Abstract
The proprotein convertase subtilisin/kexin (PCSK) enzymes proteolytically convert immature proproteins into bioactive molecules and thereby they serve as key regulators of cellular homeostasis. The archetype PCSK, FURIN is a direct target gene of the IL-12/STAT4 pathway and it is upregulated in T helper 1 type cells. We have previously demonstrated that FURIN expression in T cells critically regulates the maintenance of peripheral immune tolerance and the functional maturation of pro-TGFβ-1 in vivo, but FURIN’s role in cell-mediated immunity and Th polarization has remained elusive. Here, we show that T-cell-expressed FURIN is essential for host resistance against a prototypic Th1 pathogen, Toxoplasma gondii and for the generation of pathogen-specific Th1 lymphocytes, including Th1-IL-10 cells. FURIN-deficient Th cells instead show elevated expression of IL-4 receptor subunit alpha (IL-4Rα) on cell surface, sensitized IL-4/STAT6 signaling and a propensity to polarize towards the Th2 phenotype. By exploring FURIN-interacting proteins in Jurkat T cells with Strep-Tag purification and mass-spectrometry we further identify an association with a cytoskeleton modifying RAC/DOCK2 protein complex and unravel that FURIN promotes F-actin polymerization, which has previously been shown to down-regulate IL-4Rα cell surface expression and promote Th1 responses. In conclusion, our results demonstrate that in addition to peripheral immune tolerance, T-cell-expressed FURIN is also a central regulator of cell-mediated immunity and Th1/2 cell balance.
Keywords: Th1/Th2 Cells, Parasitic-Protozoan, Cytokines, Cell Differentiation, FURIN, Proprotein Convertase
INTRODUCTION
Upon encountering the cognate antigen naïve CD4+ T helper cells (Th) activate and polarize into functionally distinct subsets that include Th type 1 (Th1), Th2, Th17 and T regulatory cells (Treg). The fate of a naïve T helper cell depends on the length and strength of the antigen stimulus as well as on surrounding cytokine milieu, both of which activate cellular signaling pathways and expression of Th subtype specific genes. (1) The first characterized subtypes, Th1 and Th2, determine the balance between cell-mediated and humoral immune responses. Th1 polarization is characteristically driven by a strong antigen stimulus in the presence of the cytokine interleukin (IL)-12. These trigger the activation of transcription factor STAT4 and elevated T-bet expression, followed by secretion of the Th1 type effector cytokines. Th2 polarization, in contrast, is initiated by a weak antigen stimulus and IL-2/STAT5 signaling resulting in upregulation of GATA-3 and IL-4 induced STAT6 activation. (2) Th1 cells secrete IFN-γ and TNF cytokines that promote for example the activation of macrophages in intracellular infections, whereas the Th2 cytokines IL-4, IL-5 and IL-13 induce IgE class switching in B cells, as well as the activation of eosinophils, both of which are required to restrain parasitic infections. (3)
Dysregulation of T helper cells is associated with immune-mediated diseases, including infections, immunodeficiencies, autoimmunity and allergic responses (4). Insufficient Th1 polarization, for example, increases susceptibility to mycobacterial infections, whereas activated Th1 cells are often overrepresented in autoimmune diseases (2). Consequently, much research effort has been aimed at identifying the detailed molecular mechanisms that control Th polarization events over the past two decades. We and others have previously shown that a proprotein convertase subtilisin/kexin (PCSK) family protease FURIN is a direct target gene for IL-12/STAT4 and TGF-β1, and that it is highly expressed in human Th1 cells (5–7). PCSK enzymes are a family of nine proteases (PCSK1-2, FURIN, PCSK4-7, MBTPS1, PCSK9) that cleave and convert their immature target proteins into biologically active forms by catalyzing endoproteolytic cleavage at a target site typically made up of basic amino acids arginine and lysine (8). Accordingly, PCSK enzymes play a key regulatory role in a multitude of biological events, including development and hormone function (9). Dysregulated proprotein convertase activity also contributes to pathogenic cell behavior, and therefore interfering with PCSK activity is currently being considered as a potential treatment for many diseases including atherosclerosis (10), rheumatoid arthritis (11) and multiple sclerosis (12).
PCSK enzymes are also central regulators in host-defense; inhibiting PCSK activity can prevent the proteolytic activation of bacterial toxins and viral entry (8), and recently a FURIN-like convertase was shown to regulate human Toll-like receptor 7 (TLR7) processing and subsequent antiviral immunity (13). In addition, deleting PCSK1 expression in mice is associated with accelerated pro-inflammatory responses (14), whereas PCSK7 participates in rescuing the unstable MHC-I molecules on dendritic cells (15) and regulates the bioavailability of TGF-β1a cytokine in zebrafish (16). Biochemical analyses have also demonstrated that the PCSK controlled proteolytic cascades are important in the functional maturation of several proteins critical for host defense such as integrins, matrix metalloproteinases and cytokines. (9, 17)
We have previously shown that the T-cell-expressed proprotein convertase FURIN is essential for maintaining Treg-mediated peripheral immune tolerance. CD4cre-furf/f mice develop an age-related systemic autoimmune disease that is characterized by circulating autoantibodies, overtly activated CD4+ and CD8+ T cells and over-production of both Th1 and Th2 cytokines, IFN-γ, IL-4 and IL-13. (18) The accelerated immune responses in CD4cre-furf/f mice could be chiefly attributed to reduced bioavailability of a central anti-inflammatory cytokine TGF-β1 in T cells. Notably, the role of FURIN in controlling the T helper cell subsets appears complex and is not limited to CD4+FoxP3+ Treg cells; inhibiting FURIN by a recombinant inhibitor (α1-antitrypsin Portland) or small interfering RNA can also abrogate the production of the Th1 effector cytokine IFN-γ by human CD4+ T cells (5). Here, we show that FURIN expression in T cells is critical for ensuring an appropriate immune response against a prototypic Th1 parasite, Toxoplasma gondii. Specifically, our data demonstrate that FURIN expression is needed for adequate antigen specific Th1 cell generation and lack of FURIN expression in T cells results in the inherent upregulation of IL-4Rα on the cell surface and dominance of Th2 polarization. Using Strep-tag purification and mass spectrometry we further show that FURIN interacts with a Th1 promoting and IL-4Rα-inhibiting RAC/DOCK2 protein complex and regulates T cell cytoskeleton dynamics by increasing its activity.
MATERIALS AND METHODS
Mice
T-cell-specific FURIN knockout (CD4cre-furf/f) mice on C57BL/6 background have been described earlier (18, 19), IL-12p40 knockout animals were purchased from Taconic. For ovalbumin specific Th polarization experiment, CD4cre-furf/f and furf/f littermate controls were crossed with mice bearing OTII TCR to generate CD4cre+/− furf/f-OTII mice, which have ovalbumin specific CD4+ T cells. All mice were housed under pathogen-free conditions in accordance with the National Institutes of Health Animal Care and Use Committee (NIH, US) or National Animal Experiment Board (Finland).
Cell culture and transfections
Jurkat E6-1 T cells (ATCC: TIB-152) were cultured in RPMI 1640 medium and HeLa cells (ATCC: CCL2) in DMEM medium, both supplemented with 10% fetal bovine serum (FBS), L-glutamine and antibiotics. To generate the stable wild type (wt) and inactive FURIN (mut) expressing cell lines, Jurkat E6-1 T cells were electroporated (1025 uF, 260 V) with pcDNA3.1-hFURINwt-StrepIII, pcDNA3.1-hFURIN-D153Amut-StrepIII, or pcDNA3.1-StrepIII as control (a kind gift from Prof. Jukka Westermarck, Turku Centre for Biotechnology, Finland). Geneticin antibiotic (600 μg/ml) was added to the cell cultures three days post transfection. cDNA expressing cell lines were then selected with clonal dilution and constant expression of recombinant protein was evaluated by western blot analysis (anti-FURIN MON-152, Enzo LifeSciences). Cell lines with equal wt and mut FURIN expression were selected for the interactome and F-actin polymerization studies. HeLa cells were transiently transfected with 0.5–1 μg pcDNA-RAC1-V5, pCI-DOCK2-FLAG (kind gifts from Prof. Yoshinori Fukui, Kyushu University, Japan), and pcDNA3.1-hFURIN-MYC-His using FuGENE® 6 Transfection Reagent (Promega).
Cell purification, cytokine measurements and flow cytometry
To study ovalbumin induced Th1/Th2 polarization, naïve CD4+CD44lowCD62L+ T cells were purified using flow cytometry from CD4cre-furf/f or littermate control mice on an OTII background. Cells were labeled with CFSE (Invitrogen) and stimulated with graded concentrations of OVA-peptide (5–1000 nM) and sorted splenic CD11c+CD49b− dendritic cells (DC) under neutral conditions. After 3.5 days the cells were activated with PMA (10 ng/ml) and ionomycin (1 μM) (EMD), and intracellular expression of IFN-γ, IL-4 and CFSE was analyzed with flow cytometry.
For polyclonal T cell activation studies naïve T cells were isolated with CD4+CD62L+ T Cell Isolation Kit (Miltenyi) from spleen and lymph nodes. Cells were activated for 72 hours with plate-bound anti-CD3 and -CD28 (10+10 μg/ml) antibodies in serum-free X-VIVO 20 medium (Lonza). IL-12 (10 ng/ml), TGF-β1 (0.5 ng/ml) or anti-IL-4 (2 μg/ml) were added to the cell cultures during the activation. Cytokines from cell culture medium were measured with CBA Th1/2/17 kit (BD) or IL-4 ELISA (Peprotech), and RNA was collected for Q-RT-PCR experiments.
For the detection of phosphorylated ERK, CD4+ T Isolation Kit (L3T4, Miltenyi) was used to isolate CD4+ T cells from spleen and lymph nodes. Cells were stimulated with soluble anti-CD3 (10 μg/ml) for 5 to 60 minutes, and phosphorylation of ERK was detected by western blot using ERK and pERK primary antibodies (Cell Signaling Technology), and anti-rabbit HRP-biotin conjugated secondary antibody (R&D Systems). Signal intensities were analyzed using NIH ImageJ software. IL-4Rα cell surface expression was analyzed from spleens and lymph nodes by staining the cells with anti-IL-4Rα (BD), CD4 and CD62L (eBiosciences) antibodies, and analyzed by flow cytometry. To detect tyrosine phosphorylated STAT6, naïve CD4+ T cells from spleen and lymph nodes were purified with the CD4+CD62L+ T cell isolation kit (Miltenyi) and activated with IL-4 (1 or 10 ng/ml) for 15 minutes. The cells were fixed with 4% paraformaldehyde and permeabilized with cold 90% methanol overnight. Staining was performed in 0.5% Triton X-100/0.1% BSA/PBS with anti-phospho-STAT6 antibody (BD).
All FACS analyses were performed using FACSCalibur or FACSCanto instrument (BD Pharmingen), and data was analyzed with Flow-Jo software (Treestar).
Quantitative real-time PCR
Total RNA was isolated with RNeasy kit (Qiagen), and reverse transcribed into cDNA with iScript kit (BioRad). Gene expressions were analyzed using SsoFast EvaGreen supermix and CFX96 instrument (BioRad). Primer sequences in 5′-3′ –direction and size of amplicons: T-bet (110 bp) FOR_TCAACCAGCACCAGACAGAG, REV_AAACATCCTGTAATGGCTTGTG; Gata-3 (75 bp) FOR_TTATCAAGCCCAAGCGAAG, REV_TGGTGGTGGTCTGACAGTTC; Il4ra (102 bp) FOR_GACTGGATCTGGGAGCATCA, REV_CAGTCCACAGCGCTATCCAG; 18s (143 bp) FOR_GTGATCCCTGAGAAGTTCCAG, REV_TCGATGTCTGCTTTCCTCAAC. The expression of each gene was normalized to ribosomal 18s gene. For FURIN and B2M housekeeping gene expression analysis in splenocytes after T. gondii infection pre-designed primers and probes from Applied Biosystems were used.
Immunofluorescence
Three days post transfection HeLa cells were fixed on coverslips with paraformaldehyde and stained with rabbit anti-FURIN (a kind gift from Prof. John Creemers, KU Leuven, Belgium) and mouse anti-V5 for RAC (Invitrogen). Nucleus was stained with DAPI, and specific protein expression was visualized with anti-rabbit TexasRed or anti-mouse Alexa-488 (both LifeTechnologies), using ApoTome microscope and AxioVision software (Zeiss).
Immunoprecipitation and western blotting
Transfected HeLa cells were lysed (lysis buffer: 50 mM Tris pH 7.5, 10 % glycerol, 150 mM NaCl, 1 mM EDTA, 1 % Triton X-100, 50 mM NaF, 1 mM TCEP, and Complete Mini protease inhibitor from Roche) and pre-cleared with Protein G sepharose 4 FastFlow beads (GE). Anti-FLAG antibody was used to capture DOCK2 and anti-MYC for FURIN, in parallel with antibody isotype and resin controls (all from Sigma). Protein elutes were separated with SDS–PAGE gel and transferred to nitrocellulose membrane. Immunodetection was performed using anti-FLAG or anti-MYC primary antibodies and anti-mouse HRP-conjugated secondary antibody (R&D Systems) Visualization was done using the ECL™ Western Blotting Detection –kit (GE Healthcare) and AGFA CP1000 imaging system.
F-actin polymerization
FURIN wt and control Jurkat E6-1 T cell lines were starved in RPMI supplemented with 1% FBS and stimulated with 250 ng/ml SDF-1α (Peprotech) for 0–120 seconds. Cells were immediately fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100, stained for polymerized F-actin (Phalloidin-FITC, Sigma) and analyzed with flow cytometry.
In vivo challenge with Toxoplasma gondii
CD4cre-furf/f, IL-12p40 KO and littermate control mice (6–10 weeks old) were challenged i.p. with 20 pepsin-treated cysts of the avirulent ME49 strain of T. gondii. Nine days post infection the FURIN expression was quantified in splenic CD4+ T cells by Q-RT-PCR and serum cytokines were measured with CBA (BD). Survival of FURIN T cell KO and littermate control animals was monitored for 100 days and quantified using Log-rank (Mantel-Cox) test. Some of the infected animals were sacrificed on 38 days post infection to analyze the number of T. gondii cysts in brain as well as antigen-specific Th1/Th1-IL-10 polarization and cytokine productions. Briefly, brains were isolated and homogenized by sequential passage through 19- and 21-gauge needles, and the number of cysts was determined microscopically. Antigen dependent cytokine production was induced ex vivo by stimulating splenocytes in complete RPMI + 10 % FBS with soluble T. gondii antigen (STAg, 5 μg/ml) for 72 hours. For flow cytometric analysis cells were stained for CD4, IFN-γ and IL-10 (BD). Cytokines were measured from cell culture supernatants with CBA (BD).
Sample preparation for mass spectrometry
Cell membrane fractions from Jurkat T cell lines were isolated (Mem-PER Eukaryotic Membrane Protein Extraction Kit, Thermo Scientific) and FURIN as well as associated proteins were affinity purified from lysates with Strep-tag columns (IBA). The presence and purity of recombinant FURIN in elutes was verified both with anti-FURIN (MON-152, Enzo Life Sciences) and anti-Strep (IBA) antibodies (data not shown). Eluted proteins were separated by 1D SDS-PAGE gel (Miniprotean precast gel, Biorad), and visualized by silver staining.
Mass spectrometry
Target bands were cut from silver stained gels, and after enzymatic protein digestion and extraction peptides were identified by MS (Proteomics Facility, Turku Centre for Biotechnology, Finland). Analysis was performed by LC/ESI-MS/MS on a nanoflow HPLC system coupled online to an Orbitrap Velos MS instrument. Database searches were performed by Mascot (version 2.2.6) against SwissProt (UniProt) protein sequence database (version 2010_09). Scaffold 3 software (Proteome Software, Inc) was used to further analyze identified proteins. Data was filtered through validation parameters (i.e. molecular weight match, min. 2 unique peptides, min. ~10% coverage).
Statistical analysis
Data represent mean ± standard error of the mean (SEM). Statistical significance was determined by non-parametric Mann-Whitney test for mouse experiments, and by two-tailed Student’s t test for cell line experiments, if not indicated otherwise. Survival after T. gondii infection was analyzed with Log-rank (Mantel-Cox) test. P-values less than 0.05 were considered statistically significant.
RESULTS
T-cell-expressed FURIN is essential for host resistance against Toxoplasma gondii
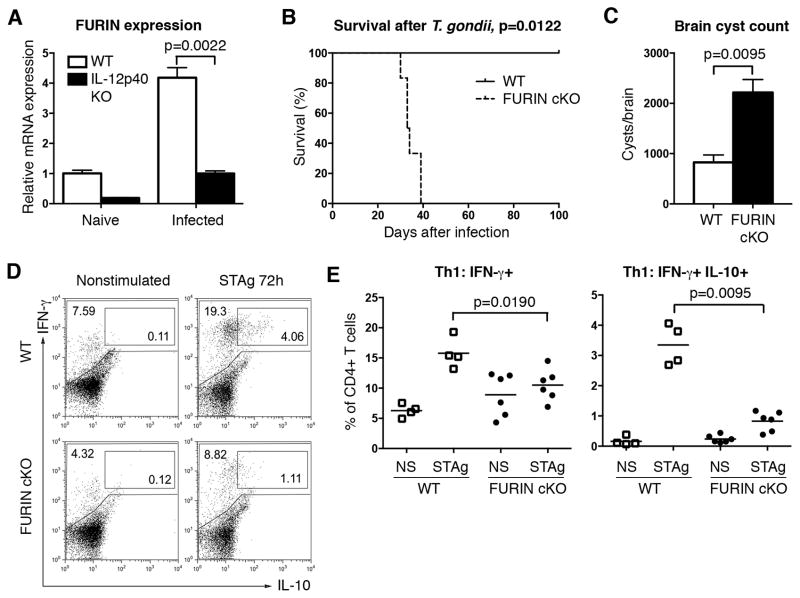
We initially identified FURIN as a novel IL-12 target gene using microarray expression analysis on human peripheral blood T lymphocytes (5). Subsequent experiments showed that the FURIN mRNA and protein are particularly highly expressed in Th1 cells and that FURIN is directly regulated by STAT4 chromatin binding, rather than in a T-bet dependent manner (20). Toxoplasma gondii is an obligate intracellular parasite that triggers a profound Th1-mediated cellular immune response characterized by elevated production of IFN-γ and TNF cytokines. We first assessed whether T. gondii infection induces FURIN expression in splenic CD4+ T cells by infecting both wild type and IL-12 deficient mice with avirulent T. gondii ME-49 strain parasites. In line with previous in vitro data showing an IL-12-dependent FURIN regulation (5), parasite-induced FURIN expression was significantly impaired in IL-12 deficient animals at 9 days post infection (Fig. 1A).
Figure 1. T-cell-expressed FURIN is critical for the host defense to Toxoplasma gondii.
(A) Wild type and IL-12p40 KO (n=6) mice were left uninfected (naïve, n=1) or infected (n=5) i.p with 20 T. gondii cysts (ME-49). Nine days after infection FURIN and B2M housekeeping gene expressions in splenic CD4+ T cells were determined with Q-RT-PCR. FURIN expression was normalized to B2M and relative expression in uninfected wild type CD4+ cells was arbitrarily set as 1. (B) Ten weeks old CD4cre-furf/f and littermate control mice were inoculated i.p. with 20 T. gondii ME-49 cysts. Survival was analyzed with Log-rank (Mantel-Cox) test between FURIN cKO mice (n=6) and littermate controls (n=3), experiment was repeated twice with identical results. (C) Brain cysts were calculated from moribund FURIN cKO mice (n=6) and littermate controls (n=4) on day 38 post infection. (D–E) Splenocytes from T. gondii infected FURIN cKO (n=6) and littermate (n=4) mice were left nonstimulated (ns) or stimulated ex vivo with soluble T. gondii antigen (STAg, 5 μg/ml) for 72 hrs. Production of IFN-γ and IL-10 was measured with flow cytometry in CD4+ T cells, representative cytokine stainings (D) and scatter blots (E) are shown. All figures represent mean ± SEM. Statistics were calculated with Mann-Whitney test except for survival analysis.
To study the potential role of FURIN in this prototypic Th1-inducing infection, we next infected mice in which FURIN was deleted specifically in T cells (CD4cre-furf/f) with T. gondii. Presence or absence of FURIN in T cells did not significantly influence survival or serum cytokine levels in the IL-12-dependent (21, 22), acute phase of parasite infection (Fig. 1B and Fig. S1). However, T. gondii infected CD4cre-furf/f mice had modestly reduced numbers of IL-10 producing Th1 cells at 9 days post infection (data not shown). Later in the course of infection all infected CD4cre-furf/f animals became moribund and ultimately succumbed to the infection (Fig. 1B). The lethality associated with the absence of FURIN in T cells could be due to either a host defense defect or an exaggerated inflammatory response (18). Analysis of parasite burden in moribund animals showed a significantly higher number of intracranial cysts in T cell FURIN KO mice suggesting inadequate protective host response (Fig. 1C) (23).
To better define the role of FURIN in mounting appropriate T. gondii-specific CD4+ T cell responses, splenocytes from infected CD4cre-furf/f or littermate mice were stimulated with soluble T. gondii antigen (STAg) for 72 hrs (Fig. 1D). Total splenocyte cell number was reduced in the declining CD4cre-furf/f mice, but CD4+/splenocyte ratios in the spleen were similar to those of the littermate control animals. The resistance to T. gondii infection is critically dependent on an adequate Th1 response and IFN-γ/TNF cytokine production (24–26), but also on the generation of Th1-IL-10+ cells, which restrict the magnitude of the infection-induced inflammation (24, 27). STAg stimulation resulted in an increase of the numbers of both Th1 and Th1-IL-10 cells in control mice, whereas the generation of antigen specific Th1 and Th1-IL-10 cells was significantly reduced in splenocytes isolated from CD4cre-furf/f mice, indicating that FURIN promotes cell-mediated immune responses (Fig. 1D–E). Profiling the antigen stimulated splenic cytokine production further showed a lower trend of IFN-γ levels accompanied with a significantly reduced production of another Th1 cytokine, TNF, but unchanged secretion of the innate immunity cytokines IL-6, IL-12 and MCP-1 (Fig. S2). Together, our data demonstrate a critical role for proprotein convertase FURIN in T cell-mediated host resistance against T. gondii and in the generation of pathogen specific Th1 and Th1-IL-10 responses.
FURIN regulates the Th1/Th2 balance
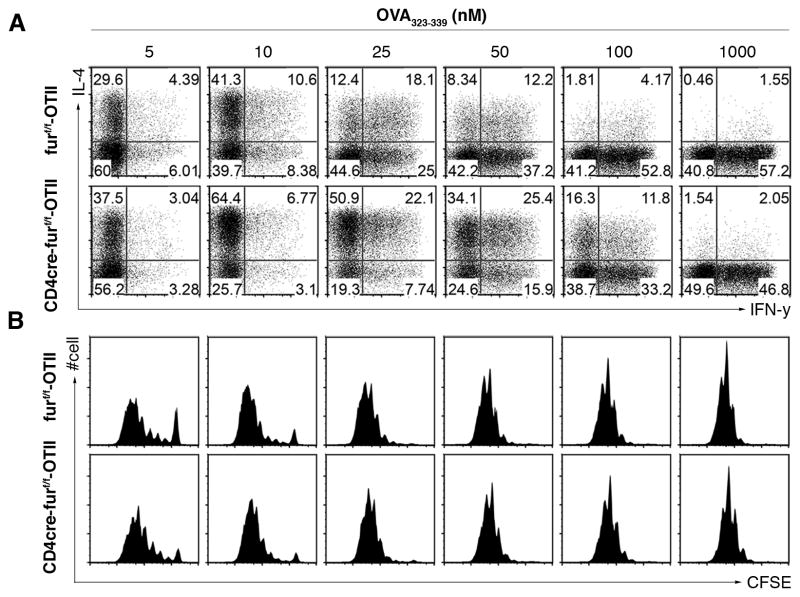
The failure of FURIN-deficient T cells to generate appropriate protective Th1 response prompted further investigation. To this end, we crossed CD4cre-furf/f mice with TCR transgenic (OTII) mice and stimulated the antigen-specific naïve CD4+ T cells (OTII+CD4+CD62L+CD44−) with varying concentrations of cognate antigenic peptide (ovalbumin, OVA), and analyzed the production of Th subtype cytokines and cell proliferation. As reported previously (28), in neutral culture conditions low dose antigen (5 nM) drove a predominant Th2 polarization of wild type CD4+ cells, as attested by high IL-4/IFN-γ ratio (Fig. 2). Conversely, increasing antigen (10–100 nM) favored generation of Th1 (IFN-γ+) cells. At a low OVA concentration FURIN cKO CD4+ cells showed modestly enhanced cell proliferation, and increased the IL-4+ Th2 cell proportions. Strikingly, stimulation of FURIN-deficient T cells to increasing antigen (10–100 nM) failed to induce normal proportions of IFN-γ+IL-4- Th1 cells. In contrast, a marked overabundance of IL-4 production sustained upon increasing the antigen dose up to 100 nM. These data show that in neutral culture conditions FURIN-deficient T cells have an intrinsic impairment in Th1 and dominance of Th2 differentiation, which could be a result of altered sensitivity to the effects of antigen dose or dysregulated cytokine signaling.
Figure 2. FURIN expression in CD4+ T cells inhibits Th2 polarization.
Naïve CD4+ T cells (CD4+CD44lowCD62L+) from CD4cre-furf/f -OTII or littermate control (furf/f –OTII) mice were labeled with CFSE and stimulated with graded concentrations of OVA-peptide/splenic dendritic cells under neutral cell culture conditions. After 3.5 days the primed T cells were stained for intracellular IFN-γ and IL-4. Cytokine production (A) and proliferation rate (B) were measured with flow cytometry. Experiment was performed twice with identical results.
Th2 polarization in FURIN-deficient CD4+ T cells is independent of TGF-β1 and resistant to IL-12 inhibition
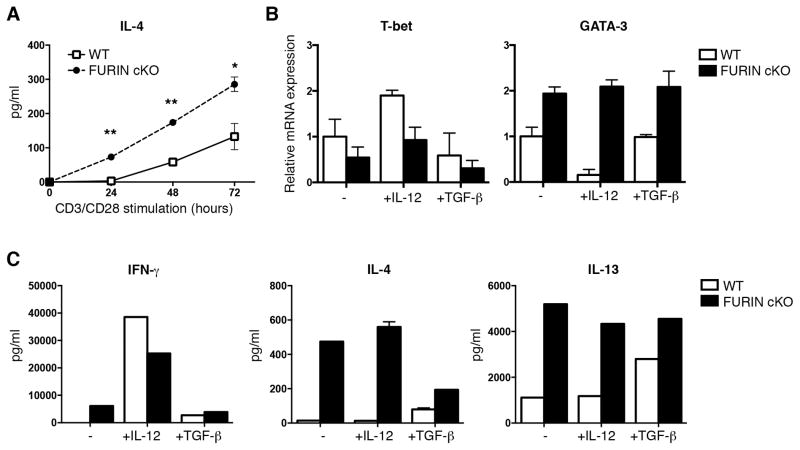
One aspect of FURIN’s function is its role in the maturation of pro-TGF-β1 through a site-specific proteolytic cleavage (29). Because TGF-β1 can have diverse effects on lymphocyte activation, we next addressed the possibility that the effect of FURIN was related to TGF-β1 or other serum cytokines (30). Naïve wild type and FURIN cKO T cells were activated with a strong polyclonal T cell receptor stimulus (anti-CD3/28 10+10μg/ml) in serum free cell culture conditions, and IL-4 production was measured at different time points (Fig. 3A). Lack of FURIN expression was not associated with enhanced apoptosis, since cell numbers and Bcl-2 mRNA expression were unaffected (data not shown). Naïve FURIN cKO CD4+ cells produced significantly higher amounts of IL-4 as early as 24 hrs after TCR activation indicating that FURIN deficiency results in accelerated Th2 responses also upon a strong polyclonal TCR activation and independently of serum cytokines.
Figure 3. Th2 polarization in FURIN deficient CD4+ T cells is independent on serum cytokines and resistant to IL-12 and TGF-β1.
Naïve CD4+CD62L+ T cells from CD4cre-furf/f (cKO) or littermate control (WT) mice were stimulated with plate-bound anti-CD3/28 (10+10 μg/ml) and cultured in serum-free medium in the presence or absence of IL-12 (10 ng/ml) or TGF-β1 (0.5 ng/ml) as indicated for 72 hours. (A) IL-4 production was measured with ELISA at different time points (n=2–3 for both groups). P values were determined with Student t test, * for p-value <0.05, ** for <0.01. (B) T-bet and GATA-3 expressions were measured with Q-RT-PCR. (C) Cytokines from cell culture supernatants were measured with CBA Th1/2/17 kit (BD). Shown are representative experiments of two identical performed (B–C), error bars indicate SEM.
We next investigated whether FURIN deficient T cells respond correctly to Th1 and Treg/Th17 inducing cytokines IL-12 and TGFβ-1, respectively (Fig. 3B–C). In keeping with the elevated levels of IL-4, activated FURIN cKO CD4+CD62L+ T cells also had increased the GATA-3/T-bet ratio in neutral, serum-free cell culture conditions. However, addition of IL-12 to the cell cultures promoted IFN-γ production in both wild type and FURIN cKO cells (Fig. 3C). Further, the lack of FURIN did not abolish the IL-12 triggered induction of STAT4 tyrosine phosphorylation in CD4+ T cells or Granzyme B expression in CD8+ T cells (data not shown), which collectively indicates that FURIN is not critical for responsiveness to IL-12. In contrast, the production of Th2 type cytokines IL-4 and IL-13 or elevated GATA-3 expression could not be markedly inhibited by adding IL-12 into the FURIN deficient CD4+ T cell cultures. Previously, we have observed that FURIN deficient T cells respond normally to TGF-β1 by upregulating the FoxP3 expression (18). FURIN deficient cells remained Th2 biased (elevated GATA3 expression and IL-13 levels) also in the presence of exogenous TGF-β1, but responded by showing downregulation of IL-4. Altogether, we conclude that the FURIN deficient T cells respond correctly to IL-12 and TGF-β1 cytokines, and the inherent Th2 bias is independent of serum and resistant to Th1 and Th17/Treg polarization inducing cytokines. These findings suggest that the FURIN deficient T helper cells could hyperpolarize to Th2 phenotype as a result of sensitized responses to Th2 promoting signals, such as autocrine IL-4 production.
FURIN inhibits the cell surface expression of IL-4Rα in naïve CD4+ cells
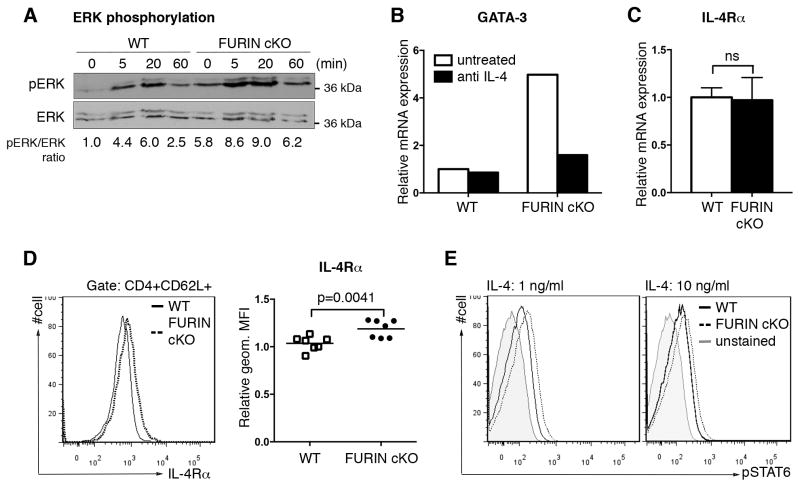
Previous work has also shown that the strength of TCR signaling can affect Th1 versus Th2 commitment by influencing the degree of ERK (extracellular-signal-regulated kinase) activation; early Th2 polarization events are inhibited by an elevated ERK activation upon a strong antigen stimulus (28). To investigate mechanisms by which FURIN normally inhibits Th2 differentiation, we measured TCR-dependent ERK activation and found that ERK phosphorylation was enhanced, not impaired in FURIN deficient CD4+ cells (Fig. 4A). Moreover, FURIN dependent Th2 bias was evident also in the presence of Th1 favoring, strong TCR induction (Fig. 3). Thus, it seems unlikely that impairment of TCR signaling underlies derangement of Th1/Th2 differentiation. IL-2-mediated STAT5 activation and upregulation of GATA-3 expression is essential for the early Th2 commitment, independently of the IL-4/STAT6 signaling cascade (2, 31). To investigate if FURIN regulates fate determining transcription factors during T cell development, we measured thymic expression of GATA-3 and T-bet and found that FURIN deficiency did not significantly affect the expression levels of either factor in single positive CD4+CD8− cells (Fig. S3). Collectively, these data suggest that the FURIN deficiency causes Th2 hyperpolarization by regulating the Th2 phenotype amplification phase through the IL-4/STAT6 route rather than by disrupting the normal thymic development or down-modulating the initial TCR signal transduction.
Figure 4. FURIN inhibits IL-4Rα cell surface expression and IL-4 induced STAT6 phosphorylation.
(A) CD4+ T cells were isolated from CD4cre-furf/f (cKO) and littermate control (WT) mice, and stimulated with soluble anti-CD3 (10 μg/ml) for indicated times. Total ERK and phosphorylated ERK (pERK) were detected with western blot, and pERK/ERK-ratio was determined by measuring the intensity of the bands. Ratio from unstimulated WT sample was arbitrarily set as 1. Experiment was repeated twice with similar results. (B) Naïve CD4+CD62L+ T cells from FURIN cKO and WT mice were cultured in serum-free media for 72 hours in the presence of plate-bound anti-CD3/28 (10+10 μg/ml) activation and neutralizing anti-IL-4 antibody (2 μg/ml) as indicated. GATA-3 and housekeeping gene 18s expressions were determined by Q-RT-PCR, and relative expression in untreated WT sample was arbitrarily set as 1. Experiment was repeated twice with identical results. Error bars indicate SEM. (C) mRNA expression of Il4ra and 18s housekeeping gene was measured from steady state naïve CD4+CD62L+ T cells from FURIN cKO (n=4) and littermate control (n=3) mice. WT cell population was arbitrarily set as 1, and no statistical differences (ns) were observed between the groups (Mann-Whitney test). Error bars indicate SEM. (D) Steady state expression of IL-4Rα on naïve CD4+CD62L+ T cells was determined with flow cytometry (n=7 for both groups). Relative IL-4Rα expression (geometric median fluorescence, MFI) in WT live cell population was arbitrary set as 1, and statistics were calculated with Mann-Whitney test. (E) Phosphorylation of STAT6 was detected in CD4+CD62L+ T cells from FURIN cKO and littermate control mice. Cells were activated with IL-4 (1 or 10 ng/ml) for 15 minutes, and pSTAT6 was stained for FACS analysis. Geometric MFI values for pSTAT6: unstained 22, 26; WT 68, 85; and FURIN cKO 111, 121, (IL-4: 1, 10 ng/ml, respectively). Shown is a representative experiment of two performed.
To test directly whether the skewed Th2 phenotype of FURIN cKO cells was due to cytokine signaling, we inhibited the IL-4/STAT6 pathway by adding IL-4 neutralizing antibody (2 μg/ml) into serum-free CD4+CD62L+ T cell cultures and measured the mRNA expression of GATA-3. Blocking IL-4 function prevented the GATA3 over-expression in in vitro activated FURIN cKO T cells (Fig. 4B). Interestingly, when the expression of IL-4 receptor alpha chain (IL-4Rα) was analyzed we observed that FURIN did not regulate Il4ra mRNA levels (Fig. 4C), but significantly inhibited the IL-4Rα protein expression on the naïve CD4+ T cell surface presumably through a post-translational mechanism (Fig. 4D). To verify the biological relevance of the increased IL-4Rα surface expression we measured the IL-4 induced STAT6 activation by flow cytometry. As expected, naïve CD4+CD62L+ T cells from FURIN cKO mice repeatedly showed more phosphorylated STAT6 after a short IL-4 stimulus when compared to littermate control mice (Fig. 4E). In conclusion, we show that the lack of FURIN upregulates the expression of IL-4Rα on naïve Th cells, which then contributes to sensitized IL-4/STAT6 signal transduction and a shift in Th1/Th2 balance.
FURIN interacts with RAC/DOCK2 and regulates T cell cytoskeleton dynamics
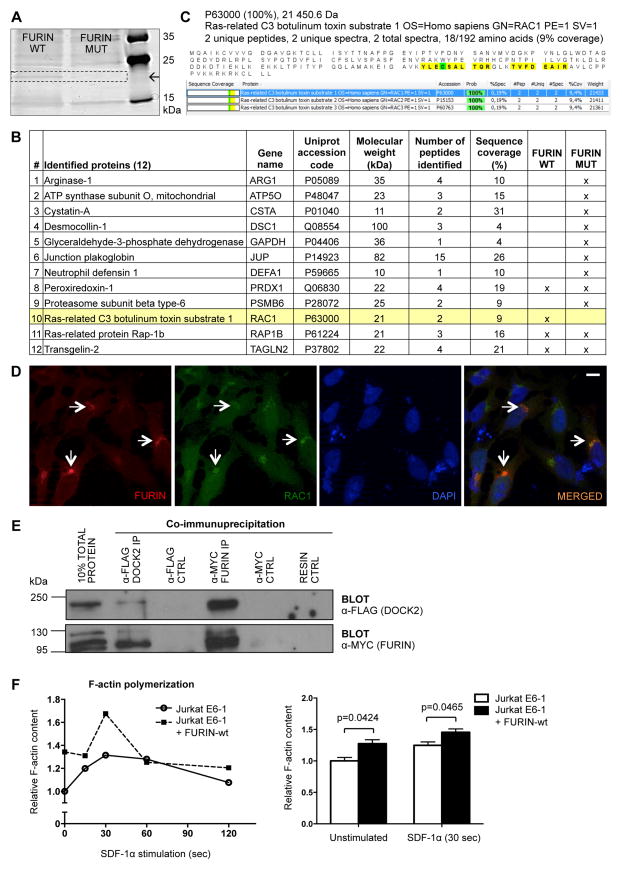
To find a potential mechanistic explanation for the FURIN-dependent dysregulation of IL-4Rα and consequent Th2 bias we sought to identify novel FURIN-interacting proteins in T cells. To this end, we first generated human Jurkat E6-1 T cell lines that stably expressed either wild type or enzymatically inactive mutant (32) human FURIN-Strep-Tag fusion proteins. FURIN interactomes were purified from cell membrane fractions using Strep-tag affinity purification and SDS-PAGE gels followed by peptide identification with mass spectrometry (MS) (33). Analysis of a 20-kDa band, the intensity of which was higher in the wild type FURIN containing elutes, resulted in the identification of 12 potential interaction partners for FURIN (Fig. 5A–C). Out of these only small GTPase RAC (Ras-related C3 botulinum toxin substrates 1–3) fulfilled the identification criteria (i.e. correct molecular weight, sufficient sequence coverage, two or more identified peptides) and was specifically present in lysates containing the enzymatically active FURIN. Of note, RAC has been reported to directly promote Th1 polarization by upregulating IFN-γ (34), and together with its activator DOCK2 (Dedicator of Cytokinesis 2), RAC is also directly implicated in the regulation of IL-4Rα recycling and degradation in CD4+ T cells (35, 36).
Figure 5. FURIN interacts with RAC/DOCK2 complex and regulates F-actin polymerization.
Jurkat E6-1 T cell lines stably expressing either Strep-tagged human wild type FURIN (pcDNA3.1-hFURINwt-StrepIII) or enzymatically inactive (D153A) mutant (pcDNA3.1-hFURINmut-StrepIII) were generated as described in methods. Cell membrane fractions were isolated and FURIN as well as associated proteins were affinity purified from lysates with Strep-tag columns. (A) Eluted proteins were separated by 1D SDS-PAGE gel, and visualized by silver staining. (B) A 20 kDa band, the intensity of which was higher in the FURINwt containing cell elutes, was cut from the gel and following enzymatic protein digestion and extraction from the gel peptides were identified by MS (Proteomics Facility, Turku Centre for Biotechnology, Finland), shown is a list of 12 potential FURIN interaction partners. (C) Peptide sequence coverages for FURINwt specific interaction candidate RAC isoforms 1–3 are shown. HeLa cells were transiently transfected with pcDNA3.1-hFURINwt-MYC, pcDNA-RAC1-V5, pCI-DOCK2-FLAG encoding constructs. (D) Co-localization of RAC1 and FURIN was detected with immunofluorescence using anti-MYC and anti-V5 antibodies and DAPI staining. Representative image is shown; arrows identify FURIN and RAC1 co-localization (scale bar=10 μm). (E) Interaction between DOCK2 and FURIN was evaluated with co-immunoprecipitation and western blotting using anti-FLAG and anti-MYC antibodies. Experiment was repeated twice with identical results. (F) F-actin polymerization was analyzed in wild type FURIN overexpressing and control (pcDNA3.1) Jurkat E6-1 T cells that were left untreated or stimulated with SDF-1α (250 ng/ml) for 15 to 120 seconds. Polymerized actin levels were quantified with flow cytometry (n=4 for both groups). Error bars represent SEM, Student’s t test was used to calculate statistical significance.
First to validate our MS finding, HeLa cells were transfected with FURIN and RAC1 or DOCK2 encoding cDNAs. RAC1 and FURIN were found to be co-localized (Fig. 5D) and the association of FURIN and DOCK2 proteins was detected by co-immunoprecipitation (Fig. 5E), which collectively indicates the existence of FURIN/RAC1/DOCK2 complexes in the cells. Because the RAC/DOCK2 complex regulates the T cell cytoskeleton dynamics (36), we then investigated whether FURIN can also promote F-actin polymerization. The stable overexpression of wild type FURIN significantly enhanced the level of F-actin polymerization in Jurkat T cells at both steady state and in response to chemotactic signal SDF-1α (Fig. 5F). Taken together, our data show that FURIN interacts with RAC/DOCK2 complex and positively regulates the F-actin polymerization in T cells, which has previously been linked with downregulation of IL-4Rα on Th cell surface and inhibition of Th2 type immunity in vivo (34, 36, 37).
DISCUSSION
Although the proprotein convertase FURIN has been shown to critically regulate diverse functions in homeostasis and pathology (9), its role in the T-cell-mediated immune regulation is incompletely understood. Our initial studies revealed that FURIN is highly expressed in human Th1 type cells, where it promotes IFN-γ production (5). However, mice lacking T-cell-expressed FURIN surprisingly presented with overly activated Th1 and Th2 cells and inappropriate Treg function (18), which implied that FURIN has a multifaceted role in the CD4+ T cell biology. Here, when mice were infected with a prototypic Th1 pathogen T. gondii, FURIN was found to be upregulated in CD4+ T cells in an IL-12 dependent manner. T-cell-expressed FURIN was shown to be essential for host defense against the parasitic infection and the generation of antigen specific Th1 and Th1-IL-10 cell responses. Activated FURIN cKO CD4+ cells showed propensity to polarize towards Th2 phenotype in vitro, which was accompanied with elevated cell surface IL-4Rα expression on naïve T helper cells. Finally, exploring the FURIN interactome using Strep-tag purification and proteomics resulted in identification of cytoskeleton modifying RAC/DOCK2 complex as a novel FURIN interaction partner. FURIN promoted actin polymerization in T cells, which has previously been shown to mediate IL-4Rα internalization. Altogether, our results unravel an IL-12/TGF-β1 induced protease FURIN as a central regulator of T helper cell polarization and demonstrate its criticality in cell-mediated immune responses.
Transcriptional profiling studies on IL-12 target genes have offered mechanistic insights into Th1 differentiation. Cytokine receptors like IL-2Rα, IL-12Rβ2, and IL-18Rα, TPL2 kinase and transcription factors such as interferon regulatory factor 1 (IRF-1) are all induced by IL-12, and required for optimal IFN-γ production and cell-mediated immunity (5, 24). Here, we showed that IL-12 is important for FURIN upregulation upon T. gondii infection in vivo, which suggested a regulatory role for this protease in host defense against intracellular pathogens. FURIN is thought to be widely expressed, and it autoregulates its own enzymatic activity (8). Thus, FURIN’s expression levels serve as a critical determinant for its substrate processing activity also during an immune response. Recent findings show that not only IL-12 and TCR signals in T cells, but also LPS stimulation in macrophages can upregulate FURIN (10, 38). FURIN is also secreted from cells upon immune activation, and elevated serum levels were recently found in chronic typhoid carriers (39). Assessing serum FURIN levels has thus potential to become a future biomarker in detecting the activation of immune systems in host.
FURIN has an essential function in proteolytic processing of the inactive pro-TGF-β1 precursor into its bioactive form (40, 41). Consequently, CD4cre-furf/f mice largely phenocopy the immunological abnormalities (e.g. age-related systemic autoimmunity) seen in CD4cre-tgfbf/n mice (18, 42). However, FURIN deficient Treg cells were partly functional, and FURIN cKO effector CD4+ cells appeared more resistant to the suppressive activity of wild type Tregs (18) than what was reported in TGF-β1 deficiency, which implies the existence of additional targets for FURIN in T cells. PCSK substrate molecules and interaction candidates have been investigated by a variety of methods, including gene expression correlations, peptide sequencing and microarrays (43–45). In this study we used Strep-tag affinity purification and MS to identify T cell specific FURIN interactions (33). By using both wild type and inactive mutant forms of FURIN we could distinguish proteins that specifically interact with the active FURIN enzyme in human Jurkat T cells. Although we carefully optimized the cell lysis protocols to catch FURIN protein efficiently, it is possible that the relatively high stringency that was required for effective lysis may have resulted in loss of some transient interaction partners. In our experiments the FURIN interacting proteins were first separated in 1D SDS-PAGE gel and identifications were focused on the silver-stained bands with different intensities, which may obviously limit the number of identifiable interaction partners.
The identified novel FURIN associated proteins, small GTPase RAC and its activator DOCK2 have diverse roles in T cell biology. In peripheral T cells, RACs are essential for TCR signaling (46) and reactivation induced apoptosis (47), but RAC can also promote Th1 polarization by upregulating signaling via p38 and NF-κB pathways and IFN-γ production (34). At the molecular level, the divergent functions of the RAC/DOCK2 complex are mediated through the modulation of actin rearrangements and associated with T cell cytoskeleton dynamics (48). Indeed, it has been previously described that FURIN regulates the actin cytoskeleton dynamics by processing integrins in vascular smooth muscle cells (49). Our data show that FURIN promotes actin polymerization in Jurkat T cells, but decoding the detailed molecular mechanisms and associated target molecules remain uncharacterized.
The cytoskeleton modifying activity of RAC/DOCK2 complex regulates the IL-4Rα trafficking and its surface expression on activated T cells, which then contributes to the strength of IL-4/STAT6 signaling and ensuing Th2 polarization (35, 36). Our flow cytometric analyses showed that FURIN deficiency significantly upregulates IL-4Rα protein levels on naïve CD4+ T cells, which inherently express only low levels of this receptor chain (31). Accordingly, FURIN deficient CD4+ T cells mimicked the phenotype of DOCK2 knockouts by showing elevated Th2 responses, such as high serum IgE level, but in contrast to DOCK2 deficient animals (36), the antigen specific IFN-γ response to chronic intracellular parasite infection was reduced in FURIN cKO mice. The Il4ra mRNA expression in CD4+ cells is initially primed by IL-2 induced STAT5 activation (31). FURIN deficient Th cells showed unchanged Il4ra and Il2ra mRNA expressions, and the IL-2 induced STAT5 phosphorylation in CD4+ cells was intact (Fig. 4C and data not shown). In contrast, neutralizing IL-4 effectively blocked the excessive GATA-3 expression in FURIN cKO T cells, and the IL-4 induced STAT6 activation was sensitized. Collectively, our results thus suggest that FURIN regulates Th1/Th2 balance by coordinating cytoskeleton dynamics and the IL-4Rα expression on the cell surface, which is essential for cytokine dependent amplification of Th2 type cells. However, it is possible that by promoting RAC activity FURIN enhances also directly IFN-γ and associated Th1 polarization (34). Further studies using endogenously expressed proteins and primary T cells are clearly required to decode the physiological molecular mechanisms by which the FURIN mediated RAC activation regulates T helper cell balance.
Cell-mediated immune responses are essential for host control over intracellular pathogens, such as Mycobacteria, Toxoplasma, Leishmania, and certain viruses. CD4cre-furf/f mice succumbed to Toxoplasma gondii infection arguing for the criticality of T-cell-expressed FURIN in the generation of protective immunity. In the acute phase of T. gondii infection, FURIN was dispensable for survival, generation of Th1 cells and normal serum cytokine responses. The early Th1 cell generation has been shown to depend on high levels of APC-produced IL-12 (21), which functions FURIN independently. However, elevated T. gondii burdens in the brains of CD4cre-furf/f mice demonstrated that FURIN has a T cell intrinsic role in controlling parasite growth. The analysis of antigen-specific Th responses showed that FURIN regulated the generation of sustainable protective (Th1) and tolerogenic (Th1-IL-10+) cell populations. An analogous dual role has previously been reported for IL-27, which was initially characterized as a driver of Th1 type responses (50) and later shown to support suppressive IL-10-producing Treg cells in T. gondii infection (51). In contrast, the lack of FURIN in T cells did not appear to promote the generation of single positive CD4+IL-10+ cells in T. gondii-infected mice. These data could indicate that in the presence of elevated parasite burdens FURIN deficiency is not able to contribute to the complimentary induction of Th2 polarization. Importantly, a direct measurement of IL-4 and other Th2 cytokines after infection would be required to clarify this aspect. Also cytotoxic CD8+ T cells critically restrict T. gondii in the chronic phase, and the exhaustion of CD8+ T cells leads to uncontrollable parasite growth and encephalitis (52). Notably, in recall assays we used soluble T. gondii antigen to induce T cell responses, which activates CD4+ cells through MHC-II. However, the role of FURIN in the regulation of CD8+ T cell biology is currently unknown and we can thus not rule out the fact that T. gondii-infected CD4cre-furf/f mice could die due to impaired CD8+ T cell function. Importantly, our previous data demonstrate that FURIN deficiency accelerates T cell responses (18), which in theory can promote the exhaustion of CD8+ T cell responses in the brain.
Inhibiting the PCSK function has a potential to become a future means to treat immune-mediated diseases such as cancers, atherosclerosis and infections (10–12). In addition, administering recombinant FURIN can alleviate the over-active immune cells in autoimmune diseases (11). Understanding how FURIN regulates cell-mediated immunity and Th balance is thus critical when such treatments are considered for clinical use. Our earlier results demonstrate that deleting FURIN activates T cells, which could be beneficial in treatment of cancers, for example. However, our results here show that T cell-expressed FURIN is critical for host resistance to T. gondii and inhibits Th2 polarization. Therefore, interfering with the PCSK activity in patients may compromise protection against intracellular pathogens and result in allergic responses.
Supplementary Material
Acknowledgments
Financial support:
This study was financially supported by the Academy of Finland (projects 128623, 135980 (MP)), a Marie Curie International Reintegration Grant within the 7th European Community Framework Programme (MP), Emil Aaltonen Foundation (MP, AO), Sigrid Jusélius Foundation (MP, IJ), Tampere Tuberculosis Foundation (MP, IJ), Competitive Research Funding of the Tampere University Hospital (grants 9M080, 9N056 (MP); grants 9N018, Fimlab X51409 (IJ)), Doctoral Programme in Biomedicine and Biotechnology - University of Tampere (AO), Finnish Cultural Foundation (AO), and Laboratoriolääketieteen edistämissäätiö (AO).
Authors thank the members of the Immunoregulation group for helpful discussions and Dr. William E. Paul (NIAID, National Institutes of Health, US), Prof. Mika Rämet (University of Tampere, Finland) and Prof. Olli Silvennoinen (University of Tampere, Finland) for comments on the manuscript. Dr. Hidehiro Yamane (NIAID, National Institutes of Health, US) is acknowledged for his contribution to ovalbumin induced Th differentiation experiment and helpful comments on the manuscript. Prof. Yoshinori Fukui (Kyushu University, Japan), Prof. Jukka Westermarck (Turku Centre for Biotechnology, Finland) and Prof. John Creemers (KU Leuven, Belgium) are thanked for providing reagents. Mass spectrometry analyses were performed at the Turku Proteomics Facility (Turku Centre for Biotechnology, Finland).
Nonstandard abbreviations
- cKO
conditional knockout
- DOCK2
dedicator of cytokinesis 2
- GATA-3
GATA binding protein 3
- MS
mass spectrometry
- MUT
mutant
- PCSK
proprotein convertase
- RAC
Ras-related C3 botulinum toxin substrate
- SDF-1α
stromal cell-derived factor-1 alpha
- STAg
soluble T. gondii antigen
- T-bet
T-box 21
- T. gondii
Toxoplasma gondii
- Treg
regulatory T cell
- WT
wild type
References
- 1.O’Shea JJ, Paul WE. Mechanisms Underlying Lineage Commitment and Plasticity of Helper CD4(+) T Cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J, Yamane H, Paul WE. Differentiation of Effector CD4 T Cell Populations. Annual Review of Immunology. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan YY, Flavell RA. How Diverse-CD4 Effector T Cells and their Functions. Journal of Molecular Cell Biology. 2009;1:20–36. doi: 10.1093/jmcb/mjp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirahara K, Poholek A, Vahedi G, Laurence A, Kanno Y, Milner JD, O’Shea JJ. Mechanisms underlying helper T-cell plasticity: Implications for immune-mediated disease. J Allergy Clin Immunol. 2013;131:1276–1287. doi: 10.1016/j.jaci.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pesu M, Muul L, Kanno Y, O’Shea JJ. Proprotein convertase furin is preferentially expressed in T helper 1 cells and regulates interferon gamma. Blood. 2006;108:983–985. doi: 10.1182/blood-2005-09-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois C, Blanchette F, Laprise M, Leduc R, Grondin F, Seidah N. Evidence that furin is an authentic transforming growth factor-beta 1-converting enzyme. Am J Pathol. 2001;158:305–316. doi: 10.1016/s0002-9440(10)63970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund RJ, Chen Z, Scheinin J, Lahesmaa R. Early target genes of IL-12 and STAT4 signaling in Th cells. Journal of Immunology. 2004;172:6775–6782. doi: 10.4049/jimmunol.172.11.6775. [DOI] [PubMed] [Google Scholar]
- 8.Thomas G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nature Reviews Molecular Cell Biology. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artenstein AW, Opal SM. MECHANISMS OF DISEASE Proprotein Convertases in Health and Disease. N Engl J Med. 2011;365:2507–2518. doi: 10.1056/NEJMra1106700. [DOI] [PubMed] [Google Scholar]
- 10.Turpeinen H, Raitoharju E, Oksanen A, Oksala N, Levula M, Lyytikainen L, Jarvinen O, Creemers JWM, Kahonen M, Laaksonen R, Pelto-Huikko M, Lehtimaki T, Pesu M. Proprotein convertases in human atherosclerotic plaques: The overexpression of FURIN and its substrate cytokines BAFF and APRIL. Atherosclerosis. 2011;219:799–806. doi: 10.1016/j.atherosclerosis.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Lin H, Kioon MA, Lalou C, Larghero J, Launay J, Khatib A, Cohen-Solal M. Protective Role of Systemic Furin in Immune Response-Induced Arthritis. Arthritis Rheum. 2012;64:2878–2886. doi: 10.1002/art.34523. [DOI] [PubMed] [Google Scholar]
- 12.Shiryaev SA, Remacle AG, Savinov AY, Chernov AV, Cieplak P, Radichev IA, Williams R, Shiryaeva TN, Gawlik K, Postnova TI, Ratnikov BI, Eroshkin AM, Motamedchaboki K, Smith JW, Strongin AY. Inflammatory Proprotein Convertase-Matrix Metalloproteinase Proteolytic Pathway in Antigen-presenting Cells as a Step to Autoimmune Multiple Sclerosis. J Biol Chem. 2009;284:30615–30626. doi: 10.1074/jbc.M109.041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hipp MM, Shepherd D, Gileadi U, Aichinger MC, Kessler BM, Edelmann MJ, Essalmani R, Seidah NG, Reis E Sousa C, Cerundolo V. Processing of Human Toll-like Receptor 7 by Furin-like Proprotein Convertases Is Required for Its Accumulation and Activity in Endosomes. Immunity. 2013;39:711–21. doi: 10.1016/j.immuni.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Refaie S, Gagnon S, Gagnon H, Desjardins R, D’Anjou F, D’Orleans-Juste P, Zhu X, Steiner DF, Seidah NG, Lazure C, Salzet M, Day R. Disruption of Proprotein Convertase 1/3 (PC1/3) Expression in Mice Causes Innate Immune Defects and Uncontrolled Cytokine Secretion. J Biol Chem. 2012;287:14703–14717. doi: 10.1074/jbc.M111.323220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonhardt RM, Fiegl D, Rufer E, Karger A, Bettin B, Knittlert MR. Post-Endoplasmic Reticulum Rescue of Unstable MHC Class I Requires Proprotein Convertase PC7. Journal of Immunology. 2010;184:2985–2998. doi: 10.4049/jimmunol.0900308. [DOI] [PubMed] [Google Scholar]
- 16.Turpeinen H, Oksanen A, Kivinen V, Kukkurainen S, Uusimaki A, Ramet M, Parikka M, Hytonen VP, Nykter M, Pesu M. Proprotein Convertase Subtilisin/Kexin Type 7 (PCSK7) Is Essential for the Zebrafish Development and Bioavailability of Transforming Growth Factor beta1a (TGFbeta1a) J Biol Chem. 2013;288:36610–36623. doi: 10.1074/jbc.M113.453183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidah NG, Sadr MS, Chretien M, Mbikay M. The multifaceted proprotein convertases: their unique, redundant, complementary, and opposite functions. The Journal of Biological Chemistry. 2013;288:21473–21481. doi: 10.1074/jbc.R113.481549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pesu M, Watford WT, Wei L, Xu L, Fuss I, Strober W, Andersson J, Shevach EM, Quezado M, Bouladoux N, Roebroek A, Belkaid Y, Creemers J, O’Shea JJ. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455:246–U73. doi: 10.1038/nature07210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roebroek AJM, Taylor NA, Louagie E, Pauli I, Smeijers L, Snellinx A, Lauwers A, Van de Ven WJM, Hartmann D, Creemers JWM. Limited Redundancy of the Proprotein Convertase Furin in Mouse Liver. Journal of Biological Chemistry. 2004;279:53442–53450. doi: 10.1074/jbc.M407152200. [DOI] [PubMed] [Google Scholar]
- 20.Thieu VT, Yu Q, Chang H, Yeh N, Nguyen ET, Sehra S, Kaplan MH. Signal Transducer and Activator of Transcription 4 Is Required for the Transcription Factor T-bet to Promote T Helper 1 Cell-Fate Determination. Immunity. 2008;29:679–690. doi: 10.1016/j.immuni.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-Induced Il-12 Stimulates Early Ifn-Gamma Synthesis and Resistance during Acute Infection with Toxoplasma-Gondii. Journal of Immunology. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 22.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki Y. Host resistance in the brain against Toxoplasma gondii. J Infect Dis. 2002;185:S58–S65. doi: 10.1086/337999. [DOI] [PubMed] [Google Scholar]
- 24.Watford WT, Hissong BD, Durant LR, Yamane H, Muul LM, Kanno Y, Tato CM, Ramos HL, Berger AE, Mielke L, Pesu M, Solomon B, Frucht DM, Paul WE, Sher A, Jankovic D, Tsichlis PN, O’Shea JJ. Tpl2 kinase regulates T cell interferon-gamma production and host resistance to Toxoplasma gondii. J Exp Med. 2008;205:2803–2812. doi: 10.1084/jem.20081461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yap GS, Sher A. Cell-mediated immunity to Toxoplasma gondii: Initiation, regulation and effector function. Immunobiology. 1999;201:240–247. doi: 10.1016/S0171-2985(99)80064-3. [DOI] [PubMed] [Google Scholar]
- 26.Denkers E, Gazzinelli R. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–+. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sheri A. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamane H, Zhu JF, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4(+) T cells to generate a Th2-inducing cytokine environment. J Exp Med. 2005;202:793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanchette F, Day R, Dong W, Laprise M, Dubois C. TGF beta 1 regulates gene expression of its own converting enzyme furin. J Clin Invest. 1997;99:1974–1983. doi: 10.1172/JCI119365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghoreschi K, Laurence A, Yang X, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun H, Eberl G, Shevach E, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–U144. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh T, Zhao K, Leonard WJ. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9:1288–1296. doi: 10.1038/ni.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creemers J. Modulation of Furin-Mediated Proprotein Processing Activity by Site-Directed Mutagenesis. J Biol Chem. 1993;268:21826–21834. [PubMed] [Google Scholar]
- 33.Junttila M, Saarinen S, Schmidt T, Kast J, Westermarck J. Single-step Strep-tag (R) purification for the isolation and identification of protein complexes from mammalian cells. Proteomics. 2005;5:1199–1203. doi: 10.1002/pmic.200400991. [DOI] [PubMed] [Google Scholar]
- 34.Li B, Yu H, Zheng W, Voll R, Na S, Roberts A, Williams D, Davis R, Ghosh S, Flavell R. Role of the guanosine triphosphatase Rac2 in T helper 1 cell differentiation. Science. 2000;288:2219. doi: 10.1126/science.288.5474.2219. [DOI] [PubMed] [Google Scholar]
- 35.Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, Inayoshi A, Noda M, Oike M, Shirai T, Sasazuki T. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka Y, Hamano S, Gotoh K, Murata Y, Kunisaki Y, Nishikimi A, Takii R, Kawaguchi M, Inayoshi A, Masuko S, Himeno K, Sasazuki T, Fukui Y. T helper type 2 differentiation and intracellular trafficking of the interleukin 4 receptor-alpha subunit controlled by the Rac activator Dock2. Nat Immunol. 2007;8:1067–1075. doi: 10.1038/ni1506. [DOI] [PubMed] [Google Scholar]
- 37.Croker BA, Handman E, Hayball JD, Baldwin TM, Voigt V, Cluse LA, Yang FC, Williams DA, Roberts AW. Rac2-deficient mice display perturbed T-cell distribution and chemotaxis, but only minor abnormalities in T(H)1 responses. Immunol Cell Biol. 2002;80:231–240. doi: 10.1046/j.1440-1711.2002.01077.x. [DOI] [PubMed] [Google Scholar]
- 38.Meissner F, Scheltema RA, Mollenkopf HJ, Mann M. Direct proteomic quantification of the secretome of activated immune cells. Science. 2013;340:475–478. doi: 10.1126/science.1232578. [DOI] [PubMed] [Google Scholar]
- 39.Kumar A, Singh S, Ahirwar SK, Nath G. Proteomics-based identification of plasma proteins and their association with the host–pathogen interaction in chronic typhoid carriers. International Journal of Infectious Diseases. 2014;19:59–66. doi: 10.1016/j.ijid.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF beta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 41.Mark Travis A, Sheppard Dean. TGF-β Activation and Function in Immunity. Annual Review of Immunology. 2014:32. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta 1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Shiryaev SA, Chernov AV, Golubkov VS, Thomsen ER, Chudin E, Chee MS, Kozlov IA, Strongin AY, Cieplak P. High-Resolution Analysis and Functional Mapping of Cleavage Sites and Substrate Proteins of Furin in the Human Proteome. Plos One. 2013;8:e54290. doi: 10.1371/journal.pone.0054290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turpeinen H, Kukkurainen S, Pulkkinen K, Kauppila T, Ojala K, Hytonen VP, Pesu M. Identification of proprotein convertase substrates using genome-wide expression correlation analysis. BMC Genomics. 2011;12:618. doi: 10.1186/1471-2164-12-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian S, Huajun Wang, Wu J. Computational prediction of furin cleavage sites by a hybrid method and understanding mechanism underlying diseases. Scientific Reports. 2012;2:261. doi: 10.1038/srep00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arrieumerlou C, Randriamampita C, Bismuth G, Trautmann A. Rac is involved in early TCR signaling. Journal of Immunology. 2000;165:3182–3189. doi: 10.4049/jimmunol.165.6.3182. [DOI] [PubMed] [Google Scholar]
- 47.Ramaswamy M, Dumont C, Cruz AC, Muppidi JR, Gomez TS, Billadeau DD, Tybulewicz VLJ, Siegel RM. Cutting edge: Rac GTPases sensitize activated T Cells to die via Fas. Journal of Immunology. 2007;179:6384–6388. doi: 10.4049/jimmunol.179.10.6384. [DOI] [PubMed] [Google Scholar]
- 48.Nishikimi A, Kukimoto-Niino M, Yokoyama S, Fukui Y. Immune regulatory functions of DOCK family proteins in health and disease. Exp Cell Res. 2013;319:2343–2349. doi: 10.1016/j.yexcr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Kappert K, Furundzija V, Fritzsche J, Margeta C, Krueger J, Meyborg H, Fleck E, Stawowy P. Integrin cleavage regulates bidirectional signalling in vascular smooth muscle cells. Thromb Haemost. 2010;103:556–563. doi: 10.1160/TH09-07-0478. [DOI] [PubMed] [Google Scholar]
- 50.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 51.Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhadra R, Khan I. Redefining Chronic Toxoplasmosis—A T Cell Exhaustion Perspective. PLoS Pathog. 2012;8(10):e1002903. doi: 10.1371/journal.ppat.1002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.