Abstract
We provide evidence at the molecular level that ubiquitinated proteins are present in exosomes shed by myeloid-derived suppressor cells (MDSC). Ubiquitin was selected as a post-translational modification of interest because it is known to play a determinant role in the endosomal trafficking that culminates in exosome release. Enrichment was achieved by two immunoprecipitations, first at the protein level and subsequently at the peptide level. Fifty ubiquitinated proteins were identified by tandem mass spectrometry filtering at a 5% spectral false discovery rate and using the conservative requirement that glycinylglycine-modified lysine residues were observed in tryptic peptides. Thirty five of these proteins have not previously been reported to be ubiquitinated. The ubiquitinated cohort spans a range of protein sizes and favors basic pI values and hydrophobicity. Five proteins associated with endosomal trafficking were identified as ubiquitinated, along with pro-inflammatory high mobility group protein B1 and proinflammatory histones.
Keywords: Exosomes, ubiquitinated proteins, proinflammatory against, MDSC, endosomal pathway, aberrant tryptic digestion, immunoaffinity enrichment, LC−MS/MS
Introduction
Exosomes are extracellular vesicles 30–100 nm in diameter that are shed by most cells.1,2 They were first observed in 1987 in maturing reticulocytes,3 and recently they have gained attention as agents of intercellular communication4 and as potential prognostic tools.5 In previous studies, we interrogated the content of myeloid-derived suppressor cells (MDSC) and exosomes shed by MDSC. We focused on these cells because of their widespread presence in most cancer patients and their critical role in promoting tumor progression through their inhibition of innate and adaptive antitumor immunity.6 We have reported that exosomes shed by MDSC contain proinflammatory molecules that drive the accumulation and immune suppressive potency of MDSC and macrophages, respectively, and have identified the specific proteins responsible for these bioactivities.7 On the basis of these and other potential functions, there is global interest in the nature of the protein cargo carried by exosomes.8−12
Exosomes are formed by a series of intracellular events initiated by the invagination of the plasma membrane to form endosomes.1,2 Within endosomes, proteins are sorted into luminal vesicles to form late endosomes or multivesicular bodies.13,14 These luminal vesicles and their cargo are then incorporated into the lysosome for degradation, recycled to the plasma membrane, or exocytosed as exosomes. Ubiquitination has been shown to signal both the internalization of surface proteins and the sorting of endosomal proteins into luminal vesicles.13,15−17
Given the strategic role of ubiquitination in intracellular protein trafficking, the present study seeks to confirm the presence and identify ubiquitinated proteins in exosomes derived from MDSC. A previous study using western blot analyses indicated that exosomes and their parental cells contain distinct populations of ubiquitinated proteins;18 however, the conjugated proteins were not identified. Identification of the conjugated proteins allows assignment of their original locations in the parent cell, their original cell functions, and the range of protein sizes and pI values and may contribute to understanding the complexity of the endosomal pathway. In addition, enriching for ubiquitinated proteins allows detection of proteins that may be relatively low in abundance but play an important role in exosome structure and function and that contribute to MDSC function.
In the present work, ubiquitinated proteins have been recognized in MDSC-derived exosomes by identifying peptides that carry glycinylglycine-modified lysine residues as remnants of the ubiquitin carboxyl terminus. Additionally, we have used Gene Ontology annotations and the UniProt database to look for trends in the source, function, size, and pI values of the ubiquitinated species.
Mass spectrometry-based bottom-up proteomics has proven to be a powerful tool for recognizing ubiquitinated tryptic peptides and identifying sites of ubiquitination on these peptides and their related proteins. Tryptic digestion of ubiquitinated proteins cleaves ubiquitin at R74, which leaves two glycine residues on the modified lysine of the substrate peptide.19 Enrichment of ubiquitinated proteins from MDSC-derived exosomes was accomplished using two immunoprecipitation steps: immunoprecipitation of ubiquitinated proteins followed by tryptic digestion and immunoprecipitation of peptides containing glycinylglycine-modified lysine residues. In a parallel workflow, immunoprecipitation of ubiquitinated proteins was followed by 1D gel electrophoresis and in-gel digestion. In each case, enriched peptides were analyzed using LC–MS/MS and a bioinformatic search program that allowed for the variable modification of glycinylglycine-modified lysine residues (KGG).20 Here, we identify 50 ubiquitinated proteins carried by MDSC-derived exosomes. These ubiquitinated proteins constitute a small subset of proteins in these exosomes, originate from a diversity of subcellular locations, and have a variety of functions.
Experimental Section
Myeloid-Derived Suppressor Cells
BALB/c mice were injected in the mammary fat pad with approximately 7000 wild-type 4T1 mammary carcinoma cells stably transfected to express interleukin-1β (IL-1β). When tumors were greater than approximately 8 mm in diameter (about 3–4 weeks after initial inoculation), MDSC were harvested from the blood, stained with fluorescently labeled monoclonal antibodies against markers of MDSC (Gr1 and CD11b), and analyzed by flow cytometry.21 Cell populations that were greater than 90% Gr1+CD11b+ were used in all experiments.21 For each experiment, a total of about 1 × 108 MDSC were pooled from 2 to 3 mice. The UMBC and UMCP Institutional Animal Care and Use Committees approved all procedures with animals and animal-derived materials.
Exosomes
MDSC were plated in serum-free HL-1 medium (BioWhittaker, Walkersville, MD) and maintained at 37 °C with 5% CO2. After 18 h, the cultures were centrifuged at 805g for 5 min (Eppendorf 5810 rotor, Eppendorf, Hamburg), the pellets were discarded, and the supernatants were centrifuged at 2090g for 30 min (Sorvall RC5C, SS34 rotor, DuPont, Wilmington, DE). The supernatants were then ultracentrifuged at 100 000g for 20 h at 10 °C (Beckman L8, SW40Ti rotor, Beckman, Pasadena, CA). The supernatants were discarded, and the pellets containing the exosomes were resuspended in PBS. Absorbances were measured at 260 and 280 nm. Exosomes were stored at −80 °C until use.
Exosomes were lysed in an optimized lysis buffer of 8 M urea in 50 mM ammonium bicarbonate with 50 μM of deubiquitinase inhibitor PR-619 (LifeSensors, Malvern, PA) and 1% of a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). They were centrifuged at 14 000g for 30 min with a 3 kDa molecular weight cut off filter, and the supernatants were discarded. This process was done three times. After lysis, the buffer was diluted to 0.8 M urea in 50 mM ammonium bicarbonate. Protein content before and after immunoprecipitation was measured by the Quick Start Bradford Assay (Bio-Rad, Hercules, CA).
Immunoprecipitation of Ubiquitinated Proteins
Ubiquitinated proteins were enriched using Protein A-Sepharose 4B beads (Invitrogen, Carlsbad, CA) that had been incubated with anti-ubiquitin antibody 3933 (Cell Signaling Technology, Danvers, MA) in a 1:600 dilution with rotation for 4 h at 4 °C. Excess antibody was removed from the beads by washing with 0.8 M urea in 50 mM ammonium bicarbonate and centrifuging three times at 3000g for 2 min. One-hundred micrograms of exosome lysate was added to the Sepharose bead slurry and incubated with rotation overnight at 4 °C. The unbound fraction was collected via centrifugation at 500g for 5 min. The Sepharose bead slurry was washed with 50 mM ammonium bicarbonate and centrifuged at 1000g for 5 min to remove nonspecifically bound proteins. Bound proteins were eluted by incubating the Sepharose bead slurry in 0.2 M glycine, pH 2.6, for 1 h at 4 °C and collected via centrifugation at 13 000g for 5 min. The elution was repeated, and the two elution fractions were combined.22 Enriched fractions of ubiquitinated exosomal proteins were subsequently processed either by tryptic digestion in gel or in solution and immunoprecipitation of peptides with glycinylglycine-modified lysine residues.
In-Gel Tryptic Digestion of Ubiquitinated Proteins
Proteomic studies were conducted on exosomal proteins enriched for ubiquitin conjugates by immunoprecipitation. Three biological replicates were resuspended in 2% SDS, 5% β-mercaptoethanal, and 62.5 mM Tris HCl and reduced at 90 °C for 5 min. The samples were then loaded onto 8–16% polyacrylamide gels (Bio-Rad) and subjected to electrophoresis for approximately 50 min at 200 V, 15 mA, and 50 W. The gels were stained using Coomassie blue (40% methanol, 20% acetic acid, 0.1% m/v Coomassie blue reagent 250; Thermo Scientific, San Jose CA) stain and then cut into 13 slices. After destaining, tryptic digestion was performed on each gel slice overnight at 37 °C.23 The extracted tryptic peptides were resuspended in 0.1% formic acid for injection into the LC–MS/MS. (See below for instrumental conditions.)
Tryptic Digestion and Immunoprecipitation of Glycinylglycine-Tagged Peptides
Enriched fractions of ubiquitinated exosomal proteins from five biological replicates were frozen, lyophilized, and resuspended in 50 mM ammonium bicarbonate. Proteins were reduced with 20 mM dithiothreitol for 30 min at 56 °C and alkylated with 10 mM methylmethanethiosulfonate for 45 min. One microgram of trypsin was added to each fraction, and digestion was performed overnight at 37 °C. As a positive control, a ubiquitin dimer linked with an isopeptide bond at K48 (Life Sensors, Malvern, PA) was also digested with trypsin under these conditions.
Peptides with glycinylglycine-modified lysine residues were enriched using Protein A-Sepharose 4B beads coupled to anti-diglycyl-lysine antibody GX41 (Millipore, Billerica, MA) using the same procedure as that with the anti-ubiquitin antibody, except the anti-diglycyl-lysine antibody was prepared at a 1:1000 dilution. The fractions of immunoprecipitated ubiquitinated proteins were added to the Sepharose bead slurry and incubated with rotation overnight at 4 °C. The unbound fraction was removed via centrifugation at 500g for 5 min. The Sepharose bead slurry was washed with 50 mM ammonium bicarbonate and centrifuged at 1000g for 10 s to remove nonspecifically bound peptides. Bound peptides were eluted by incubating the Sepharose bead slurry in 0.2 M glycine, pH 2.6, for 1 h at 4 °C and collected via centrifugation at 13 000g for 5 min. The elution was repeated, and the two elution fractions were combined. Prior to LC–MS/MS analysis, all fractions were desalted with C18 TopTip spin columns (Glygen, Columbia, MD) and resuspended in 100 μL of 0.1% formic acid.
Western Blotting
All fractions were subjected to one-dimensional gel electrophoresis on an 8–16% Criterion precast gel (Bio-Rad) at 200 V, 50 mA, and 15 W for 56 min, followed by transfer to a PVDF membrane (EMD Millipore, Billerica, MA) at 100 V, 350 mA, and 35W for 1 h. Free ubiquitin, polyubiquitin, and ubiquitinated proteins were detected by blotting with anti-ubiquitin antibody 3933 (Cell Signaling Technology) followed by anti-mouse IgG-HRP (Cell Signaling Technology). Protein bands were visualized with an Image Lab System (Bio-Rad, Hercules, CA) using the Gel-Doc program (Kodak Molecular Imaging Systems) and the SuperSignal West Dura chemiluminescent substrate (Thermo Fisher Scientific, Waltham, MA).
Extraction of Histones
Exosomal histones were extracted using the EpiQuick Total Histone Extraction Kit (Epigentek, Farmingdale, NY) according to the manufacturer’s instructions and analyzed for ubiquitination via western blotting with anti-ubiquitin antibody 3933 as previously described.
LC–MS/MS and Bioinformatics Analysis
LC–MS/MS analyses were performed on a Shimadzu Prominence nano HPLC (Shimadzu Scientific Instruments, Columbia, MD) in-line with an LTQ Orbitrap XL (Thermo Fisher Scientific). A 10 μL aliquot of tryptic peptides was injected onto an Acclaim PepMap 300 C18 precolumn (Dionex, Sunnyvale, CA) followed by desalting with 10% solvent A (97.5% H2O, 2.5% CAN, and 0.1% formic acid) for 20 min. Peptides were fractionated on a C18 analytical column (150 × 0.15 mm, 300 Å, Grace Davidson Discovery Sciences, Deerfield, IL) with a linear gradient increasing from 0 to 40% solvent B (97.5% ACN, 2.5% H2O, and 0.1% formic acid) in 85 min, followed by an increase from 40 to 85% solvent B in 20 min. The flow rate was 500 nL/min. Precursor scans were acquired in the orbitrap with a resolution of 30 000 at m/z 400. In each cycle, the nine most abundant ions were selected for fragmentation by collisional induced dissociation, and product ion scans were acquired in the LTQ. A dynamic exclusion of 1 repeat count over 180 s was used.
Peptide and protein identifications were made by the PepArML24,25 meta-search engine against the UniProt mouse database (July 2014). For the in-gel digestion, all peptide identifications were filtered at 10% spectral FDR, and proteins were required to be supported by at least 2 unshared peptides, bounding a protein FDR at 1%. For the two-step immunoaffinity enrichment of glycinylglycine-modified lysine-containing peptides, all peptide identifications were filtered at a 5% spectral FDR. Fixed modifications listed methylthio modification of cysteine, and variable modifications included oxidation of methionine and glycinylglycine modification of lysine. Proteins with at least one peptide with a lysine residue tagged with glycinylglycine were considered to be ubiquitinated. When a protein identification was based on a single peptide from the double immunoaffinity workflow, spectra are presented in the Supporting Information. Subcellular location and function assignments of the identified proteins were made using the Protein Information Resource GO Slim (http://pir.georgetown.edu) using UniProt Gene Ontology annotations (July 2014).
Results and Discussion
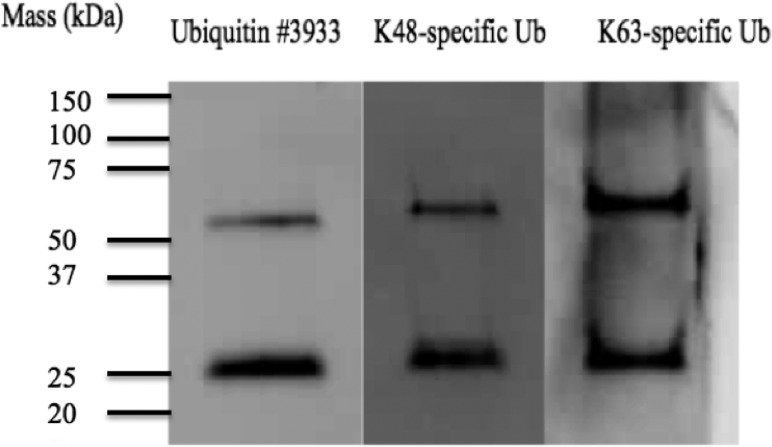
Western blotting was used to determine if MDSC-derived exosomes contain ubiquitinated proteins. A general ubiquitin antibody was used as well as antibodies that recognize K48 and K63 linkages in ubiquitin chains. Figure 1 confirms the presence of ubiquitinated proteins, including proteins with K48- and K63-linked branched ubiquitins. Consequently, mass spectrometry-based proteomic strategies were used to identify conjugated proteins. In-gel digestion of immunoprecipitated ubiquitin-conjugated proteins was performed and evaluated. Table 1 lists 16 ubiquitinated proteins identified from glycinylglycine-tagged peptides recovered from tryptic digestion in gel. Table 2 lists 38 proteins identified from glycinylglycine-modified peptides isolated after tryptic digestion in solution. The experimental design, with immunoaffinity isolation of glycinylglycine-modified peptides, leads to several protein identifications that are each based on a single tagged peptide and thus are less reliable than those listed in Table 1. These are listed in Table 2, and annotated spectra are provided in Supporting Information.
Figure 1.
Western blots of lysates from MDSC-derived exosomes. Antibodies used are (left) anti-ubiquitin 3933, (middle) anti-K-48-linked polyubiquitin, and (right) anti-K-63-linked polyubiquitin.
Table 1. Ubiquitinated Proteins and Peptides Identified from In-Gel Digestion of Exosomal Proteins with Glycinylglycine-Modified Lysine Residues.
| protein accession | protein name | no. of nonoverlapping peptides identified | protein FDR | peptide sequence |
|---|---|---|---|---|
| F6XI62 | 60S ribosomal protein L7 (Fragment)a | 2 | 2.450 × 10–5 | REKKKKVATVPGTLKKKVPAGPKTLK(GG)K |
| P61161 | Actin-related protein 2 | 3 | 6.486 × 10–4 | VVVCDNGTGFVK(GG) |
| P26040 | Ezrin | 3 | 6.490 × 10–4 | EELMLRLQDYEQK(GG)TKR |
| P17156 | Heat shock-related 70 kDa protein 2 | 5 | 4.860 × 10–6 | HWPFRVVSEGGK(GG)PK(GG) |
| P63158 | High mobility group protein B1 | 4 | 5.614 × 10–4 | WK(GG)TMSAK(GG) |
| P10922 | Histone H1.0 | 4 | 5.610 × 10–5 | AAKPKKAASK(GG)APSK |
| K(GG)KPAATPK(GG)K | ||||
| KAKKPK(GG)VVK | ||||
| ASK(GG)PKKAKTVKPK | ||||
| P15864 | Histone H1.2 | 11 | 3.920 × 10–14 | K(GG)ATGAATPKKAAK |
| AKKPAAAAVTK(GG)K | ||||
| K(GG)VAKSPK | ||||
| KAK(GG)VTKPKK | ||||
| AAK(GG)PKVAK | ||||
| P43277 | Histone H1.3 | 9 | 2.738 × 10–10 | TPVKK(GG)KAK(GG) |
| SPKKVKAAK(GG)PK | ||||
| KAAKSPAKAK(GG) | ||||
| AKASK(GG)PKASKPK | ||||
| P43274 | Histone H1.4 | 4 | 1.250 × 10–10 | AKKPAGAAK(GG) |
| TVKPKAAKPK(GG)TSK(GG) | ||||
| P43276 | Histone H1.5 | 10 | 3.040 × 10–18 | AKK(GG)TGAAKAK |
| AKKPAGATPKKPKK(GG) | ||||
| K(GG)PAAAGVK | ||||
| VTKPKTAKPK(GG)AAKAK | ||||
| Q07133 | Histone H1t | 3 | 6.486 × 10–4 | GKGK(GG)KSASAK(GG) |
| TK(GG)AVKKPKATPTK(GG) | ||||
| P27661 | Histone H2A.x | 5 | 4.860 × 10–6 | K(GG)SSATVGPK(GG)APAVGKK |
| P62806 | Histone H4 | 6 | 4.207 × 10–7 | GKGGK(GG)GLGK(GG)GGAK |
| Q6IFX2 | Keratin, type I cytoskeletal 42a | 9 | 2.729 × 10–10 | NK(GG)ILAATIDNASIVLQIDNAR |
| P08071 | Lactotransferrin | 29 | 1.522 × 10–31 | GDADAMSLDGGYIYTAGK(GG) |
| P52480 | Pyruvate kinase isozymes M1 | 7 | 3.640 × 10–8 | GPEIRTGLIKGSGTAEVELK(GG)K |
Indicates that the protein has been reported previously to be ubiquitinated.
Table 2. Ubiquitinated Proteins and Peptides Identified Following Immunoaffinity Enrichment of Tryptic Peptides with Glycinylglycine-Modified Lysines.
| protein accession | protein name | no. of peptides identified | no. of nonoverlapping K(GG) containing peptides | peptide FDR | peptide sequence |
|---|---|---|---|---|---|
| F6XI62 | 60S ribosomal protein L7 (Fragment)a,b | 2 | 1 | 7.83 × 10–2 | REKKKKATVPGTLKKKVPAGPKTLK(GG)K |
| Q5SWU9 | Acetyl-CoA carboxylase 1 | 1 | 1 | 4.48 × 10–2 | FGGNKVIEKVLIANNGIAAVK(GG)CMRSIR |
| E0CYH9 | Carboxyl-terminal PDZ ligand of neuronal nitric oxide synthase protein | 1 | 1 | 5.10 × 10–2 | KKKVSIMVSVDGVKVILK(GG)KKKKLLLLQK |
| Q6P925 | Cysteine-rich perinuclear theca 4 | 1 | 1 | 5.10 × 10–2 | AK(GG)RSKLKKKRNPRSKLPK(GG)RSRHSLIR |
| Q9CQJ6 | Density-regulated protein | 2 | 1 | 5.10 × 10–2 | QKK(GG)K(GG)TVPQKVTIAKIPRAKKKYVTR |
| P08113 | Endoplasmin | 2 | 1 | 3.63 × 10–2 | LLKVIRK(GG)KLVR |
| P43275 | Histone H1.1 | 10 | 2 | 5.10 × 10–2 | KTVK(GG)TPKKPKKPAVSKKTSKSPKKPKVVK |
| 5.10 × 10–2 | AKKVAKSPAKAKAVKPKASKAKVTKPK(GG)TPAKPK | ||||
| P15864 | Histone H1.2b | 11 | 5 | 8.66 × 10–2 | K(GG)ATGAATPKKAAK |
| 8.66 × 10–2 | AKKPAAAAVTK(GG)K | ||||
| 8.66 × 10–2 | K(GG)VAKSPK | ||||
| 8.66 × 10–2 | KAK(GG)VTKPKK | ||||
| 8.66 × 10–2 | AAK(GG)PKVAK | ||||
| P43274 | Histone H1.4b | 4 | 2 | 8.66 × 10–2 | AKKPAGAAK(GG) |
| 8.66 × 10–2 | TVKPKAAKPK(GG)TSK(GG) | ||||
| P43276 | Histone H1.5b | 10 | 7 | 8.66 × 10–2 | AKK(GG)TGAAKAK |
| 8.66 × 10–2 | AKKPAGATPKKPKK(GG) | ||||
| 8.66 × 10–2 | K(GG)PAAAGVK | ||||
| 8.66 × 10–2 | VTKPKTAKPK(GG)AAKAK | ||||
| P15975 | Inactive ubiquitin carboxyl-terminal hydrolase 53 | 1 | 1 | 4.48 × 10–2 | MAWVK(GG)FLRKPSGNLGK |
| B2RXC2 | Inositol 1,4,5-trisphosphate 3-kinase B | 1 | 1 | 2.15 × 10–2 | GTPASPRCGSPTPMETDK(GG)RVAPSLER |
| Q61781 | Keratin type I cytoskeletal 14 | 2 | 1 | TIEDLKSK(GG)ILAATVDNANVLLQIDNAR | |
| Q6IFX2 | Keratin, type I cytoskeletal 42a,b | 9 | 1 | 8.66 × 10–2 | NK(GG)ILAATIDNASIVLQIDNAR |
| Q924L1 | LETM1 domain-containing protein 1 | 1 | 1 | 8.52 × 10–3 | MKGIQMLWADGKK(GG)AR |
| Q0P5X1 | Leucine-rich repeat and IQ domain-containing protein 1 | 1 | 1 | 5.10 × 10–2 | KLRKKLEPSVRLALFKKAK(GG)NK(GG)VSVTK |
| P51960 | Myb-related protein A | 1 | 1 | 8.52 × 10–3 | WSLIAK(GG)HLK(GG)GR |
| E9Q5F6 | Polyubiquitin-C (Fragment) | 4 | 2 | 1.87 × 10–3 | TLSDYNIQK(GG)ESTLHLVLR |
| 5.10 × 10–2 | MQIFVK(GG)TLTGK | ||||
| Q9Z100 | Probable carboxypeptidase X1 | 2 | 1 | 2.60 × 10–3 | LRVIKKKKIVVKKRK(GG)KLR |
| H3BKN5 | Probable global transcription activator SNF2L2 | 1 | 1 | 9.13 × 10–3 | VLGRK(GG)LPKKKRVRKKAMK(GG)KR |
| H3BL88 | Protein 9930021J03Rik | 2 | 1 | 5.10 × 10–2 | K(GG)LKLTKMRAKKKKKKK |
| E9Q6J5 | Protein Bod1l | 2 | 1 | 3.99 × 10–3 | IKEVLKERKVLEKKV(GG)ALSKRRRK |
| J3QQ16 | Protein Col6a3 | 1 | 1 | 3.63 × 10–2 | DLK(GG)IMVLMLTGDMQR |
| A2AU83 | Protein GM14124 | 1 | 1 | 1.02 × 10–2 | AFSSPSGFLYHK(GG)R |
| E9PZM7 | Protein Scaf11 | 1 | 1 | 5.10 × 10–2 | RK(GG)SVRRGRK(GG)PPLLKKKLRR |
| G3UWJ2 | Protein Zfp69 | 1 | 1 | 2.93 × 10–2 | GEGPCMAESQGPEDPILDVKNKLETK(GG) |
| F6SB18 | RNA-binding protein 28 | 2 | 1 | 5.10 × 10–2 | KVLALPSHRGPKIRRLKERLRRIRQK(GG) |
| Q8C4U3 | Secreted frizzled-related protein 1 | 2 | 1 | 1.29 × 10–5 | IVPKKKKPLKLGPIKKK(GG)ELKRLVLFLK |
| Q9CZ91 | Serum response factor-binding protein 1 | 2 | 1 | 4.05 × 10–4 | KEVKRIRVLVIRK(GG)LVRSVGRLKSKK |
| Q9CXH7 | Shugoshin-like 1 | 2 | 1 | EKRNKNLAGIGK(GG) | |
| G5E861 | Sodium channel and clathrin linker 1 | 1 | 1 | 3.77 × 10–2 | LQQENEQLQKETEDLRKVALEAQK(GG) |
| Q6PHS6 | Sorting nexin-13 | 1 | 1 | 5.28 × 10–3 | DDQVK(GG)GTAEDLVETFFEVEVEMEK |
| D3Z1Z3 | Sphingosine-1-phosphate lyase 1 | 1 | 1 | 5.10 × 10–2 | KKLFKLIRKMPFIGRKVSKAK(GG)KDLVK(GG) |
| Q9CSP9 | Tetratricopeptide repeat protein 14 | 1 | 1 | 5.10 × 10–2 | TK(GG)K(GG)IETRAEKLRKLLKEEKRLKKK |
| P40630 | Transcription factor A, mitochondrial | 2 | 1 | 2.45 × 10–5 | QRRLKKKALVKRRELILLGKPK(GG)R |
| Q5HZG4 | Transcription initiation factor TFIID subunit 3 | 2 | 1 | 5.10 × 10–2 | LPSSVDVKKKLKKELKTKLKK(GG)KEKQR |
| Q6ZPJ3 | Ubiquitin-conjugating enzyme E2 O | 1 | 1 | 5.10 × 10–2 | KKSIPLSIKNLK(GG)RK(GG)HKRKKNKVTR |
Indicates that the protein has been reported previously to be ubiquitinated.
Indicates that the protein was also identified in the in-gel digestion.
To report a protein as being ubiquitinated, reliable identification was required of at least one peptide containing a KGG residue. Initial identifications were made by the PepArML meta-search engine (see Experimental section), and all candidate tandem mass spectra were confirmed manually (Supporting Information Figure S1). A combined total of 65 tryptic peptides containing modified lysines correspond to 50 ubiquitinated proteins (Supporting Information Table S1). Only 10 of the 50 proteins identified were previously reported in an MDSC-derived exosome lysate,7 demonstrating that enrichment for ubiquitinated proteins enabled the identification of low-abundance proteins in exosomes. As suggested by the western blots in Figure 1, a polyubiquitin fragment was characterized with multiple branch sites. Several histones were also observed to be conjugated at multiple unique and nonoverlapping sites, and the pro-inflammatory high mobility group protein B1 (HMG B1) was found to be ubiquitinated. The glycinylglycine-lysine sites identified experimentally were compared to ubiquitination sites predicted in silico by a ubiquitination prediction tool, UbiProber.26 Of the 65 peptides identified, the glycinylglycine-lysine sites in 42 peptides are ubiquitination sites predicted with probabilities > 0.7, UbiProber’s confidence level.
Tables 1 and 2 also present the glycinylglycine-tagged peptides, where it can be seen that 15 of the 65 peptides have been formed by tryptic cleavage at GG-derivatized lysine residues. This unexpected tryptic cleavage has been observed previously by others,27,28 and a control experiment was also carried out to confirm its occurrence under the conditions of the present investigation. A commercial ubiquitin dimer linked with an isopeptide bond at K48 was subjected to tryptic digestion as described in the Experimental Section. Both the expected peptide LIFAGKGGQLEDGR and the irregular peptide LIFAGKGG were identified by tandem mass spectrometry in approximately a 3:1 ratio (data not shown). This further supports the assignments in Tables 1 and 2.
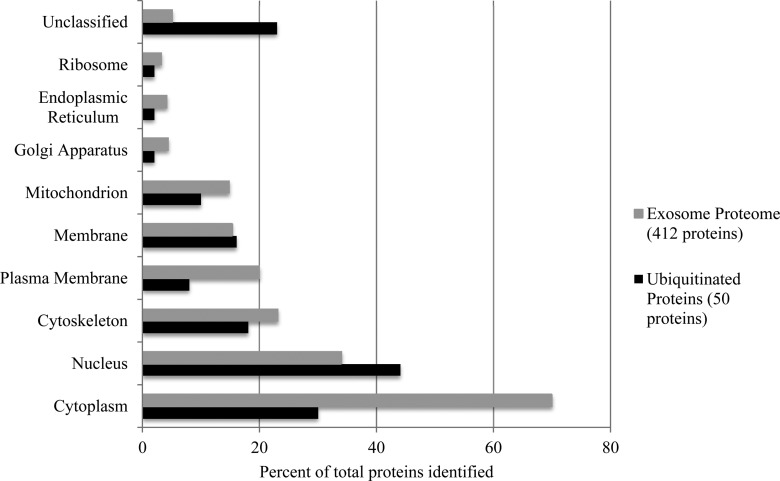
Several comparisons were made between the ubiquitinated proteins and a larger set of 412 proteins identified in an earlier study of MDSC exosome lysates.7 Figure 2 presents a comparison of the UniProt-derived locations of exosome proteins referenced to parental MDSC and illustrates an increased percentage of nuclear proteins in the ubiquitinated cohort and significantly lower percentages of cytosolic and plasma membrane proteins. The ubiquitinated nuclear proteins include nine histones and isoforms as well as other nucleic acid binding proteins (transcription factor A, mitochondrial, density regulated protein, transcription initiation factor TFIID subunit 3, and protein Bodl1) (Tables 1 and 2). It should be noted that histones, especially linker histones such as the histone H1 family, have been reported to be located in the cytoplasm and cell surface as well as the nucleus29 and that several histones are already known to be ubiquitinated, e.g., refs (30−32). The observation of ubiquitinated histones was confirmed by western blotting using anti-ubiquitin antibody 3933 on the histone fraction recovered using a total histone extraction kit (see Experimental Section) (data not shown). Figure 2 also indicates that 12 of the ubiquitinated proteins have no assigned cellular location.
Figure 2.
Protein locations assigned to MDSC-derived exosomal lysate (412 proteins) in gray and the ubiquitinated cohort (50 proteins) in black. Some proteins have multiple locations.
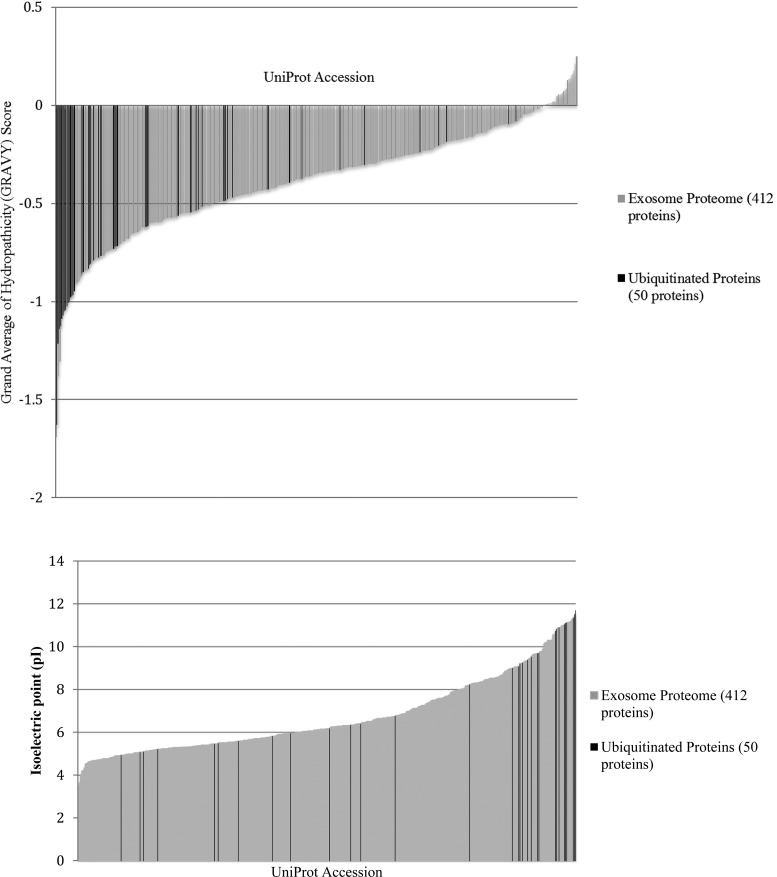
Gene Ontology annotations and the UniProt database were used to compare distributions of protein sizes (without ubiquitin), grand average of hydropathicity (GRAVY) scores, and isoelectric points (pI) of the 50 ubiquitinated proteins identified. The intact masses of the proteins are somewhat evenly distributed between 11 and 327 kDa (excluding ubiquitination). The GRAVY scores and pI distributions are shown in Figure 3, panels a and b, respectively. Although both sets of data illustrate a wide range of GRAVY scores (−0.081 to −1.627) and pI values (4.72 to 11.71), proteins that are ubiquitinated in MDSC-derived exosomes tend to cluster as hydrophilic and basic proteins. Seventy-two percent of the proteins identified have a GRAVY score less than −0.5, and 50% of the proteins have a pI greater than 9.00. (Ubiquitin is not included in these calculations; its monomer has a GRAVY score of −0.489 and a pI value of 6.56.) The bias toward a high pH is consistent with observations by Chen and co-workers,26 who report a greater abundance of positively charged amino acids in ubiquitinated proteins.
Figure 3.
Distribution of (top) grand average of hydropathicity score (GRAVY) and (bottom) isoelectric point of MDSC-derived exosomal lysate (412 proteins) in gray and the ubiquitinated cohort (50 proteins) in black.
Among the combined cohort of 50 proteins, 34 have not been previously reported to be ubiquitinated (Tables 1 and 2). Among these, sorting nexin 13 has been observed to participate in endosomal trafficking of ubiquitinated proteins.33 Identification of two ubiquitinated keratins is consistent with the proposed role of protein aggregation in invagination, the initial step of exosome formation.34,35 Other ubiquitinated proteins that are thought to play important roles in endosome and exosome formation include leucine zipper EF hand-containing transmembrane protein 1 (LETM1) and endoplasmin. Although the functions of the ubiquitinated proteins are not known, these two unconjugated proteins participate in transporting and maintaining the high luminal concentration of Ca2+ required for optimal exocytosis of exosomes.36
Conclusions
On the basis of protein assay results, approximately 10% of the MDSC-derived exosome lysate comprises ubiquitinated proteins. Tandem mass spectrometry coupled with immunoprecipitation has been used successfully to isolate and identify 50 ubiquitinated proteins from MDSC-derived exosomes and to determine their positions of conjugation. Five of these are associated with formation of endosomes and exosomes, consistent with earlier proposals. The skew of pI values toward basicity in the conjugated cohort of exosomal proteins may contribute to their concentration and retention by the progressive acidification that has been documented37 along the endosomal pathway. The presence of ubiquitinated histones in these exosomes should be considered in the context of heightened interest in extracellular histones and their proinflammatory activity.38−40 HMG B1 is another pro-inflammatory mediator of particular interest since it was recently established as a driver of MDSC accumulation and suppressive potency.41 Interestingly, S100 A8 and S100 A9, exosomal pro-inflammatory proteins previously demonstrated7 to contribute to the bioactivity of MDSC, have not been identified with a glycinylglycine-lysine modification, even though they are quite abundant and readily identified in the exosome data sets.
Acknowledgments
The research was supported by a grant from the National Institutes of Health, GM021248, and by a grant to the University of Maryland from the Howard Hughes Medical Institute Undergraduate Science Education Program.
Supporting Information Available
Table S1. Peptides and proteins identified. Figure S1. Annotated MS/MS spectra for all single peptide protein identifications. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Pan B.-T.; Teng K.; Wu C.; Adam M.; Johnstone R. M. Electron microscopic evidence of the transferrin receptor in vesicular form in sheep reticulocutes. J. Cell Biol. 1985, 101, 942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer K.; Kleijmeer M. J.; Heijnen H. F. G.; Stoorvogel W.; Geuze H. J. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113, 3365–3374. [DOI] [PubMed] [Google Scholar]
- Johnstone R. M.; Adam M.; Hammond J. R.; Orr L.; Turbide C. Vesicle formation during reticulocyte maturation. J. Biol. Chem. 1987, 262, 9412–9420. [PubMed] [Google Scholar]
- Simons M.; Raposo G. Exosomes—vesicular carriers for intracellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [DOI] [PubMed] [Google Scholar]
- Taylor D. D.; Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [DOI] [PubMed] [Google Scholar]
- Gabrilovich D. I.; Ostrand-Rosenberg S.; Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M.; Choksawangkarn W.; Edwards N.; Ostrand-Rosenberg S.; Fenselau C. Exosomes from myeloid-derived suppressor cells carry biologically active proteins. J. Proteome Res. 2014, 13, 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauro B. J.; Greening D. W.; Mathias R. A.; Mathivanan S.; Ji H.; Simpson R. J. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol. Cell. Proteomics 2013, 12, 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton J. L.; Khanna S.; Giles P. J.; Brennan P.; Brewis I. A.; Staffurth J.; Mason M. D.; Clayton A. Proteomics analysis of bladder cancer exosomes. Mol. Cell. Proteomics 2010, 9, 1324–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Hill S.; Luther J. M.; Hachey D. L.; Schey K. L. Proteomic analysis of urine exosomes by multidimensional protein identification technology (MudPIT). Proteomics 2012, 12, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B.; Peng P.; Chen S.; Li L.; Zhang M.; Cao D.; Yang J.; Li H.; Gui T.; Li X.; Shen K. Characterization and proteomic analysis of ovarian cancer-derived exosomes. J. Proteomics 2013, 80, 171–182. [DOI] [PubMed] [Google Scholar]
- Henderson M. C.; Azorsa D. O. The genomic and proteomic content of cancer cell-derived exosomes. Front. Oncol. 2012, 2, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann D. J.; Odorizzi G.; Emr S. D. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Bio. 2002, 3, 893–905. [DOI] [PubMed] [Google Scholar]
- Raposo G.; Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2001, 2, 195–201. [DOI] [PubMed] [Google Scholar]
- Piper R. C.; Lehner P. J. Endosomal transport via ubiquitination. Trends Cell Biol. 2011, 21, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno H.; Komada M. The ubiquitin code and its decoding machinery in the endocytic pathway. J. Biochem. 2013, 153, 497–504. [DOI] [PubMed] [Google Scholar]
- Buschow S. I.; Liefhebber J. M. P.; Wubbolts R.; Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood Cells, Mol., Dis. 2013, 35, 398–403. [DOI] [PubMed] [Google Scholar]
- Peng J.; Schwartz D.; Elias J. E.; Thoreen C. C.; Cheng D.; Marisischky G.; Roelofs J.; Finley D.; Gygi S. P. A proteomics approach to understanding protein ubiquitination. Nat. Biotechnol. 2003, 21, 921–926. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick D. S.; Denison C.; Gygi S. P. Weighing in on ubiquitin: the expanding role of mass-spectrometry-based proteomics. Nat. Cell Biol. 2005, 7, 750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt S. K.; Sinha P.; Clements V. K.; Leips J.; Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J. Immunol. 2005, 176, 284–290. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S.; Dell’Angelica E. C.; Springer T. A. Immunoprecipitation. Curr. Protoc. Immunol. 2001, 41, 8.3.1–8.3.28. [DOI] [PubMed] [Google Scholar]
- Shevchenko A.; Tomas H.; Havliš J.; Olsen J. V.; Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007, 1, 2856–2860. [DOI] [PubMed] [Google Scholar]
- Edwards N.; Wu X.; Tseng C.-W. An unsupervised, model-free, machine-learning combiner for peptide identifications from tandem mass spectra. Clin. Proteomics 2009, 5, 23–36. [Google Scholar]
- Risk B. A.; Edwards N. J.; Giddings M. C. A peptide-spectrum scoring system based on ion alignment, intensity, and pair probabilities. J. Proteome Res. 2013, 12, 4240–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Qiu J.-D.; Shi S.-P.; Suo S.-B.; Huang S.-Y.; Liang R.-P. Incorporating key position and amino acid residue features to identify general and species-specific ubiquitin conjugation sites. Bioinformatics 2013, 29, 1614–1622. [DOI] [PubMed] [Google Scholar]
- Denis N. J.; Vasilescu J.; Lambert J. P.; Smith J. C.; Figeys D. Tryptic digestion of ubiquitin standards reveals an improved strategy for identifying ubiquitinated proteins by mass spectrometry. Proteomics 2007, 7, 868–874. [DOI] [PubMed] [Google Scholar]
- Xu G.; Paige J. S.; Jaffrey S. R. Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nat. Biotechnol. 2010, 28, 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parseghian M. H.; Luhrs K. A. Beyond the walls of the nucleus: the role of histones in cellular signaling and innate immunity. Biochem. Cell Biol. 2006, 84, 589–604. [DOI] [PubMed] [Google Scholar]
- Haas A. L.; Bright Reback P.; Chau V. Ubiquitin conjugation by the yeast RAD6 and CDC34 gene products. J. Biol. Chem. 1991, 266, 5104–5112. [PubMed] [Google Scholar]
- Wang H.; Zhai L.; Xu J.; Joo H.-Y.; Jackson S.; Erdjument-Bromage H.; Tempst P.; Xiong Y.; Zhang Y. Histone H3 and H4 ubiquitylation by the CUL4-DDB-ROC1 ubiquitin ligase facilitates cellular response to DNA damage. Mol. Cell 2006, 22, 383–394. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Zhu P.; Wang J.; Pascual G.; Ohgi K. A.; Lozach J.; Glass C. K.; Rosenfeld M. G. Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcription elongation. Mol. Cell 2008, 29, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilthorpe J. D.; Oozeer F.; Nash J.; Calvo M.; Bennett D. L.; Lumsden A.; Pini A. Extracellular histone H1 is neurotoxic and drives a pro-inflammatory response in microglia. F1000Research 2013, 2, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z.; Liu Y.; Li F.; Ren F.; Chen D.; Li X.; Wen T. Circulating histones exacerbate inflammation in mice with acute liver failure. J. Cell. Biochem. 2013, 114, 2384–2391. [DOI] [PubMed] [Google Scholar]
- Allam R.; Kumar S. V.; Darisipudi M. N.; Anders H. Extracellular histones in tissue injury and inflammation. J. Mol. Med. 2014, 92, 465–472. [DOI] [PubMed] [Google Scholar]
- Zheng B.; Tang T.; Tang N.; Kudlicka K.; Ohtsubo K.; Ma P.; Marth J. D.; Farquhar M. G.; Lehtonen E. Essential role of RGS-PX1/sorting nexin 13 in mouse development and regulation of endocytosis dynamics. Proc. Nat. Acad. Sci. U.S.A. 2006, 103, 16776–16781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J.; Scott C. C. Ion flux and the function of endosomes and lysosomes: pH is just the start. BioEssays 2011, 33, 103–110. [DOI] [PubMed] [Google Scholar]
- Vidal M.; Mangeat P.; Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J. Cell Sci. 1997, 110, 1867–1877. [DOI] [PubMed] [Google Scholar]
- Janig E.; Stumptner C.; Fuchsbichler A.; Denk H.; Zatloukal K. Interaction of stress proteins with misfolded keratins. Eur. J. Cell Biol. 2005, 84, 329–339. [DOI] [PubMed] [Google Scholar]
- Sannerud R.; Saraste J.; Goud B. Retrograde traffic in the biosynthetic-secretory route: pathways and machinery. Curr. Opin. Cell Biol. 2003, 15, 438–445. [DOI] [PubMed] [Google Scholar]
- Parker K.; Sinha P.; Horn L. A.; Clements V. K.; Yang H.; Li J.; Tracey K. J.; Ostrand-Rosenberg S. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014, 85, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.