Abstract
Ribulose bisphosphate carboxylase, a key enzyme in the photosynthetic carboxylation process, has been localized through an indirect immunofluorescent technique in the guard cells of some of the 41 species of plants examined. This sample includes 17 families of both dicotyledons and monocotyledons, one gymnosperm, and one pteridophyte. Plants were selected to represent all of the three major photosynthetic categories, namely C3, C4, and Crassulacean acid metabolism. Antibodies raised against tobacco (Nicotiana tabacum L.) ribulose bisphosphate carboxylase were used for this immunofluorescent study. A good degree of fluorescence was observed in the guard cells of seven out of 21 species exhibiting Crassulacean acid metabolism. C3 plants exhibited a very low degree (almost negligible) of fluorescence, while the C4 species did not exhibit any fluorescence.
Full text
PDF
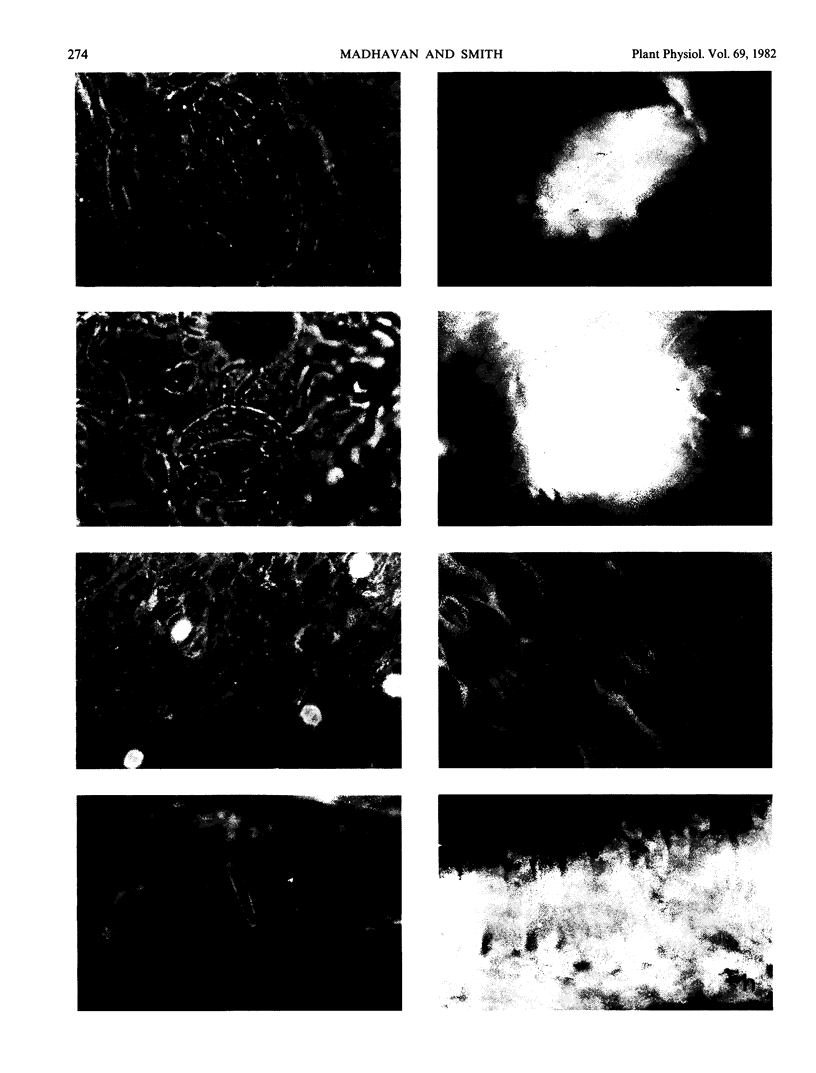
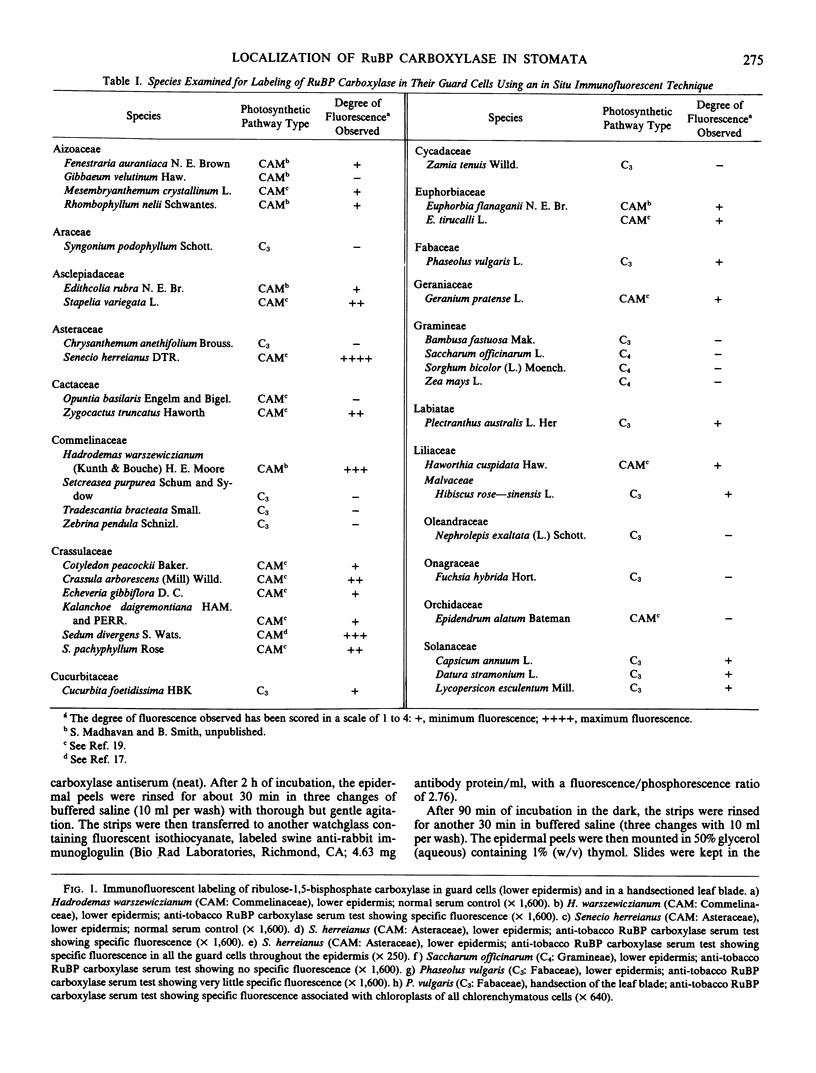


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan P. H., Sakano K., Singh S., Wildman S. G. Crystalline fraction I protein: preparation in large yield. Science. 1972 Jun 9;176(4039):1145–1146. doi: 10.1126/science.176.4039.1145. [DOI] [PubMed] [Google Scholar]
- Cockburn W. Relationships between Stomatal Behavior and Internal Carbon Dioxide Concentration in Crassulacean Acid Metabolism Plants. Plant Physiol. 1979 Jun;63(6):1029–1032. doi: 10.1104/pp.63.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Outlaw W. H., Lowry O. H. Organic acid and potassium accumulation in guard cells during stomatal opening. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4434–4438. doi: 10.1073/pnas.74.10.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J., Dicamelli C. A., Randall D. D., Rapp B., Veith G. M. Photosynthetic carbon reduction pathway is absent in chloroplasts of Vicia faba guard cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6371–6375. doi: 10.1073/pnas.76.12.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kirk C. A., Raschke K. Presence of Chloride Reduces Malate Production in Epidermis during Stomatal Opening. Plant Physiol. 1978 Mar;61(3):361–364. doi: 10.1104/pp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer C. M., Pallas J. E., Black C. C. Carbon dioxide metabolism in leaf epidermal tissue. Plant Physiol. 1973 Nov;52(5):448–452. doi: 10.1104/pp.52.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]



