Abstract
Cells respond to mechanical signals, but the subcellular mechanisms are not well understood. The nucleus has recently emerged as an important mechanosensory organelle in the cell, as it is intimately connected to the cytoskeleton. Mechanical forces applied to cells that act on membrane-embedded receptors are transmitted through the cytoskeleton to the nuclear surface. Interfering with linkers of the nucleus to the cytoskeleton causes defects in cell mechanosensing and cell function. In this chapter, we discuss recent work in this area, highlighting the role that the nuclear linkages with the cytoskeleton play in cellular mechano-transduction.
1. Introduction
Cells in the body are exposed to both ‘active’ and ‘passive’ mechanical stimuli. For example, endothelial cells that line the blood vessels are constantly exposed to shear stresses and cyclic stretching imposed by the pulsatile flow of blood, actively remodeling their cytoskeleton and overall morphology. Adherent cells are also exposed to widely varying mechanical cues from the extracellular matrix (ECM) depending on the organ they inhabit-neurons, for example, are surrounded by much softer tissue than smooth muscle cells or osteoblasts. These passive cues also elicit a response from cells. The mechanisms by which cells respond to mechanical stimuli are of strong current interest in the emerging area of mechanobiology.
Cells can transduce mechanical signals into a biochemical response. This process is known as mechano-transduction; however there is no singular mechanism by which this happens. Mechanical forces applied to cells, which transmit signals ~40-fold faster than diffusion of some chemical signals 1, can cause conformational changes in heterodimeric integrin proteins in cell-matrix adhesion sites. This can ultimately alter signaling pathways and gene expression. Mechanical stimuli can open ion channels (see review by Morris 2), alter binding of proteins in focal adhesions 3, and cause changes in overall cell morphology 4 (also see review by Ingber 5). In recent years, it has become recognized that the nucleus of the cell can act as a mechanosensory organelle (see review by Wang et al.6), which not only experiences and transmits forces directly but also influences cell mechanosensing, through mechanisms that are beginning to be understood.
In this chapter, we review the evidence that supports the concept that the nucleus mediates mechanosensing. We discuss how force propagation occurs to the nuclear surface, how cytoskeletal coupling to the nucleus is necessary for mechanosensing and how the nucleus should be seen as an integrated component with the cytoskeleton in models for cell mechanosensing.
2. Cytoskeletal forces are exerted on the nucleus
Early evidence that the nucleus is under tension came from Ingber and coworkers in 1992, who showed that perturbing actomyosin forces altered cell and nuclear shape 7. In a landmark paper in 1997, they showed that tugging on integrin receptors in the cell membrane causes nuclear distortion and motion 8. This established the concept that forces applied externally to the cell are propagated to the nuclear surface, consistent with mechanical models of the cell cytoskeleton that are ‘hardwired’ to the nuclear envelope 9,10,11,12,13,14. These external forces have now been shown to induce clearly-detectable nuclear deformation 8,15,16,17,18,19,20,21,22. The F-actin cytoskeleton plays a major role in propagating the mechanical forces from integrin receptors to the nuclear surface, although the molecules which connect the nucleus to the cytoskeleton have only recently been identified.
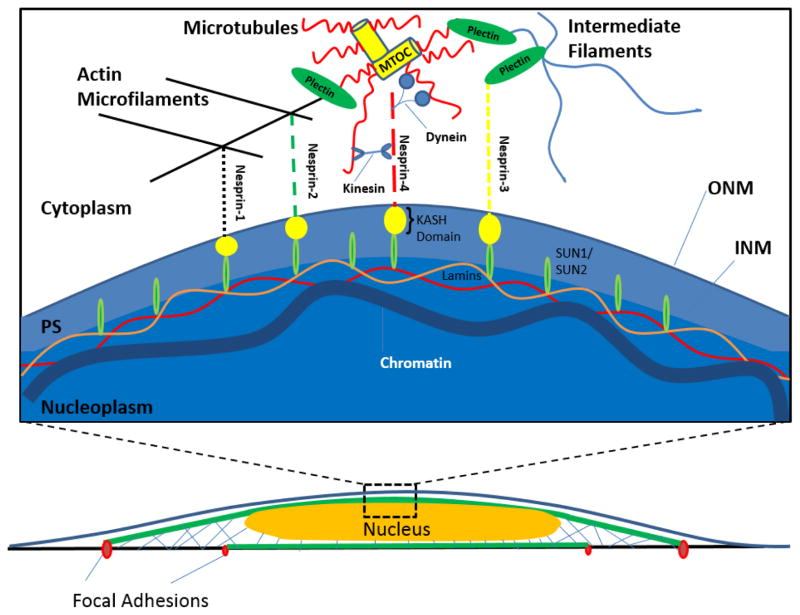
In recent years, members of the so-called LINC complex (for Linker of Nucleoskeleton to the Cytoskeleton) have been discovered in the nuclear envelope 23,24,25,26. The LINC complex is comprised of two protein families that span the nuclear envelope, and physically connect the cytoskeleton to the nucleoskeleton. The SUN (Sad1p, UNC-84) domain proteins span the inner nuclear membrane (INM) and translumenally bind the KASH (Klarsicht/ANC-1/Syne Homology) domain proteins that span the outer nuclear membrane (ONM) (Figure 1). In this way the KASH and SUN domain proteins create a mechanical tether that connects both membranes of the nuclear envelope. The KASH domain proteins bind to various cytoskeletal constituents, whereas the SUN domain proteins associate with the nuclear lamina. Thus, the mechanical connections created by the LINC complex can integrate the forces of the cytoskeleton and the nucleus.
Figure 1.
The LINC complex is formed by the SUN domain proteins spanning the inner nuclear membrane (INM) and translumenally binding the KASH domain proteins that span the outer nuclear membrane (ONM). The SUN domain proteins associate with the nuclear lamina, whereas the KASH domain proteins bind to various cytoskeletal constituents through the nesprin protein family (nesprin 1–4). Nesprin 1 and nesprin 2 bind to actin, and to dynein and kinesin, whereas nesprin 3 binds plectin, an intermediate filament-associated cytolinker.
The LINC complex is functionally well conserved in eukaryotes, including single celled organisms such as yeasts, however the number and nature of the KASH and SUN domain constituents varies between divergent species. In mammals there are five Sun domain proteins (SUNs 1–5), but only SUN 1 and SUN2 appear widely expressed. SUNs 3–5 are predominantly, if not exclusively expressed in the testis. SUN1 and SUN2 have been shown to associate with the nuclear lamina, however the role of any additional nucleoplasmic associations of SUN domain proteins in LINC complex function remains unclear. The crystal structure of the large SUN 2 lumenal domain has revealed that the protein forms a trimeric 27,28 oligomer, mediated by a lumenal coiled-coil domain. Each of the three SUN domains in this trimer binds to a unique KASH domain. This oligomeric association likely enhances the physical strength the LINC complex to transfer forces between the cytoskeleton and nucleus.
There are six mammalian KASH domain proteins (nesprins 1–4, LRMP and KASH5), only three of which (nesprins 1–3) are generally ubiquitous. Nesprin 1 and nesprin 2 bind to actin, and to dynein and kinesin 29,30, whereas nesprin 3 binds plectin, an intermediate filament-associated cytolinker. Nesprin 4 is predominately expressed in highly polarized epithelial cells and its loss leads to hearing defects in mice and humans associated with perturbation of nuclear positioning 31,32. KASH5 expression appears limited to meiotic cells where it binds dynein to move chromosomes during homologous recombination 33,34. Predominantly expressed in lymphocytes and taste cells, LRMP does not appear to interact with the cytoskeleton but instead binds to the calcium channel IP3 receptors 33,35. There are multiple splice isoforms of nesprin 1 and nesprin 2 that do not contain the ONM-targeting KASH domain. Many of these KASH-less isoforms likely have functions other than formation of the LINC complex 36,37,38,39,40.
The LINC complex is the only known structure by which cytoskeletal stresses can be directly transferred to the nuclear surface (Fig. 1). Since the cytoskeleton ultimately connects to focal adhesions 41,42 (also see review by Geiger, Spatz and Bershadsky 43), the LINC complex enables a mechanical linkage between the nucleus, the cytoskeleton, and the extracellular matrix 20,25,26.
3. The LINC complex transmits cytoskeletal forces to the nuclear surface
The nucleus has been suggested to be a cellular mechanosensor 6, and besides being a key player in the physical signaling pathway, it is possible that the LINC complex is involved in chemical signal transduction pathways as well 44,45,46. The LINC complex is essential for efficient migration 17,47,48, normal structure 48,49, function 17,48, and maintaining nuclear shape and position 17,48,50,51,52. For a review on disrupted LINC complexes causing defects in mechano-transduction, see the recent review by Jaalouk and Lammerding 53. The LINC complex has been found to be a key player in force transmission between the nucleus and cytoskeleton 17,48, and an intact LINC complex is required for nuclear positioning, cell polarization, and normal propagation of cytoskeletal forces 17.
It has been demonstrated that external forces applied to the apical surface of a living cell propagate through the cytoskeleton and all the way to the nucleus 8 and that forces applied to integrins can cause motion of intranuclear organelles 22. Chancellor et al. recently showed that nesprin-1 knockdown significantly increased the nuclear height, suggesting an essential role of nesprin-1 in flattening the nucleus in endothelial cells 54,55. Inhibiting myosin activity similarly produced a vertically rounded nucleus. Chancellor et al. proposed a model in which actomyosin force pulls laterally on the nucleus and flattens it to the shape of a disk. In this model, the nucleus acts as a scaffold that balances actomyosin forces internally while the substrate balances them externally. In the absence of nesprin-1, the pulling force on the nucleus is substantially reduced and the nucleus is free to relax vertically into a rounded shape 54 while the excess force is now balanced at an increased number of adhesion sites with the substratum.
In a series of recent papers, Gundersen and coworkers showed that dorsal actin bundles (on top of the nuclear surface) are directly linked to the nucleus via TAN (for Transmembrane Actin-associated Nuclear) lines assembled from nesprin2giant and SUN2 proteins 47,56. The authors suggest that the TAN lines across the nuclear membrane function in a manner similar to focal adhesions across the cellular membrane in that both assemblies are linked to actin cables and transmit mechanical force 47,57. They also showed in fibroblasts that emerin and myosin IIB function to polarize nuclear movement and flow of actin, which suggests a new role of the nuclear envelope in establishing cytoskeletal polarity and directional actin flow 58.
Recently, Khatau et al. suggested that apical stress fibers on top of the nucleus shape it by squeezing from the top as the fibers contract 59. The authors call these distinct bundles the ‘actin cap’. These bundles terminate at a small distinct subset of focal adhesions, which are proposed to regulate mechanosensing via the actin cap. The actin cap associate focal adhesions have been shown to be larger in size than conventional focal adhesions, are located only at the periphery, and experience fast turnover dynamics60. Wirtz and coworkers have proposed that the actin cap and its associated focal adhesions play a key role in the fast and efficient physical pathway for mechano-transduction by providing a continuous mechanical linkage from the ECM to the nucleus 60,61,62. Coupling of the actin cap to the nuclear lamina occurs via the LINC complex 59,63,64 and its associated nesprins.
Like actin bundles, microtubule motor proteins can also generate tension that is transmitted to the nuclear surface through the LINC complex. For example, Splinter et al. showed that by binding to the nuclear membrane at their cargo end, dynein and kinesin-1 can pull the nucleus in opposite directions as they walk. Kinesin-1 pulls the nucleus away from the centrosome, while dynein pulls it towards the centrosome 65. The combined activities of these two processive motor proteins control nuclear translation and rotation 66,67,68. Wu et al. showed that dynein walking on microtubules in the vicinity of the nucleus can produce nuclear rotations 69. They also modeled the rotation of the nucleus computationally to show how this could mechanically occur in the cell. As mentioned previously, nesprin 4, and likely nesprins 1 and 2, function to position the nucleus within the cell through the action of microtubule motors. KASH5 (and its orthologs) functions to transmit microtubule motor forces through the nuclear envelope directly to meiotic chromosomes 33,34,70,71,72.
4. The role of the nucleus in cell mechanosensing
Although the exact mechanisms are not completely understood, cells have been shown to sense and respond to different mechanical cues from their surroundings such as shear stress 73, substrate strain 74, and substrate rigidity 75. Evidence that the nuclear force balance is important in this cell mechanosensing has come from studies in which the LINC complex was disrupted. For example, endothelial cells were found to lose their ability to re-orient in response to uniaxial cyclic strain upon knockdown of nesprin-1 54. Because the nucleus no longer acts as an internal scaffold to balance actomyosin tension, the nesprin-1 deficient cells apply increased traction on the substratum, resulting in stronger adhesion and less propensity to reorient in response to mechanical strain.
There is evidence that nuclear forces might regulate gene expression. Changing nuclear shape by controlling the degree of cell spreading alters protein synthesis 76. In response to cyclic strain, lamin A/C deficient and emerin-deficient mouse embryonic fibroblasts (MEF) have impaired expression of genes lex-1 and Egr-1 20,55. Philip et al. showed that in response to shear stress, lamins are upregulated and reorganized 77. More recently, defects in lamin A/C have been demonstrated to impair nuclear translocation of MKL-1, a transcription factor 78.
In NIH/3T3 fibroblasts, the nuclear shape has been shown to depend on the rigidity of the underlying substrate 79. The nucleus takes a spherical shape on soft substrates, likely due to the smaller actomyosin tension and a flattened ellipsoid on rigid substrates where higher forces are generated in cells 79. Tuning the actomyosin tension by changing the substrate rigidity enables control of nuclear shape. Disrupting the LINC complex via KASH overexpression or inhibiting myosin II eliminates this shape dependence of nucleus on substrate rigidity, suggesting that the coupling of the nucleus to the cytoskeleton is essential for mechanosensing 79. The cell motility and the cell spreading area both correlate with the underlying-substrate rigidity in a manner that depends on the nuclear linkages to the cytoskeleton; on softer substrates, cells have lower speed and spreading area, whereas they have higher speed and spreading area on stiffer substrates, and KASH overexpression ablated this trend 79. Separately, Swift and coworkers have demonstrated that the lamin A levels are proportional to the tissue rigidity 80, indicating that lamin-A stabilizes the nucleus under stress. Consistent with this, LINC complex disruption decreases cellular mechanical stiffness 49.
CONCLUSIONS
In conclusion, physical connectivity from the nucleus to the cytoskeleton and cell membrane is required for normal cell mechanosensing. Force transmission from external receptors to the nuclear surface requires an intact nuclear lamina and physical connectivity between the nucleus and the cytoskeleton by the LINC complex. Ultimately, how the integrated nuclear-cytoskeleton integrates mechanical stimuli with complex intracellular signaling pathways and gene regulatory networks will require a systems biology approach that seeks to understand cell mechanosensing in an integrated manner.
Acknowledgments
This work is supported by the National Science Foundation under award CMMI 0954302 (TPL) and by the National Institutes of Health under awards R01GM102486-01 (TPL, KR and RBD) and 5R01EB014869-02 (TPL and KR).
References
- 1.Na S, Collin O, Chowdhury F, et al. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A. 2008 May 6;105(18):6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris CE. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- 3.Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber DE. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol. 2006 Apr;207(1):187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- 4.Yeung T, Georges PC, Flanagan LA, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005 Jan;60(1):24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 5.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006 May;20(7):811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 6.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nature reviews. Molecular cell biology. 2009 Jan;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 7.Sims JR, Karp S, Ingber DE. Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeletal and nuclear shape. Journal of cell science. 1992 Dec;103( Pt 4):1215–1222. doi: 10.1242/jcs.103.4.1215. [DOI] [PubMed] [Google Scholar]
- 8.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences of the United States of America. 1997 Feb 4;94(3):849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 10.Chen CS, Ingber DE. Tensegrity and mechanoregulation: from skeleton to cytoskeleton. Osteoarthritis Cartilage. 1999 Jan;7(1):81–94. doi: 10.1053/joca.1998.0164. [DOI] [PubMed] [Google Scholar]
- 11.Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003 Apr 1;116(Pt 7):1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- 12.Wang N, Naruse K, Stamenovic D, et al. Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci U S A. 2001 Jul 3;98(14):7765–7770. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamenovic D, Mijailovich SM, Tolic-Norrelykke IM, Chen J, Wang N. Cell prestress. II. Contribution of microtubules. Am J Physiol Cell Physiol. 2002 Mar;282(3):C617–624. doi: 10.1152/ajpcell.00271.2001. [DOI] [PubMed] [Google Scholar]
- 14.Wang N, Tolic-Norrelykke IM, Chen J, et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol. 2002 Mar;282(3):C606–616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 15.Guilak F, Tedrow JR, Burgkart R. Viscoelastic properties of the cell nucleus. Biochem Biophys Res Commun. 2000 Mar 24;269(3):781–786. doi: 10.1006/bbrc.2000.2360. [DOI] [PubMed] [Google Scholar]
- 16.Guilak F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech. 1995 Dec;28(12):1529–1541. doi: 10.1016/0021-9290(95)00100-x. [DOI] [PubMed] [Google Scholar]
- 17.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem. 2011 Jul 29;286(30):26743–26753. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lammerding J, Lee RT. Mechanical properties of interphase nuclei probed by cellular strain application. Methods Mol Biol. 2009;464:13–26. doi: 10.1007/978-1-60327-461-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lammerding J, Fong LG, Ji JY, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 2006 Sep 1;281(35):25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 20.Lammerding J, Schulze PC, Takahashi T, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004 Feb;113(3):370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A. 2007 Oct 2;104(40):15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poh YC, Shevtsov SP, Chowdhury F, et al. Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nat Commun. 2012;3:866. doi: 10.1038/ncomms1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke B, Roux KJ. Nuclei take a position: managing nuclear location. Dev Cell. 2009 Nov;17(5):587–597. doi: 10.1016/j.devcel.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Crisp M, Liu Q, Roux K, et al. Coupling of the nucleus and cytoplasm: role of the LINC complex. The Journal of cell biology. 2006 Jan 2;172(1):41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol. 2010 Nov 10;26:421–444. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon DN, Wilson KL. The nucleoskeleton as a genome-associated dynamic ‘network of networks’. Nat Rev Mol Cell Biol. 2011 Nov;12(11):695–708. doi: 10.1038/nrm3207. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Shi Z, Jiao S, et al. Structural insights into SUN-KASH complexes across the nuclear envelope. Cell Res. 2012 Oct;22(10):1440–1452. doi: 10.1038/cr.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sosa BA, Rothballer A, Kutay U, Schwartz TU. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell. 2012 May 25;149(5):1035–1047. doi: 10.1016/j.cell.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J, Lei K, Zhou M, et al. KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum Mol Genet. 2011 Mar 15;20(6):1061–1073. doi: 10.1093/hmg/ddq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Lei K, Yuan X, et al. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009 Oct 29;64(2):173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horn HF, Brownstein Z, Lenz DR, et al. The LINC complex is essential for hearing. J Clin Invest. 2013 Feb 1;123(2):740–750. doi: 10.1172/JCI66911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roux KJ, Crisp ML, Liu Q, et al. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proceedings of the National Academy of Sciences of the United States of America. 2009 Feb 17;106(7):2194–2199. doi: 10.1073/pnas.0808602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horn HF, Kim DI, Wright GD, et al. A mammalian KASH domain protein coupling meiotic chromosomes to the cytoskeleton. J Cell Biol. 2013 Sep 30;202(7):1023–1039. doi: 10.1083/jcb.201304004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morimoto A, Shibuya H, Zhu X, et al. A conserved KASH domain protein associates with telomeres, SUN1, and dynactin during mammalian meiosis. J Cell Biol. 2012 Jul 23;198(2):165–172. doi: 10.1083/jcb.201204085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shindo Y, Kim MR, Miura H, et al. Lrmp/Jaw1 is expressed in sweet, bitter, and umami receptor-expressing cells. Chem Senses. 2010 Feb;35(2):171–177. doi: 10.1093/chemse/bjp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi Y, Katanosaka Y, Iwata Y, Matsuoka M, Shigekawa M, Wakabayashi S. Identification and characterization of GSRP-56, a novel Golgi-localized spectrin repeat-containing protein. Exp Cell Res. 2006 Oct 1;312(16):3152–3164. doi: 10.1016/j.yexcr.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Rajgor D, Mellad JA, Autore F, Zhang Q, Shanahan CM. Multiple novel nesprin-1 and nesprin-2 variants act as versatile tissue-specific intracellular scaffolds. PLoS One. 2012;7(7):e40098. doi: 10.1371/journal.pone.0040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cottrell JR, Borok E, Horvath TL, Nedivi E. CPG2: a brain- and synapse-specific protein that regulates the endocytosis of glutamate receptors. Neuron. 2004 Nov 18;44(4):677–690. doi: 10.1016/j.neuron.2004.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marme A, Zimmermann HP, Moldenhauer G, et al. Loss of Drop1 expression already at early tumor stages in a wide range of human carcinomas. Int J Cancer. 2008 Nov 1;123(9):2048–2056. doi: 10.1002/ijc.23763. [DOI] [PubMed] [Google Scholar]
- 40.Warren DT, Tajsic T, Mellad JA, Searles R, Zhang Q, Shanahan CM. Novel nuclear nesprin-2 variants tether active extracellular signal-regulated MAPK1 and MAPK2 at promyelocytic leukemia protein nuclear bodies and act to regulate smooth muscle cell proliferation. J Biol Chem. 2010 Jan 8;285(2):1311–1320. doi: 10.1074/jbc.M109.032557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 42.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May 21;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 43.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009 Jan;10(1):21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 44.Bissell MJ, Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–262. [PubMed] [Google Scholar]
- 45.Bissell MJ, Barcellos-Hoff MH. The influence of extracellular matrix on gene expression: is structure the message? J Cell Sci Suppl. 1987;8:327–343. doi: 10.1242/jcs.1987.supplement_8.18. [DOI] [PubMed] [Google Scholar]
- 46.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 47.Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010 Aug 20;329(5994):956–959. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lombardi ML, Lammerding J. Keeping the LINC: the importance of nucleocytoskeletal coupling in intracellular force transmission and cellular function. Biochem Soc Trans. 2011 Dec;39(6):1729–1734. doi: 10.1042/BST20110686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp Cell Res. 2008 May 1;314(8):1892–1905. doi: 10.1016/j.yexcr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer-Vize JA, Mosley KL. Marbles mutants: uncoupling cell determination and nuclear migration in the developing Drosophila eye. Development. 1994 Sep;120(9):2609–2618. doi: 10.1242/dev.120.9.2609. [DOI] [PubMed] [Google Scholar]
- 51.Malone CJ, Fixsen WD, Horvitz HR, Han M. UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development. 1999 Jun;126(14):3171–3181. doi: 10.1242/dev.126.14.3171. [DOI] [PubMed] [Google Scholar]
- 52.Mosley-Bishop KL, Li Q, Patterson L, Fischer JA. Molecular analysis of the klarsicht gene and its role in nuclear migration within differentiating cells of the Drosophila eye. Curr Biol. 1999 Nov 4;9(21):1211–1220. doi: 10.1016/s0960-9822(99)80501-6. [DOI] [PubMed] [Google Scholar]
- 53.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009 Jan;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chancellor TJ, Lee J, Thodeti CK, Lele T. Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophysical journal. 2010 Jul 7;99(1):115–123. doi: 10.1016/j.bpj.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005 Aug 29;170(5):781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gundersen GG, Worman HJ. Nuclear positioning. Cell. 2013 Mar 14;152(6):1376–1389. doi: 10.1016/j.cell.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luxton GW, Gomes ER, Folker ES, Worman HJ, Gundersen GG. TAN lines: a novel nuclear envelope structure involved in nuclear positioning. Nucleus. 2011 May-Jun;2(3):173–181. doi: 10.4161/nucl.2.3.16243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang W, Folker ES, Worman HJ, Gundersen GG. Emerin organizes actin flow for nuclear movement and centrosome orientation in migrating fibroblasts. Mol Biol Cell. 2013 Dec;24(24):3869–3880. doi: 10.1091/mbc.E13-06-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khatau SB, Hale CM, Stewart-Hutchinson PJ, et al. A perinuclear actin cap regulates nuclear shape. Proceedings of the National Academy of Sciences of the United States of America. 2009 Nov 10;106(45):19017–19022. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim DH, Khatau SB, Feng Y, et al. Actin cap associated focal adhesions and their distinct role in cellular mechanosensing. Scientific reports. 2012;2:555. doi: 10.1038/srep00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chambliss AB, Khatau SB, Erdenberger N, et al. The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction. Sci Rep. 2013;3:1087. doi: 10.1038/srep01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim DH, Chambliss AB, Wirtz D. The multi-faceted role of the actin cap in cellular mechanosensation and mechanotransduction. Soft Matter. 2013 Jun 21;9(23):5516–5523. doi: 10.1039/C3SM50798J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khatau SB, Kusuma S, Hanjaya-Putra D, et al. The differential formation of the LINC-mediated perinuclear actin cap in pluripotent and somatic cells. PLoS One. 2012;7(5):e36689. doi: 10.1371/journal.pone.0036689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khatau SB, Bloom RJ, Bajpai S, et al. The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration. Sci Rep. 2012;2:488. doi: 10.1038/srep00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Splinter D, Tanenbaum ME, Lindqvist A, et al. Bicaudal D2, dynein, and kinesin-1 associate with nuclear pore complexes and regulate centrosome and nuclear positioning during mitotic entry. PLoS Biol. 2010;8(4):e1000350. doi: 10.1371/journal.pbio.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson MH, Holzbaur EL. Opposing microtubule motors drive robust nuclear dynamics in developing muscle cells. Journal of cell science. 2012 Sep 1;125(Pt 17):4158–4169. doi: 10.1242/jcs.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fridolfsson HN, Ly N, Meyerzon M, Starr DA. UNC-83 coordinates kinesin-1 and dynein activities at the nuclear envelope during nuclear migration. Dev Biol. 2009 Feb 15;338(2):237–250. doi: 10.1016/j.ydbio.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fridolfsson HN, Starr DA. Kinesin-1 and dynein at the nuclear envelope mediate the bidirectional migrations of nuclei. The Journal of cell biology. 2010 Oct 4;191(1):115–128. doi: 10.1083/jcb.201004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu J, Lee KC, Dickinson RB, Lele TP. How dynein and microtubules rotate the nucleus. J Cell Physiol. 2011 Oct;226(10):2666–2674. doi: 10.1002/jcp.22616. [DOI] [PubMed] [Google Scholar]
- 70.Sato A, Isaac B, Phillips CM, et al. Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell. 2009 Nov 25;139(5):907–919. doi: 10.1016/j.cell.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miki F, Kurabayashi A, Tange Y, Okazaki K, Shimanuki M, Niwa O. Two-hybrid search for proteins that interact with Sad1 and Kms1, two membrane-bound components of the spindle pole body in fission yeast. Mol Genet Genomics. 2004 Jan;270(6):449–461. doi: 10.1007/s00438-003-0938-8. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida M, Katsuyama S, Tateho K, et al. Microtubule-organizing center formation at telomeres induces meiotic telomere clustering. J Cell Biol. 2013 Feb 18;200(4):385–395. doi: 10.1083/jcb.201207168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiu JJ, Usami S, Chien S. Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Ann Med. 2009;41(1):19–28. doi: 10.1080/07853890802186921. [DOI] [PubMed] [Google Scholar]
- 74.Seliktar D, Nerem RM, Galis ZS. Mechanical strain-stimulated remodeling of tissue-engineered blood vessel constructs. Tissue Eng. 2003 Aug;9(4):657–666. doi: 10.1089/107632703768247359. [DOI] [PubMed] [Google Scholar]
- 75.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005 Nov 18;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 76.Thomas CH, Collier JH, Sfeir CS, Healy KE. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proceedings of the National Academy of Sciences of the United States of America. 2002 Feb 19;99(4):1972–1977. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Philip JT, Dahl KN. Nuclear mechanotransduction: response of the lamina to extracellular stress with implications in aging. J Biomech. 2008 Nov 14;41(15):3164–3170. doi: 10.1016/j.jbiomech.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 78.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013 May 23;497(7450):507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lovett DB, Shekhar N, Nickerson JA, Roux KJ, Lele TP. Modulation of Nuclear Shape by Substrate Rigidity. Cell Mol Bioeng. 2013 Jun;6(2):230–238. doi: 10.1007/s12195-013-0270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013 Aug 30;341(6149):1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]