequently found in 40% of the malignant ovarian cells. Nine out of 14 positive patients had a serous cystadenocarcinomas. There is a statistically significant difference in the expression of p53 between malignant and benign tumors (p< 0.005, chi square test).
bcl-2
Bcl-2 immunoreactive products appeared as brown granules that were predominantly localized in the perinuclear locations and cytoplasmic regions of epithelial cells, which presumably are locations for mitochondria and endoplasmic reticulum. Bcl-2 cytoplasmic staining was only observed in 17 of the 75 (22.7%) patient tumors. Twelve of the 18 benign tumors and 5 of the 10 borderline tumors demonstrated bcl-2 expression. Overexpression of bcl-2 was most frequently observed in serous cystadenomas and this association was highly significant (p<0.001). Malignant tumors showed no bcl-2 reactivity (Table 1, Figure 2).
Table 1.
P53, bcl-2, Ki-67 Li (labeling index) status in benign, proliferative, and malignant ovarian surface epithelial neoplasms
| P53 | Bcl-2 | Ki-67 | |||
|---|---|---|---|---|---|
| Tumor type (n) | Negative | Positive | Negative | Positive | Labeling Index |
| Benign (30) | 30 | 0 | 18 | 12 | 14.9% |
| Borderline (10) | 10 | 0 | 5 | 5 | 22,8% |
| Malign (35) | 21 | 14 | 35 | 0 | 42,8% |
| Grade I (14) | 13 | 1 | 14 | 0 | 25.9% |
| Grade II (8) | 4 | 4 | 8 | 0 | 49.8% |
| Grade III (13) | 4 | 9 | 13 | 0 | 52.7% |
Fig. 2.
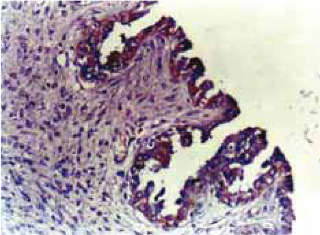
Positive bcl-2 immunostaining.
Ki-67
The staining pattern of the Ki-67 antigen is shown in figure 3 and Ki-67 LI is shown in table 2. The mean Ki-67 LI in benign tumors was 14.9% (0.7–32.5%); in borderline tumors it was 22.8% (0.2–61.2%); in malign tumors it was 42.8% (0.4–84.2%). When compared with the benign tumors, Ki-67 LI was found to be significantly increased in the malign tumors. For example, Ki-67 LI was 52.7% in serous cystadeno-carcinoma, 39.7% in endometrioid carcinoma and 33.2% in mucinous cystadenocarcinoma.
Fig. 3.
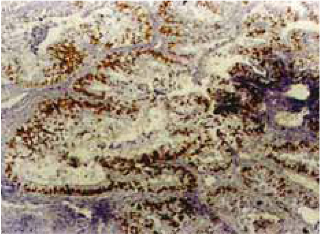
Positive Ki-67 immunostaining.
For all of the malignant ovarian tumors examined, high grade tumors were associated with a high mean Ki-67 LI. Among the malignant ovarian tumors examined, the mean Ki-67 LI for grade III tumors (52.7%) was the highest, whereas the Ki-67 LI was the lowest for grade I tumors (25.9%) and the index for grade II tumors was an intermediate value (49.8%).
Expression of p53 and Ki-67 LI were significantly higher in the bcl-2 negative tumors than in the bcl-2 positive ones (p<0.005).
Discussion
More than 100 cancer related genes have been discovered, several of which have been implicated in the natural history of human cancer because they consistently are found to be mutated in tumors [1,3,4]. The p53 tumor suppressor gene is the most striking example because it is mutated in approximately half of a wide spectrum of tissues [6,25,26]. The two main methods for studying p53 are sequence analysis and immunohistochemical staining. Immunohistochemistry is used to detect approximately 90% of the p53 mutations [1]. Positive p53 staining has been associated with more frequent cancer relapse and aggressive tumors [1,6,27]. Marks et al. [28] analyzed p53 staining in ovarian tissue and found positive staining in 50% of malignant tumors but no staining in benign tissue. The presence of mutant p53 protein has been significantly associated with high histological grade. p53 gene mutations are most typically observed in ovarian cancer patients with stage III or IV disease and appear at a lower frequency in the early stages of the disease [1]. Wen [29] noted that positive staining was related to stage. Michael et al. [3] noted that the rate of p53 overexpression was 5%, 17%, 57%, 67% for stage I–IV, respectively (p<0.002).
The relationship between p53 mutation or p53 protein expression and the histopathological subtype in ovarian carcinomas is still controversial [6]. The highest incidence of p53 positivity was reported in serous adenocarcinomas (61%) by Milner et al [30] and by Gotlier and Berek [31]; whereas in other studies, no significant relationship between histopathological subtype and p53 expression could be found [1,6,26]. The lowest rate of p53 expression was found in endometroid carcinoma. In our series, no significant relationship between histopathological subtype and p53 expression could be found. The P53 gene is not important in the pathogenesis of borderline ovarian tumors [2]. In a study of 79 LMP ovarian tumors, Kupryjanczyk et al. [32] 40 of the tumors were observed to be immunoreactive for anti-p53 in 14% of the tumors with no significant differences among various histological variants. Michael [1] demonstrated that 10.5% of borderline ovarian tumors and 1 of 48 ovarian cancers presented in a study by Wertheim et al. [29] showed anti-p53 reactivity. In this series, none of the ten proliferative tumors showed reactivity. Therefore, immunohistochemistry is a good screening method that can be used to predict malignant versus proliferative tumors. Expression of bcl-2 has been correlated with improved survival in ovarian cancer [8]. The strongest bcl-2 immunoreactivity was detected in benign and borderline tumors, whereas malignant tumors showed moderate immunoreactivity [2,33,34]. Bcl-2 expression has been associated with the histopathological subtype, and positive staining has most frequently been observed in serous (77%) and endometroid (56%) carcinomas, but was less common in mucinous carcinomas (20%) [6]. Diebold et al.[16] demonstrated that intense bcl-2 expression is associated with a low tumor grade in a study of 118 patients with ovarian cancer in FIGO stages I–IV and it is seen most often in endometroid carcinomas [32]. Among the malignant tumors, the strongest bcl-2 immunoreactivity was detected in grade I tumors, whereas grade II and III malignant tumors showed moderate immunoreactivities [2]. The protein expression patterns of p53 and bcl-2 negatively correlated during ovarian carcinogenesis. Chan et al.[2] identified a p53-regulating domain present in the 5´untranslated region of bcl-2 gene that was able to inhibit bcl-2 expression and showed that downregulation of bcl-2 expression may be a result of the inhibitory effect of p53 expression on bcl-2 during ovarian carcinogenesis [2]. In our series, a significantly negative relationship between bcl-2 and p53 expression was found (p<0.005).
The proliferation index has been correlated with prognosis and other clinicopathological features in a number of human malignancies [18–20]. The tumor proliferative fraction of ovarian carcinomas has been investigated immunohistochemically by means of antibodies that recognize the nuclear antigen Ki-67 expressed in proliferating cells. Expression of the Ki-67 proliferation marker, which detects all phases of the cell cycle except G0, is known to predict disease outcome in many human malignancies [20]. Multivariate analysis revealed a significant relationship between high Ki-67 immunostaining in ovarian neoplasms and disease-free survival and also found that high proliferation was associated with poor prognosis for ovarian cancer in both univariate and multivariate analyses [5,23]. However, no association was found with age, FIGO stage, or tumor grade [21]. At the same time, Ki-67 and p53 have been reported to be used in a parallel manner [35]. We also confirmed this finding. In our study, there was a statistically significant correlation between the expression of p53 and Ki-67 LI (P<0.005).
Although the staining pattern for p53, bcl-2, and Ki-67 in predicting malignant versus borderline tumors is statistically significant, light microscopy remains the best method to evaluate these types of lesions. In difficult cases, such a panel of markers (or one similar to this) may be helpful in the diagnosis and may further define the biologic potential of a specific tumor. Also, additional studies of borderline lesions using multiple tumor markers with follow-up data and histopathological correlation are warranted to further define the true biologic behavior of these tumors.been shown [16,17]. For smaller vessels (e.g., coronary or cerebral arteries) imaging of the lumen, neointimal hyperplasia or in-stent re-stenosis is more challenging.
Fig. 1.

Positive p53 immunostaining.
Footnotes
Conflict interest statement The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Levesque MA, Katsaros D, Zola P, et al. Mutant p53 protein overexpression is associated with poor outcome in patients with well or moderately differentiated ovarian carcinoma. Cancer. 1995;75:1327–38. doi: 10.1002/1097-0142(19950315)75:6<1327::aid-cncr2820750615>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Chan WY, Cheung KK, Schorge JO, et al. Bcl-2 and p53 protein expression, apoptosis, and p53 mutation in human epithelial ovarian cancers. Am J Pathol. 2000;156:409–17. doi: 10.1016/S0002-9440(10)64744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox H. Obstetrical and Gynaecological Pathology. London: Wiliam Clowes; 1987. pp. 542–55. [Google Scholar]
- 4.Saretzki G, Hoffmann U, Rohlke P, et al. Identification of allelic losses in benign, borderline, and invasive epithelial ovarian tumors and correlation with clinical outcome. Cancer. 1997;80:1241–9. doi: 10.1002/(sici)1097-0142(19971001)80:7<1241::aid-cncr7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Garzetti G, Ciavattini A, Goteri G, et al. Ki-67 antigen immunostaining (MIB 1 monoclonal antibody) in serous ovarian tumours: index of proliferative activity with prognostic significance. Gynaecol Oncol. 1995;56:169–74. doi: 10.1006/gyno.1995.1026. [DOI] [PubMed] [Google Scholar]
- 6.Skirnisdottir I, Sorbe B, Seidal T. P53, bcl-2, and bax: their relationship and effect on prognosis in early stage epithelial ovarian carcinoma. Int J Gynecol Cancer. 2001;11:147–58. doi: 10.1046/j.1525-1438.2001.01003.x. [DOI] [PubMed] [Google Scholar]
- 7.Anreder MB, Freeman SM, Merogi A, Halabi S. P53, c-erb-2, and PCNA status in benign, proliferative and malignant ovarian surface epithelial neoplasms: a study of 75 cases. Arch Pathol Lab Med. 1999;123:310–6. doi: 10.5858/1999-123-0310-PCEAPS. [DOI] [PubMed] [Google Scholar]
- 8.Angelopoulou K, Rosen B, Stratis M, et al. Circulating antibodies against p53 protein in patients with ovarian carcinoma. Correlation with clinicopathologic features and survival. Cancer. 1996;78:2146–52. [PubMed] [Google Scholar]
- 9.Henriksen R, Strang P, Wilnder E, et al. P53 expression in epithelial ovarian neoplasms: relationship to clinical and pathological parameters, Ki-67 expression and flow cytometry. Gynaecol Oncol. 1994;53:301–6. doi: 10.1006/gyno.1994.1138. [DOI] [PubMed] [Google Scholar]
- 10.Levesque MA, Katsaros D, Zola D, et al. Mutant p53 protein overexpression is associated with poor outcome in patients with well or moderately differentiated ovarian carcinoma. Cancer. 1995;75:1327–38. doi: 10.1002/1097-0142(19950315)75:6<1327::aid-cncr2820750615>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Klemi P, Pylkkänen L, Kiilholma P, et al. P53 protein detected by immunohistochemistry as a prognostic factor in patients with epithelial ovarian carcinoma. Cancer. 1995;76:1201–8. doi: 10.1002/1097-0142(19951001)76:7<1201::aid-cncr2820760716>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Buttitta F, Marchetti F, Gadducci A, et al. P53 alterations are predictive of chemoresistance and aggressiveness in ovarian carcinomas: a molecular and immunohistochemical study. Br J Cancer. 1997;75:230–5. doi: 10.1038/bjc.1997.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks JR, Davidoff AM, Kerns BJ, et al. Over-expression and mutation of p53 in epithelial ovarian cancer. Cancer Res. 1991;51:2979–84. [PubMed] [Google Scholar]
- 14.Niwa K, Itoh M, Murase T. Alteration of p53 gene in ovarian carcinoma: clinicopathological correlation and prognostic significance. Br J Cancer. 1994;70:1191–7. doi: 10.1038/bjc.1994.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahin MS, Hughes JH, Sood AK, Buller RE. The prognostic significance of p53 tumor suppressor gene alterations in ovarian carcinoma. Cancer. 2000;89:2006–17. doi: 10.1002/1097-0142(20001101)89:9<2006::aid-cncr18>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Diebold J, Baretton G, Felchner M, et al. Bcl-2 expression, p53 accumulation and apoptosis in ovarian carcinomas. Am J Clin Pathol. 1996;105:341–9. doi: 10.1093/ajcp/105.3.341. [DOI] [PubMed] [Google Scholar]
- 17.Herod JO, Eliopoulos AG, Warwick J, et al. The prognostic significance of bcl-2 and p53 expression in ovarian carcinoma. Cancer Res. 1996;56:2178–84. [PubMed] [Google Scholar]
- 18.Muggia FM, Krezorsk SK, Hansen H. Cell kinetic studies in patients with small cell carcinoma of the lung. Cancer. 1974;34:1683–90. doi: 10.1002/1097-0142(197411)34:5<1683::aid-cncr2820340516>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz BR, Pinkus G, Bacus S, et al. Cell proliferation in non-Hodgkins lymphoma. Digital image analysis of Ki-67 antibody staining. Am J Pathol. 1989;134:327–36. [PMC free article] [PubMed] [Google Scholar]
- 20.Hall PA, Levison DA. Review: assessment of cell proliferation in histological material. J Clin Pathol. 1990;43:184–92. doi: 10.1136/jcp.43.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnabei VM, Miller DS, Bauer KD, et al. Flow cytometric evaluation of epithelial ovarian cancer. Am J Obstet Gynaecol. 1990;162:1584–90. doi: 10.1016/0002-9378(90)90924-v. [DOI] [PubMed] [Google Scholar]
- 22.Isola J, Kallioniemi OP, Korte JM, et al. Steroid receptors and Ki-67 reactivity in ovarian cancer and in normal ovary: correlation with DNA flow cytometry, biochemical receptor assay, and patient survival. J Pathol. 1990;162:295–301. doi: 10.1002/path.1711620404. [DOI] [PubMed] [Google Scholar]
- 23.Anttila M, Kosma VM. Clinical significance of alpha-catenin, collagen IV, and Ki-67 expression in epithelial ovarian cancer. J Clin Oncol. 1998;16:2591–600. doi: 10.1200/JCO.1998.16.8.2591. [DOI] [PubMed] [Google Scholar]
- 24.Röhlke P, Milde-Langosch K, Weyland C, et al. P53 Is a persistant and predictive marker in advanced ovarian carcinomas: multivariate analysis including comparison with Ki-67 immunoreactivity. J Cancer Res Clin Oncol. 1997;123:496–501. doi: 10.1007/BF01192204. [DOI] [PubMed] [Google Scholar]
- 25.McKee PH, Hobbs C, Hall PA. Antigen retrieval by microwave irradiation lowers immunohistological detection thresholds. Histopathology. 1993;23:377–9. doi: 10.1111/j.1365-2559.1993.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 26.Geisler JP, Geisler HE, Wiemann MC, et al. Quantification of p53 in epithelial ovarian cancer. Gynecol Oncol. 1997;66:435–8. doi: 10.1006/gyno.1997.4799. [DOI] [PubMed] [Google Scholar]
- 27.Klemi PJ, Pylkkanen L, Kiilholma P, et al. p53 protein detected by immunohistochemistry as a prognostic factor in patients with epithelial ovarian carcinoma. Cancer. 1995;76:1201–8. doi: 10.1002/1097-0142(19951001)76:7<1201::aid-cncr2820760716>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Marks JR, Davidoff AM, Kerns BJ, et al. Over-expression and mutation of p53 in epithelial ovarian cancer. Cancer Res. 1991;1:2979–84. [PubMed] [Google Scholar]
- 29.Wen WH, Reles A, Runnebaum IB, et al. p53 mutations and expression in ovarian cancers: correlation with overall survival. Int J Gynecol Pathol. 1999;18:29–41. doi: 10.1097/00004347-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Milner BJ, Allan LA, Eccles DM, et al. P53 mutations is a common genetic event in ovarian carcinoma. Cancer Res. 1993;53:2128–32. [PubMed] [Google Scholar]
- 31.Gottlieb WH, Berek JS. Advances in the biology of gynecologic cancer. Curr Opin Oncol. 1994;6:513–8. doi: 10.1097/00001622-199409000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Kupryjanczyk J, Dansonka-Mieszkowska A, et al. Spontaneous apoptosis in ovarian carcinomas: a positive association with p53 gene mutation is dependent on growth fraction. Br J Cancer. 2000;82:579–83. doi: 10.1054/bjoc.1999.0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geisler JP, Geisler HE, Miller GA, et al. P53 and bcl-2 in epithelial ovarian carcinoma: their value as prognostic indicators at a median follow-up of 60 months. Gynecol Oncol. 2000;77:278–82. doi: 10.1006/gyno.2000.5780. [DOI] [PubMed] [Google Scholar]
- 34.Kassim SK, Ali HS, Sallam MM, et al. Increased bcl-2 expression is associated with primary resistance to chemotherapy in human epithelial ovarian cancer. Clin Biochem. 1999;32:333–8. doi: 10.1016/s0009-9120(99)00026-0. [DOI] [PubMed] [Google Scholar]
- 35.Diebold J, Baretton G, Felchner M, et al. Bcl-2 expression, p53 accumulation, and apoptosis in ovarian carcinomas. Am J Clin Pathol. 1996;105:341–49. doi: 10.1093/ajcp/105.3.341. [DOI] [PubMed] [Google Scholar]


