Abstract
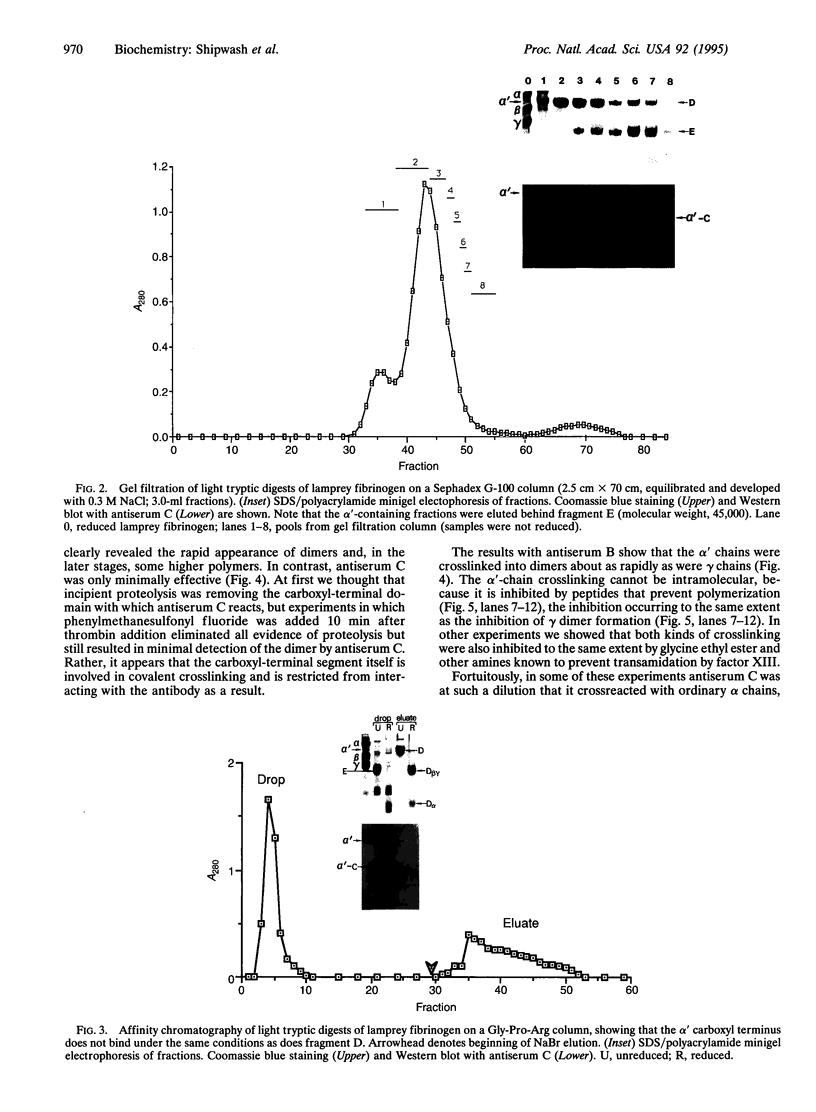
Lampreys have two genes for the alpha chains of fibrinogen, the second of which encodes a minor form with a carboxyl-terminal domain homologous to the carboxyl-terminal domains of beta and gamma chains. Initially, we referred to the alternative chain as alpha-II; we now use the designation alpha' in order to facilitate reference to crosslinked dimers. Antisera raised to synthetic peptides based on the cDNA sequence confirmed that the alpha' chain was present in fibrinogen prepared directly from plasma. The same antibodies were used to determine the size and properties of the carboxyl-terminal domain after its release by mild tryptic digestion, a fragment of apparent molecular weight 35,000-40,000 being produced. Unlike fragment D generated in the same digestions, the alpha' fragment did not bind to Gly-Pro-Arg or Gly-Val-Arg peptide affinity columns. During clotting under conditions where factor XIII is active, the alpha' chains became crosslinked very much more rapidly than ordinary alpha chains, the principal product being an apparent dimer, but smaller amounts of higher multimers being detectable. The crosslinking was inhibited by various amines, as well as by peptides that prevent polymerization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale M. D., Janmey P. A., Ferry J. D. Kinetics of formation of fibrin oligomers. II. Size distributions of ligated oligomers. Biopolymers. 1982 Nov;21(11):2265–2277. doi: 10.1002/bip.360211113. [DOI] [PubMed] [Google Scholar]
- DOOLITTLE R. F. DIFFERENCES IN THE CLOTTING OF LAMPREY FIBRINOGEN BY LAMPREY AND BOVINE THROMBINS. Biochem J. 1965 Mar;94:735–741. doi: 10.1042/bj0940735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. A detailed consideration of a principal domain of vertebrate fibrinogen and its relatives. Protein Sci. 1992 Dec;1(12):1563–1577. doi: 10.1002/pro.5560011204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Riley M., Pan Y. Direct measurement of a second fibrinogen alpha chain in lamprey blood plasma. Thromb Res. 1992 Dec 15;68(6):489–493. doi: 10.1016/0049-3848(92)90062-f. [DOI] [PubMed] [Google Scholar]
- Fu Y., Grieninger G. Fib420: a normal human variant of fibrinogen with two extended alpha chains. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2625–2628. doi: 10.1073/pnas.91.7.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Weissbach L., Plant P. W., Oddoux C., Cao Y., Liang T. J., Roy S. N., Redman C. M., Grieninger G. Carboxy-terminal-extended variant of the human fibrinogen alpha subunit: a novel exon conferring marked homology to beta and gamma subunits. Biochemistry. 1992 Dec 8;31(48):11968–11972. doi: 10.1021/bi00163a002. [DOI] [PubMed] [Google Scholar]
- Gorkun O. V., Veklich Y. I., Medved L. V., Henschen A. H., Weisel J. W. Role of the alpha C domains of fibrin in clot formation. Biochemistry. 1994 Jun 7;33(22):6986–6997. doi: 10.1021/bi00188a031. [DOI] [PubMed] [Google Scholar]
- Gray J. E., Doolittle R. F. Characterization, primary structure, and evolution of lamprey plasma albumin. Protein Sci. 1992 Feb;1(2):289–302. doi: 10.1002/pro.5560010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyas C., Haeberli A., Walder P., Straub P. W. Isolation of human fibrinogen and its derivatives by affinity chromatography on Gly-Pro-Arg-Pro-Lys-Fractogel. Thromb Haemost. 1990 Jun 28;63(3):439–444. [PubMed] [Google Scholar]
- MIHALYI E., GODFREY J. E. Digestion of fibrinogen by trypsin. II. Characterization of the large fragment obtained. Biochim Biophys Acta. 1963 Jan 8;67:90–103. doi: 10.1016/0006-3002(63)91799-2. [DOI] [PubMed] [Google Scholar]
- Pan Y., Doolittle R. F. cDNA sequence of a second fibrinogen alpha chain in lamprey: an archetypal version alignable with full-length beta and gamma chains. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2066–2070. doi: 10.1073/pnas.89.6.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Z., Patterson J., Gray J. E., Yu C., Cottrell B. A., Shimizu A., Graham D., Riley M., Doolittle R. F. Complete sequence of the lamprey fibrinogen alpha chain. Biochemistry. 1989 Dec 12;28(25):9801–9806. doi: 10.1021/bi00451a039. [DOI] [PubMed] [Google Scholar]
- Weissbach L., Grieninger G. Bipartite mRNA for chicken alpha-fibrinogen potentially encodes an amino acid sequence homologous to beta- and gamma-fibrinogens. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5198–5202. doi: 10.1073/pnas.87.13.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Doolittle R. F. Presence of a vertebrate fibrinogen-like sequence in an echinoderm. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2097–2101. doi: 10.1073/pnas.87.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazumi K., Doolittle R. F. Photoaffinity labeling of the primary fibrin polymerization site: localization of the label to gamma-chain Tyr-363. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2893–2896. doi: 10.1073/pnas.89.7.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]