Abstract
A polyethylene glycol (PEG) retinylamine (Ret-NH2) conjugate PEG-GFL-NH-Ret with a glycine-phenylalanine-leucine (GFL) spacer was synthesized for controlled oral delivery of Ret-NH2 to treat retinal degenerative diseases, including Stargardt disease (STGD) and age-related macular degeneration (AMD). The peptide spacer was introduced for sustained release of the drug by digestive enzymes in the gastrointestinal tract. The pharmacokinetics experiments showed that the PEG conjugate could control the sustained drug release after oral administration and had much lower nonspecific liver drug accumulation than the free drug in wild-type female C57BL mice. In the mean time, the conjugate maintained the same concentration of Ret-NH2 in the eye as the free drug. Also, PEG-GFL-NH-Ret at a Ret-NH2 equivalent dose of 25 mg/kg produced complete protection of Abca4–/–Rdh8–/– mouse retinas against light-induced retinal degeneration for 3 days after oral administration, as revealed by OCT retina imaging, whereas free Ret-NH2 did not provide any protection under identical conditions. The polymer conjugate PEG-GFL-NH-Ret has great potential for controlled delivery of Ret-NH2 to the eye for effective protection against retinal degenerative diseases.
Introduction
Vertebrate vision, initiated by photoisomerization of the chromophore 11-cis-retinal to all-trans-retinal (atRAL) in the retina, is maintained by continuous regeneration of 11-cis-retinal through a complex enzymatic pathway known as the retinoid (visual) cycle.1 If the conversion or clearance of atRAL in the photoreceptor cells is disrupted, this reactive aldehyde can form dimeric condensation products, including N-retinyl-N-retinylidene-ethanolamine (A2E) and A2E-like derivatives. These toxic products, free aldehyde and its condensation product, contribute to retinal degenerative diseases such as Stargardt disease (STGD) and age-related macular degeneration (AMD).2 Thus, excessive production and slow transformation of toxic atRAL are considered as one of the key factors in initiating retinal degeneration characterized by progressive photoreceptor cell death induced by both acute and chronic light exposure.3 To date, there is no effective treatment that prevents, halts, or slows down the progression of STGD, AMD, and other retinal degenerative diseases in humans.4 However, it has been reported that sequestration of atRAL can reduce the accumulation of A2E-like derivatives, prevent retinal degeneration, and preserve vision in animal models1 and, potentially, in humans.4−7 Recently, our group found that treatment with primary amines, including Ret-NH2, can lower retinal atRAL concentrations to safe levels and prevent retinal degeneration in double knockout (Abca4–/–Rdh8–/–) mice, a rodent model for STGD and AMD.1,8 Ret-NH2 is an aldehyde scavenger and an inhibitor of RPE65, a critical isomerase of the retinoid cycle. Ret-NH2 also can effectively reduce levels of free atRAL in the retinas of other animal models and holds great promise as a therapeutic agent to prevent acute light induced retinal degeneration.9,10 However, Ret-NH2 possesses shortcomings that may limit their clinical usefulness, such as poor water solubility, instability, and short circulating half-life.10 We aimed to design and develop an effective oral delivery system for Ret-NH2 to address these limitations and provide a prolonged therapeutic effect at safe doses.
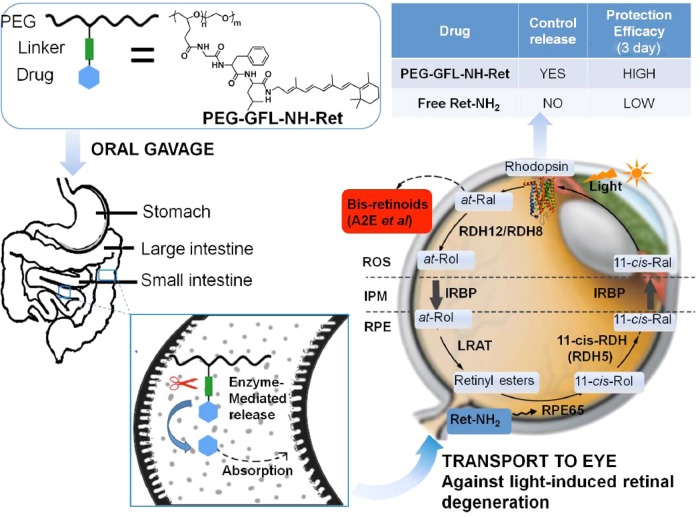
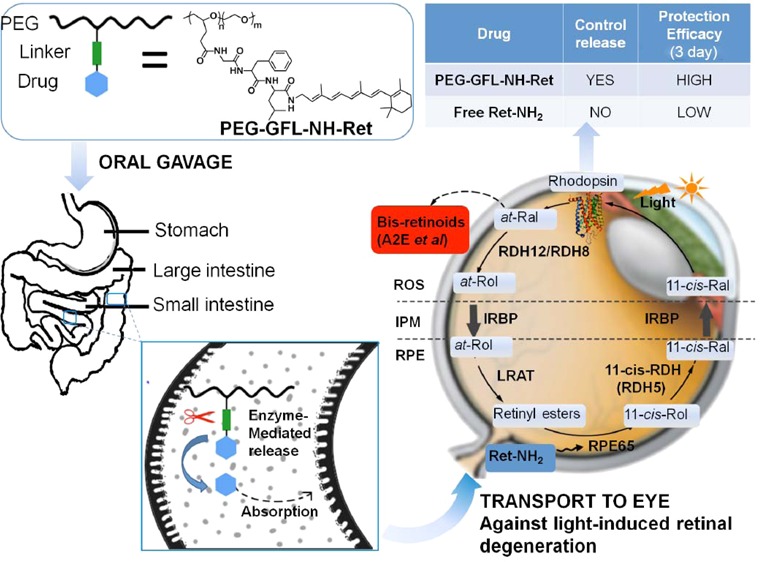
Drug delivery to the back of the eye is highly challenging due to the unique anatomy and physiology of the eye.11−13 Various new drug delivery systems, including dendrimers, mucoadhesive polymers, microspheres, nanoparticles, microneedles, and intraocular implants, have been introduced to improve the pharmacokinetics and pharmacodynamics of ocular therapeutics and to overcome barriers for intraocular drug delivery.14−21 Among these advanced delivery systems, polymer-based carriers appear especially attractive and are being extensively investigated. Oral administration is the most practical approach for drug delivery because of superior patient compliance. A biocompatible polymer conjugate of Ret-NH2 with a peptide spacer could be effective for sustained release and prolonged therapeutic efficacy of Ret-NH2. N-(2-Hydroxypropyl)methacrylamide (HPMA) polymer drug conjugates with oligopeptide spacers have been tested for site-specific release of anticancer therapeutics by pancreatic and intestinal enzymes,22,23 aminopeptidases are responsible for the cleavage of oligopeptide.23,24 Through cleavage of its peptide spacer by digestive enzymes, such a drug–polymer conjugate could effectively control the sustained release and absorption of Ret-NH2. Ret-NH2 then could be gradually released in the small intestine and colon to maintain a relatively stable effective drug concentration in the circulation over an extended period. Sustained drug release from the conjugate could reduce the overall dose and dosing frequency required to produce prolonged protection against light induced retinal degeneration. Reduced dose and dosing frequency could also minimize any dose-related toxic side effects. A representative illustration of this hypothesis and its results are shown in Figure 1.
Figure 1.
Oral PEG retinylamine conjugate for prolonged protection against light-induced retinal degeneration in Abca4–/–Rdh8–/– mice. After oral administration of PEG-GFL-NH-Ret conjugate, Ret-NH2 should be gradually released in the small intestine and colon to maintain a relatively stable effective drug concentration in the circulation and a sufficient amount of the drug in the eye for an extended period. Sustained drug release from the conjugate would reduce the overall dose and dosing frequency required and provide prolonged protection against light-induced retinal degeneration.
Materials and Methods
Materials and Equipment
All commercially available reagents and solvents were received as analytically pure substances. Polyethylene glycol with eight side-chain propionic acid groups (PEG-8PA, MW = 20000 g/mol) was obtained from Sunbio, Inc. (Anyang City, South Korea). Benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP), 1-hydroxybenzotriazole hydrate (HOBt), 2-chlorotrityl chloride resin, and Fmoc-protected amino acids were purchased from Chem-Impex International, Inc. (IL, U.S.A.). Anhydrous N,N-diisopropylethyl amine (DIPEA), p-nitrophenol, and N,N-dimethylformamide (DMF) were obtained from Sigma-Aldrich Co., LLC. 4-Dimethylaminopyridine (DMAP), N,N′-diisopropylcarbodiimide (DIC), and trifluoroacetic acid (TFA) were from Oakwood Products, Inc. (SC, U.S.A.). l-Leucine p-nitroanilide (Leu-pNA) was from Chem-Implex International, Inc. (IL, USA).
Chemical reactions were monitored by thin-layer chromatography (TLC) on silica gel plates (60 F254) with a fluorescent indicator at 254 nm. Intermediates and Ret-NH2 derivatives were purified by column chromatography on silica gels (Silica gel grade: 200–400 mesh, 40–63 μm) and characterized by 1H NMR spectroscopy and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. Proton-NMR spectra were recorded on a Varian 400 MHz NMR spectrometer. MALDI-TOF mass spectra were acquired on a Bruker Autoflex III MALDI-TOF MS in a linear mode with 2,5-dihydroxybenzoic acid (2,5-DHB) as a matrix. Final conjugate (PEG-GFL-NH-Ret) was characterized by size exclusion chromatography (SEC) on an AKTA FPLC system (Amersham Biosciences Corp., Piscataway, NJ) equipped with a Superose 12 column and a refractive index detector. Molecular weights were calibrated with standard poly[N-(2-hydroxypropyl)methacrylamide].
Animal Models
Abca4–/–Rdh8–/– mice were obtained as previously described.1 Mice were housed and cared for in the animal facility at the School of Medicine, Case Western Reserve University, according to an animal protocol approved by the CWRU Institutional Animal Care and Use Committee and conformed to recommendations of the American Veterinary Medical Association Panel on Euthanasia and the Association of Research for Vision and Ophthalmology.
Solid-Phase Synthesis of Peptide NH2-GFL-OH
The peptide spacer NH2-GFL-OH was synthesized using standard solid-phase Fmoc peptide synthesis.25 2-Chlorotrityl chloride resin was first reacted with Fmoc-Leu-OH and addition of the remaining amino acids was accomplished by repeated cycles of coupling and deprotection. Fmoc-amino acids (2-fold molar excess) were coupled to the resin in the presence of the condensation reagents, PyPOP and HOBt. The peptide was obtained after cleavage from the resin with TFA (95%) with a yield of 60%. 1H NMR (400 MHz, D2O, ppm): 8.43 (d, J = 7.8 Hz, 1H, -CONH-), 7.47–7.11 (m, 5H, -CH2C6H5), 4.73–4.54 (m, 1H, -NHCHCO-), 4.31 (dd, J = 9.0, 5.8 Hz, 1H-NHCHCO-), 3.73 (q, J = 16.3 Hz, 2H, NH3+CH2-), 3.04 (ddd, J = 22.1, 13.9, 7.5 Hz, 2H, -CH2C6H5), 1.72–1.41 (m, 3H, -CH2CH(CH3)2), 0.84 (dd, J = 19.0, 6.1 Hz, 6H, -CH(CH3)2). MALDI-TOF (m/z, M+) calcd for C17H25N3O4, 335.185; found, 335.649.
Synthesis of the PEG-GFL-NH-Ret and PEG-GFL-pNA Conjugate
PEG-8PA (5 g, 2 mmol propionic acid) was dissolved in 100 mL of dichloromethane, and 0.84 g (6 mmol) p-nitrophenol, 75 mg (0.6 mmol) 4-dimethylaminopyridine (DMAP), and 0.76 g (6 mmol) N,N′-diisopropylcarbodiimide (DIC) were added to the solution. The reaction mixture was stirred for 24 h at room temperature, and then dropped into ether. The solid was collected and washed three times with ether to obtain PEG-8(PA-ONp), yield 91.9% (4.82 g). PEG-8(PA-ONp) (4 g, 1.6 mmol ONp active ester) was dissolved in 30 mL of dimethyl sulfoxide and 880 mg (1.96 mmol) H2N-Gly-Phe-Leu-OH·TFA and 1 mL of DIPEA were added to the solution. The reaction mixture was stirred for 24 h at room temperature, and then dripped into ether. The resulting solid was collected and washed three times with ether to obtain PEG-8(PA-GFL-OH), yield 92.7% (4.0 g). 1H NMR (400 MHz, acetone-d6, ppm): 7.26 (bm, 5H, -CH2C6H5), 4.72 (s, 1H), 4.48 (s, 1H), 3.80–3.34 (m, 184H, -OCH2CH2O- and PEG-CH2CH2CONH), 3.21 (m, 1H), 2.96 (m, 1H), 1.64 (m, 3H), 1.01–0.81 (m, 6H, -CH(CH3)2).
PEG-8(PA-GFL-OH) (2.12 g, 0.8 mmol GFL) was dissolved in 30 mL of dichloromethane, and 336 mg (2.4 mmol) p-nitrophenol, 30 mg (0.24 mmol) DMAP, and 302 mg (2.4 mmol) DIC were added to the solution. The reaction mixture was stirred for 24 h at room temperature, and dripped into ether. The solid was collected and washed three times with ether to give PEG-8(PA-GFL-ONp), yield 94.3% (2.05 g). PEG-8(PA-GFL-ONp) (2.15 g, 0.8 mmol ONp active ester) was then dissolved in 30 mL of dimethyl sulfoxide, and 570 mg (2 mmol) Ret-NH2 and 0.5 mL of DIPEA were added to the mixture. The reaction mixture was stirred for 24 h at room temperature, and dripped into ether. The solid was collected and washed three times with ether, yield 70.6% (1.68 g). About four drug molecules on average were conjugated to each PEG20k (5.1% w/w), as calculated from the 1H NMR spectrum. 1H NMR (400 MHz, DMSO-d6, ppm): 7.19 (bm, 5H, -H2C6H5), 6.09 (bm, 1H, -CH=CH-), 4.53 (s, 1H), 4.26 (s, 1H), 3.91–3.09 (m, 165H, -OCH2CH2O- and PEG-CH2CH2CONH), 3.04–3.01 (bm, 2H), 2.76 (s, 1H), 1.98–1.81 (bm, 3H), 1.59 (bm, 8H), 0.99 (m, 3H, -C(CH3)2), 0.85 (m, 6H, -CH(CH3)2).
The model drug p-nitroaniline (pNA) conjugate, PEG-GFL-pNA, was synthesized through reaction between PEG-8(PA-GF-ONP) and Leu-pNA, yield 75.6%. About eight drug molecules on average were conjugated to each PEG20k (4.7% w/w), as calculated from the 1H NMR spectrum. 1H NMR (400 MHz, DMSO-d6, ppm): 8.18 (bm, 2H, -NHC2H4NO2), 7.24–7.31 (bm, 7H, -NHC2H4NO2 and -CH2C6H5), 4.47 (bm, 7H), 3.91–3.09 (m, 277H, -OCH2CH2O- and PEG-CH2CH2CONH), 0.93 (m, 6H, -CH(CH3)2).
Pharmacokinetics of PEG-GFL-NH-Ret in C57BL Mice
Female C57BL mice (4-week-old) were randomly divided into two dosing groups. One group of mice was gavaged with one dose of Ret-NH2 (50 mg/kg, 100 μL of 10 mg/mL DMSO solution per mouse), and the other group of mice was also gavaged once dose with PEG-GFL-NH-Ret (875 mg/kg, equivalent to 50 mg/kg Ret-NH2, 100 μL of 175 mg/mL DMSO solution per mouse). Then, at each predetermined time point (0, 4, 8, 24, 48, and 72 h after drug administration), six mice were sacrificed. The liver and eye balls were collected to determine tissue N-retinylamide content for pharmacokinetic analysis. A portion of the liver tissue (ca. 0.5 g) was weighed, homogenized in 2 mL of 1:1 ethanol/PBS solution, and the eye balls were similarly processed. N-Retinylamides were extracted in 4 mL of hexane, concentrated, and reconstituted to a 300 μL volume. Normal-phase HPLC (Agilent- Zorbax SIL; 5 μm; 4.5 × 250 mm; flow rate of 1.4 mL/min; 80:20 hexane/ethyl acetate (v/v); detection at 325 nm) was utilized to determine N-retinylamide concentration in these tissues.
In Vitro Drug Release by Rat Intestinal Brush Border Enzymes24
Eight-week-old rats were sacrificed and the small intestine was removed as soon as possible, rinsed with cold saline, everted, rinsed again with cold saline, and blotted with a hard paper towel to remove saline. The mucosa was scraped off gently with a glass slide, with special attention paid to preserving the fat tissue. Mucosal scrapings were homogenized at 4 °C in a Waring blender at maximum speed for 1 min. To a 0.5 g portion of each homogenate, 2 mL of conjugate PEG-GFL-pNA solution or 2 mL of Leu-pNA at equivalent 1 mM pNA in pH 6.8 isotonic phosphate buffer (33.4 mM NaH2PO4, 33.4 mM Na2HPO4, and 82.1 mM NaCl, pH 6.8) solution was added, and the mixtures were incubated for 16 h at 37 °C. At selected time intervals (0, 0.5, 1, 2, 3, 4, and 16 h), 100 μL samples were taken and 900 μL of methanol was added. After samples were centrifuged at 14000 rpm for 3 min, the organic supernatant was analyzed by HPLC.
Preventing Light-Induced Retinal Degeneration with PEG-GFL-NH-Ret in Abca4–/–Rdh8–/– Mice
Abca4–/–Rdh8–/– mice (male and female, 4-week-old, 4–5 mice in each treatment group) were kept in the dark for 48 h before each experiment. Then single dose free Ret-NH2 (25 mg/kg, 100 μL of 5 mg/mL DMSO solution per mouse) or conjugate PEG-GFL-NH-Ret (438 mg/kg, equivalent to 25 mg/kg free Ret-NH2, 100 μL of 87.5 mg/mL DMSO solution per mouse) was administered by gastric gavage. Mice were illuminated with 10000 lux light for 30 min at 1, 3, or 6 days after their gavage, and then kept in the dark for 7 days when final retinal evaluations were performed. Mice were anesthetized by intraperitoneal injection of a cocktail (20 μL/g body weight) containing ketamine (6 mg/mL) and xylazine (0.44 mg/mL) in PBS buffer (10 mM sodium phosphate and 100 mM NaCl, pH 7.2). Pupils were dilated with 0.01% tropicamide. Retinas of mice were imaged in vivo with ultrahigh resolution spectral-domain OCT (SD-OCT; Bioptigen, Irvine, CA). Five pictures acquired in the B-scan mode were used to construct each final averaged SD-OCT image. Quantitative ONL thicknesses were measured from OCT images along the vertical meridian from the superior to inferior retina. Electroretinograms (ERGs) were then recorded, as previously reported,9 24 h after the OCT test. Dark-adapted mice were anesthetized by the same protocol used for OCT recordings. All experimental procedures were performed under a safety light. A contact lens electrode was placed on the eye, and a reference electrode and ground electrode were placed underneath the skin between the two ears and in the tail, respectively. ERGs were recorded with the universal electrophysiologic system UTAS E-3000 (LKC Technologies, Inc., Gaithersburg, MD). Light intensity calibrated by the manufacturer was computer-controlled. Mice were placed in a Ganzfeld dome, and scotopic responses to flash stimuli were obtained from both eyes simultaneously.
Statistical Analyses
Statistical analyses using the one-way ANOVA were accomplished with data representing the means ± SD for the results of at least three independent experiments comparing the treatment groups. A p value of ≤0.05 was considered significant.
Results and Discussion
Synthesis of PEG-GFL-NH-Ret and PEG-GFL-pNA Conjugates
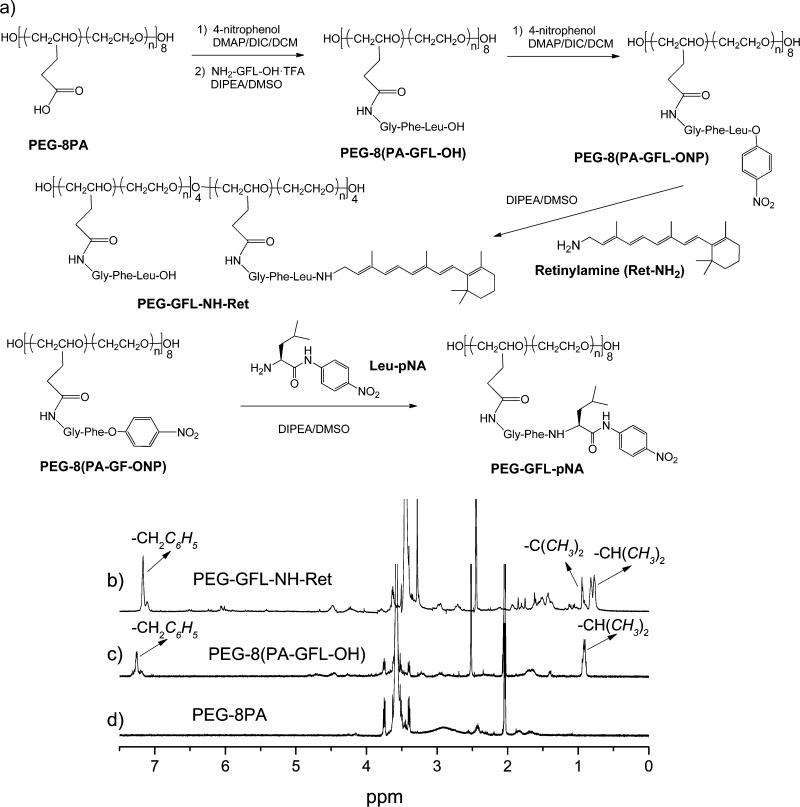
We employed 20 kDa copolymer polyethylene glycol (PEG) with eight of the pendant propionic acid groups (PA) randomly grafted on its backbone26 as our drug carrier and synthesized a polymer conjugate of Ret-NH2 with the peptide spacer, glycine-phenylalanine-leucine (GFL). PEG is one of the most commonly used biocompatible polymers used in drug delivery.27 Copolymer PEG with functionalized side chains could increase drug loading efficiency per polymer chain. Multifunctional PEG retinylamine conjugate with a Gly-Phe-Leu tripeptide spacer, PEG-GFL-NH-Ret, was synthesized from 20 kDa copolymer polyethylene glycol with eight propionic acid side chains (PEG-8-PA),26 as shown in Figure 2a. The peptide spacer NH2-Gly-Phe-Leu-OH (NH2-GFL-OH) was synthesized according to standard solid-phase peptide chemistry25 with a 60% yield. PEG-8PA was first converted to PEG-8PA p-nitrophenol active ester by reaction with excess p-nitrophenol in the presence of coupling agents. GFL peptide was then incorporated into the PEG side chains with an excess of peptide to ensure complete conjugation. The resulting PEG-8(PA-GFL-OH) was then reacted with excess p-nitrophenol in the presence of coupling agents to yield PEG-8(PA-GFL-ONp) active ester. The polymer Ret-NH2 conjugate was prepared by reacting PEG-8(PA-GFL-ONp) with Ret-NH2 and drug loading was controlled by the molar ratio of the two components. We used stepwise polymer analogous reactions for the Ret-NH2 conjugation, rather than the synthesis of GFL-NH-Ret conjugate and then one step direct conjugation to PEG. With this strategy, it was relatively convenient to purify the intermediates and the final product. All intermediates were characterized by 1H NMR spectroscopy, peptide GFL and GF were also characterized by MALDI-TOF mass spectrometry (Supporting Information, Figures S1–S11). Because PEG conjugates with more than 4 Ret-NH2 molecules per polymer chain exhibited poor water solubility, the conjugate with four molecules of Ret-NH2 on average with a water solubility of 30 mg/mL at room temperature was selected for in vivo experiments. Free Ret-NH2 was insoluble in water. The final product, namely, PEG (GFL-NH-Ret)4, was characterized by 1H NMR spectroscopy (Figure 2b–d). The number and weight-average molecular weights of polymer conjugate was 21 and 22 kDa (Mw/Mn = 1.05), as determined by size exclusion chromatography. The stability of drug Ret-NH2 has greatly improved after conjugation. The structure of PEG-GFL-NH-Ret was preserved during the storage in solid form more than 6 months at room temperature, while free Ret-NH2 decomposed in less than 1 week under the same condition.
Figure 2.
Synthetic scheme for PEG-GFL-NH-Ret and PEG-GFL-pNA (a) and 1H NMR spectra of the PEG-GFL-NH-Ret (b) and PEG-8(PA-GFL-OH) (c) intermediates and the PEG-8PA (d) starting material. Solvents: acetone-d6 for PEG-PA and PEG-PA-GFL; DMSO-d6 for PEG-GFL-NH-Ret. The multiplets around 7.25 ppm in (c) and (d) were assigned to phenylalanine aromatic protons with an integral value of five protons. The two methyl protons on Ret-NH2 in spectrum (c) appeared around 1.0 ppm with an integral value of 3. Methyl protons of leucine in (c) and (d) appeared around 0.9 ppm with an integral value of six protons. Two leucine methyl peaks with an integral value of three protons each were observed in spectrum (c), which confirms the conjugation of four drug molecules to side chains of the polymer.
Pharmacokinetics of PEG-GFL-NH-Ret in Normal C57BL Mice
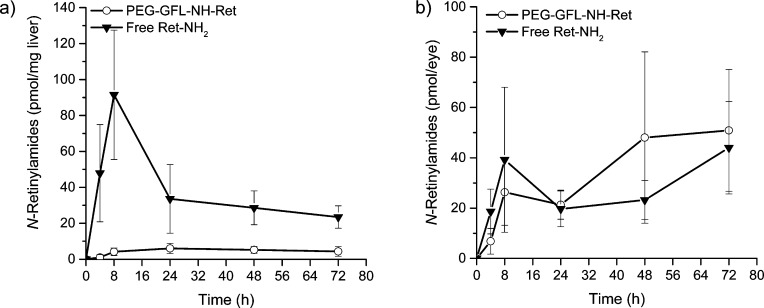
Pharmacokinetic distribution of Ret-NH2 in the liver and eye was determined after oral gavage of PEG-GFL-NH-Ret compared with free Ret-NH2 at the same equivalent dose of 1 mg (3.5 μmol) Ret-NH2 per mouse into 4-week-old C57BL6 female mice. Figure 3 shows the concentration of N-retinylamides,10 the main metabolites of Ret-NH2, in the liver (a) and eye (b) at different time points after the gavage. Mice treated with free Ret-NH2 had much higher nonspecific liver concentrations of N-retinylamides than those treated with the conjugate, especially in the first 8 h after the gavage. The high liver concentrations of N-retinylamides suggest rapid absorption and metabolism of Ret-NH2 after oral administration. In contrast, liver concentrations of N-retinylamides in mice treated with PEG-GFL-NH-Ret were below the detection limit in the first 4 h after conjugate administration and then were maintained at a stable low level from 8 to 72 h thereafter. Both the conjugate and free Ret-NH2 resulted in similar concentrations of N-retinylamides in the eye (P > 0.05) under the same conditions. The liver is a major first pass organ for drug absorption after oral administration. The pharmacokinetics in the liver indicates that the PEG conjugate could control the sustained drug release from the conjugate after oral administration; drug release from the conjugate was slow initially and then was maintained at a stable low rate for up to 72 h. The low concentrations of N-retinylamides found in the liver after conjugate administration are advantageous for minimizing potential toxic side effects associated with the drug. Although concentrations of N-retinylamides in the liver were substantially higher after gavage with free Ret-NH2 than the conjugate, both resulted in comparable concentrations of N-retinylamides in the eye over the 3 day experimental period. The result demonstrated that PEG-GFL-NH-Ret was effective in delivering sufficient Ret-NH2 into the eyes while maintaining low drug concentrations in the rest of the body.
Figure 3.
Pharmacokinetic distribution of N-retinylamides, the main metabolites of Ret-NH2, in the liver (a) and eye (b) after oral administration of Ret-NH2 and PEG-GFL-NH-Ret. The formulated conjugate, PEG-GFL-NH-Ret or Ret-NH2 (1 mg equivalent of Ret-NH2/mouse), was orally gavaged into dark-adapted 4-week-old C57BL wild-type female mice. Mice then were sacrificed at predetermined time points (4, 12, 24, 48, 72, 96, 120 h) after such treatment. N-Retinylamides were extracted from the eyes and liver and quantitatively determined by HPLC. Error bars indicate SD of the means (n = 6).
In Vitro Drug Release Studies
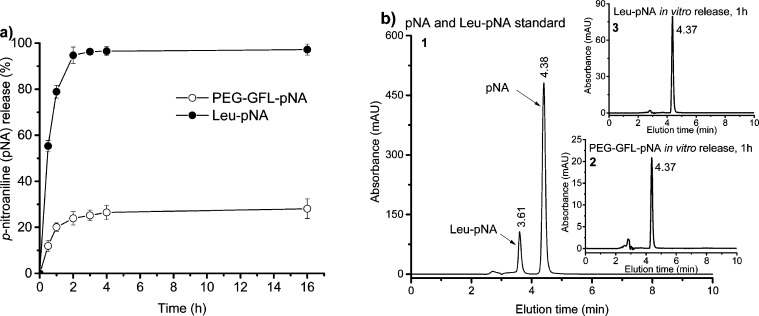
Free Ret-NH2 is highly unstable and it was difficult to accurately determine the drug release kinetics by incubating PEG-GFL-NH-Ret with the digestive enzymes from the GI tract. In order to accurately assess the drug release kinetics, a model drug p-nitroaniline (pNA) conjugate, PEG-GFL-pNA, was synthesized by reacting PEG-8(PA-GF-ONP) with Leu-pNA. Figure 4 shows the in vitro drug release kinetics of p-nitroaniline (pNA) from PEG-GFL-pNA and Leu-pNA in the presence of the homogenates of rat intestinal brush border in isotonic phosphate buffer at pH 6.8 assayed by HPLC. The HPLC elution times for pNA released from PEG-GFL-pNA and Leu-pNA were both 4.37 min consistent with the retention time of the pNA standard (4.38 min) (Figure 4b). At 16 h after the incubation, 28.1 and 97.2% free pNA was released from PEG-GFL-pNA and Leu-pNA, respectively. The polymer conjugate PEG-GFL-pNA showed a more controllable release pattern compared to the low-molecular-weight amino acid conjugate Leu-pNA.
Figure 4.
In vitro drug release kinetics of the conjugate PEG-GFL-pNA. (a) Release kinetics of pNA from PEG-GFL-pNA and Leu-pNA in the homogenates of rat intestinal brush border in isotonic phosphate buffer at pH 6.8 assayed by HPLC; (b) representative HPLC spectra of the released products: (1) pNA and Leu-pNA standard, (2) PEG-GFL-pNA in vitro release at 1 h, (3) Leu-pNA in vitro release at 1 h. HPLC conditions: analytical C18 reverse column (250 mm × 4.6 mm, i.d., 5 μm particle size) with a mobile phase of H2O/actonitrile (40:60, v/v) with 0.05% trifluoroacetic acid, flow rate of 1.0 mL/min and UV detector set at 375 nm.
Effects of PEG-GFL-NH-Ret on Preventing Light-Induced Retinal Degeneration
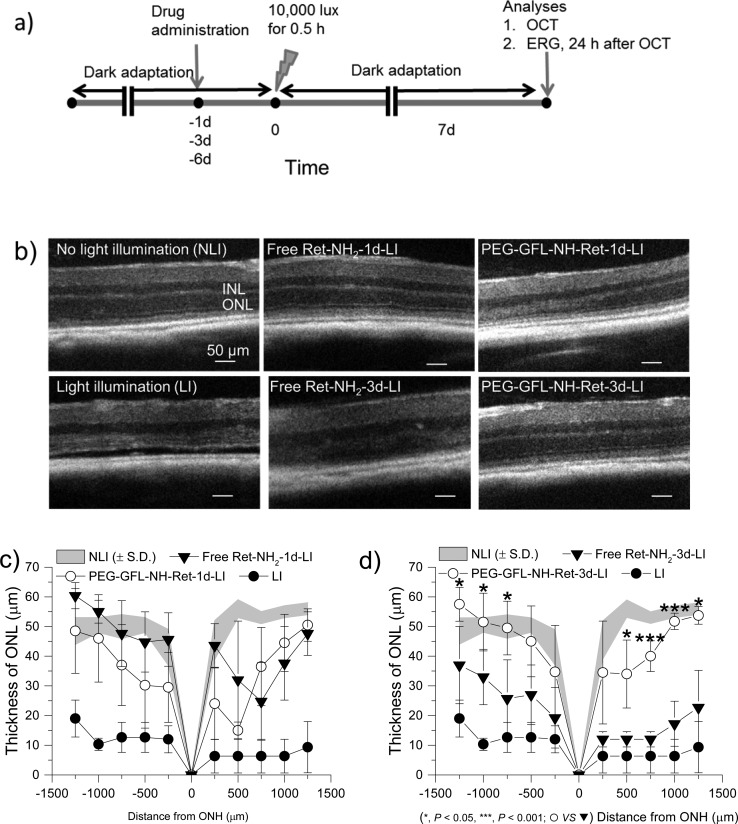
The therapeutic efficacy of PEG-GFL-NH-Ret in preventing light-induced retinal degeneration was investigated in 4-week-old male and female Abca4–/–Rdh8–/– mice. Both Abca4 and Rdh8 are the key enzymes of the visual cycle that act on atRAL.3 Bright white light induces photoreceptor cell death and retinal degeneration, signs similar to human STGD/AMD disease, in this mouse model. As compared to free Ret-NH2, sustained release of Ret-NH2 from PEG-GFL-NH-Ret in the GI tract after oral administration could provide prolonged protection against light-induced retinal degeneration in these double knockout mice. Figure 5a shows a schematic representation of our experimental design. The effectiveness of the conjugate for prolonged retinal protection was determined after the exposure of 4-week-old Abca4–/–Rdh8–/– mice to 10000 lux light for 30 min at 1, 3, and 6 days after a single oral administration of either 8.75 mg of PEG-GFL-NH-Ret or the equivalent 0.5 mg free Ret-NH2 per mouse.
Figure 5.
Protecting effects of PEG-GFL-NH-Ret against light-induced acute retinal degeneration in 4-week-old Abca4–/–Rdh8–/– mice. (a) Schematic representation of the experimental design for assessing the effectiveness of the conjugate. After 4-week-old female Abca4–/–Rdh8–/– mice were kept in the dark for 48 h, they were given either free Ret-NH2 or conjugate PEG-GFL-NH-Ret by gastric gavage at an equivalent dose of 0.5 mg Ret-NH2 per mouse. Mouse eyes were illuminated with 10000 lux light for 30 min either 1 day (1 d), 3 days (3 d), or 6 days (6 d) after the gavage. Mice then were kept in the dark for 7 days, after which final retinal evaluations were performed. (b) Representative OCT images of Abca4–/–Rdh8–/– mouse retinas in different treatment groups (NLI = no light illumination; LI = light illuminated). Scale bar indicates 50 μm in the OCT image. (c, d) Seven days after light exposure, the ONL thickness was measured from in vivo OCT images obtained along the vertical meridian from the superior to inferior retina of mice gavaged either 1 day (c) or 3 days (d) before bright light exposure. Statistical analysis was performed to compare the treatment groups using one-way ANOVA. Error bars indicate SD of the means (n = 4–5).
Retinal integrity of the treated mice was determined with ultrahigh resolution spectral-domain optical coherent tomography (OCT). Figure 5b shows representative OCT images of retinas from mice in different treatment groups. Free Ret-NH2 produced effective protection against light-induced retinal degeneration when gavaged at a dose of 0.5 mg/mouse 1 day before light exposure but did not maintain such protection when administered 3 days before the same exposure. In contrast, PEG-GFL-NH-Ret gavaged 3 days before light exposure did provide such protection at the same equivalent dose. No protective effect against light-induced retinal degeneration was imaged by OCT after pretreatment with either free Ret-NH2 or PEG-GFL-NH-Ret 6 days before light exposure (Supporting Information, Figure S12). Figure 5c,d shows the thickness of the outer nuclear layer (ONL) of retinas measured from OCT images of mice in the different treatment groups. Both the free drug and PEG-GFL-NH-Ret gavaged 1 day before light exposure similarly protected the ONL against strong light with a thickness comparable to that of mice without light illumination. The retinal ONL thickness of mice treated with conjugate 3 days before strong light illumination remained the same as that of unilluminated mice, whereas this thickness was significantly reduced in mice treated with free Ret-NH2 under the same conditions.
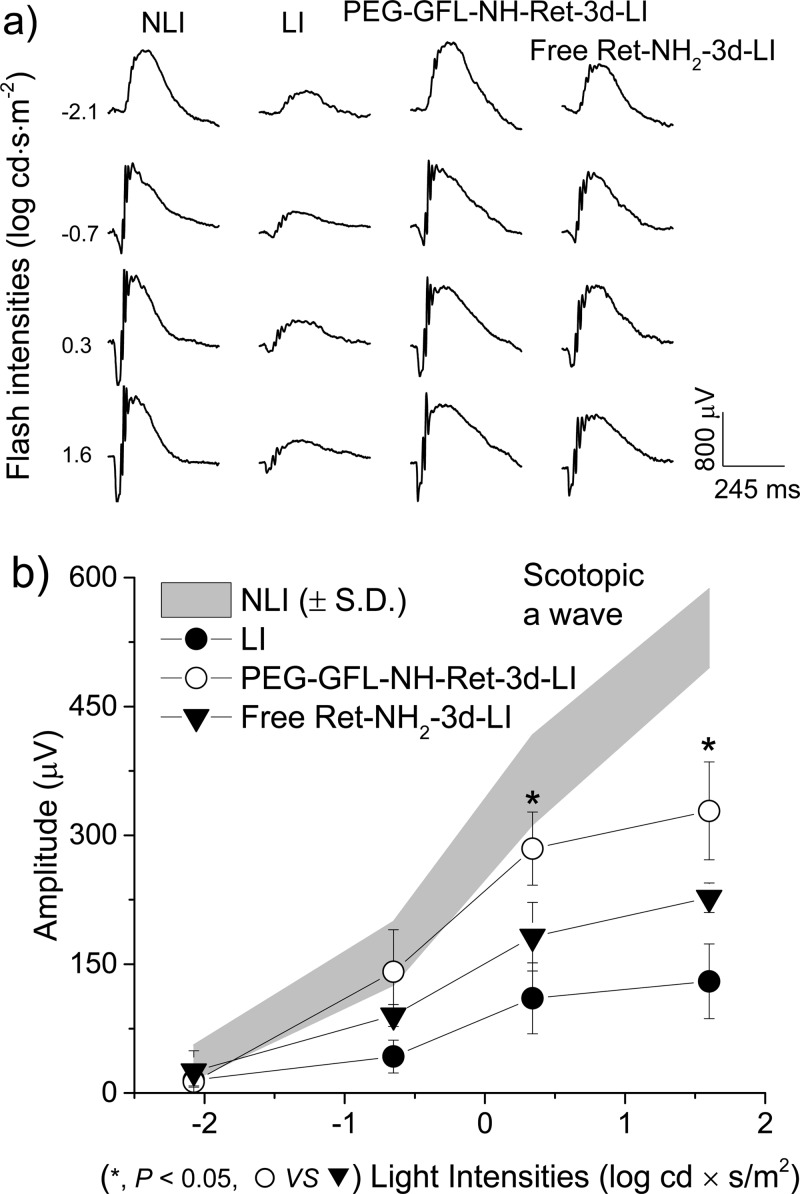
Electroretinograms (ERGs) also were recorded to evaluate retinal function in these Abca4–/–Rdh8–/– mice after treatment with free Ret-NH2 or PEG-GFL-NH-Ret followed by strong light exposure 3 days later. Figure 6 shows representative scotopic waves and average ERG peak amplitudes of these mice in different treatment groups. ERG responses of mice treated with PEG-GFL-NH-Ret and light illumination (PEG-GFL-NH-Ret-3d-LI) were virtually the same as in mice with no light-illumination (NLI), but the ERG activity in mice with strong light illumination was substantially reduced and mice treated with free Ret-NH2 also exhibited significant loss of ERG activity. The average ERG peak amplitudes of mice treated with the conjugate were significantly higher than those of mice treated with free Ret-NH2 (Figure 6b).
Figure 6.
ERG evaluation of the effectiveness of PEG-GFL-NH-Ret on preserving retina function against light-induced acute retinal degeneration in 4-week-old Abca4–/–Rdh8–/– mice. Representative ERG scotopic waves (a) and average peak amplitudes (b) (NLI = no light illumination; LI = light illuminated) of 4-week-old Abca4–/–Rdh8–/– mice pretreated with either free Ret-NH2 or PEG-GFL-NH-Ret at an equivalent dose of 0.5 mg Ret-NH2 per mouse 3 days (3 d) prior to light illumination and evaluated 7 days later. Statistical analysis was performed to compare the treatment groups using one-way ANOVA. Error bars indicate SD of the means (n = 3).
Here we have demonstrated that oral administration of the polymer Ret-NH2 conjugate, PEG-GFL-NH-Ret, provided more prolonged preservation of retinal structure and function against strong light exposure after a single oral dose than free Ret-NH2 at an equivalent dose. Ret-NH2 was previously shown to be effective in preventing light-induced retinal degeneration in animal models.1 Conjugation of the drug to biocompatible polymers resulted in controlled drug distribution in the eye and more prolonged prevention of retinal degeneration against strong light exposure 3 days after oral administration. The duration of this effect was consistent with the transition time (72 h) of undigested materials in the mouse GI tract. Because of its relatively high molecular weight (20 kDa), PEG cannot be absorbed in the intestine, and the presence of the PEG conjugate in the gastrointestinal (GI) tract allows a gradual release of Ret-NH2 through cleavage of the oligopeptide spacer by digestive peptidases in the small intestine and colon. As shown in the pharmacokinetics experiment, sustained drug release from the polymer drug conjugate in the GI tract can maintain minimally effective drug concentrations in the liver and systemic circulation, which is critical to minimize any dose-dependent toxic side effects of the drug. At the same time, a sufficient amount of Ret-NH2 can still be delivered to the eye for prolonged periods, resulting in effective protection of the retina from strong light-induced degeneration.
Oral drug delivery is the most convenient drug delivery process. Prolonged retinal protection by the polymer drug conjugate can reduce dosing frequency and the overall dose, which also helps to minimize any potential dose-dependent toxicity and increase patient compliance. PEG-GFL-NH-Ret has clearly shown several advantageous features over free Ret-NH2 for oral drug delivery, including sustained drug delivery, controlled pharmacokinetics, low drug concentrations in the systemic circulation, and prolonged effective protection against light-induced retinal degeneration. Promising results from these experiments have demonstrated that PEG-GFL-NH-Ret conjugate has the potential to effectively treat human retinal degenerative diseases, including STGD and AMD. As yet, no drug has been approved by the FDA for treatment of either STGD or the “dry” form of AMD, although several therapeutics with conventional formulations have undergone clinical trials for the diseases.4 We have demonstrated here that the efficacy of ocular therapeutics can be further improved by using cutting-edge drug delivery technologies based on biomedical polymers. The drug delivery system can also serve as a platform technology for controlled oral delivery of other ocular therapeutics to treat human retinal degenerative diseases.
Conclusion
A water-soluble PEG Ret-NH2 conjugate containing an oligopeptide spacer, namely, PEG-GFL-NH-Ret, was designed for prolonged treatment of retinal degenerative diseases by sustained release of Ret-NH2 after oral administration. Upon pretreatment by oral gavage, PEG-GFL-NH-Ret resulted in more controlled pharmacokinetics and produced a greater and more prolonged protective effect against light-induced retinal degeneration in Abca4–/–Rdh8–/– mice than free Ret-NH2 at a single equivalent 0.5 mg dose. This PEG Ret-NH2 conjugate shows promise for treating human retinal degenerative diseases, including STGD and AMD.
Acknowledgments
The authors thank Dr. Zhuxian Zhou for valuable advice on data preparation. This work was supported in part by funding from the National Eye Institute of the National Institutes of Health (Grant R24EY0211260). G.Y. is also supported in part by a Knights Templar Eye Foundation pediatric ophthalmology career-starter research grant. K.P. is John H. Hord Professor of Pharmacology. Z.-R.L. is M. Frank Rudy and Margaret Domiter Rudy Professor of Biomedical Engineering.
Supporting Information Available
Supplemental text contains 1H NMR and MALDI-TOF mass spectrometry for the conjugates and OCT images showing representative morphology of Abca4–/–Rdh8–/– mouse retinas with drug treatment 6 days before light illumination. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
‡ These authors contributed equally to this work (G.Y. and X.W.).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Maeda A.; Golczak M.; Chen Y.; Okano K.; Kohno H.; Shiose S.; Ishikawa K.; Harte W.; Palczewska G.; Maeda T.; Palczewski K. Nat. Chem. Biol. 2012, 8, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A.; Maeda T.; Golczak M.; Palczewski K. J. Biol. Chem. 2008, 283, 26684–26693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser P. D.; Golczak M.; Palczewski K. Chem. Rev. 2014, 114, 194–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. B.; Syed B. A. Nat. Rev. Drug Discovery 2013, 12, 501–502. [DOI] [PubMed] [Google Scholar]
- Dobri N.; Qin Q.; Kong J.; Yamamoto K.; Liu Z.; Moiseyev G.; Ma J. X.; Allikmets R.; Sparrow J. R.; Petrukhin K. Invest. Ophthalmol. Vis. Sci. 2013, 54, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota R.; Boman N. L.; David R.; Mallikaarjun S.; Patil S.; Birch D. Retina 2012, 32, 183–188. [DOI] [PubMed] [Google Scholar]
- Kubota R.; Al-Fayoumi S.; Mallikaarjun S.; Patil S.; Bavik C.; Chandler J. W. Retina 2014, 34, 603–609. [DOI] [PubMed] [Google Scholar]
- Palczewski K. Trends Pharmacol. Sci. 2010, 31, 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A.; Maeda T.; Golczak M.; Imanishi Y.; Leahy P.; Kubota R.; Palczewski K. Mol. Pharmacol. 2006, 70, 1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golczak M.; Imanishi Y.; Kuksa V.; Maeda T.; Kubota R.; Palczewski K. J. Biol. Chem. 2005, 280, 42263–42273. [DOI] [PubMed] [Google Scholar]
- Gaudana R.; Ananthula H. K.; Parenky A.; Mitra A. K. AAPS J. 2010, 12, 348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geroski D. H.; Edelhauser H. F. Invest. Ophthalmol. Vis. Sci. 2000, 41, 961–964. [PubMed] [Google Scholar]
- Eljarrat-Binstock E.; Pe’er J.; Domb A. J. Pharm. Res. 2010, 27, 530–543. [DOI] [PubMed] [Google Scholar]
- Vandamme T. F. Prog. Retin. Eye Res. 2002, 21, 15–34. [DOI] [PubMed] [Google Scholar]
- Kaur I. P.; Garg A.; Singla A. K.; Aggarwal D. Int. J. Pharm. 2004, 269, 1–14. [DOI] [PubMed] [Google Scholar]
- Barbu E.; Verestiuc L.; Iancu M.; Jatariu A.; Lungu A.; Tsibouklis J. Nanotechnology 2009, 20, 225108. [DOI] [PubMed] [Google Scholar]
- Hacker M. C.; Haesslein A.; Ueda H.; Foster W. J.; Garcia C. A.; Ammon D. M.; Borazjani R. N.; Kunzler J. F.; Salamone J. C.; Mikos A. G. J. Biomed. Mater. Res., Part A 2009, 88, 976–989. [DOI] [PubMed] [Google Scholar]
- Shirasaki Y. J. Pharm. Sci. 2008, 97, 2462–2496. [DOI] [PubMed] [Google Scholar]
- Achouri D.; Alhanout K.; Piccerelle P.; Andrieu V. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [DOI] [PubMed] [Google Scholar]
- Wu X.; Yu G.; Luo C.; Maeda A.; Zhang N.; Sun D.; Zhou Z.; Puntel A.; Palczewski K.; Lu Z. R. ACS Nano 2014, 8, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhauser H. F.; Rowe-Rendleman C. L.; Robinson M. R.; Dawson D. G.; Chader G. J.; Grossniklaus H. E.; Rittenhouse K. D.; Wilson C. G.; Weber D. A.; Kuppermann B. D.; Csaky K. G.; Olsen T. W.; Kompella U. B.; Holers V. M.; Hageman G. S.; Gilger B. C.; Campochiaro P. A.; Whitcup S. M.; Wong W. T. Invest. Ophthalmol. Vis. Sci. 2010, 51, 5403–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopečková P.; Longer M. A.; Woodley J. F.; Duncan R.; Kopeček J. Macromol. Rapid Commun. 1991, 12, 101–106. [Google Scholar]
- Kopečková P.; Ikesue K.; Kopeček J. Macromol. Chem. Phys. 1992, 193, 2605–2619. [Google Scholar]
- Heizmann J.; Langguth P.; Biber A.; Oschmann R.; Merkle H. P.; Wolffram S. Peptides 1996, 17, 1083–1089. [DOI] [PubMed] [Google Scholar]
- Coin I.; Beyermann M.; Bienert M. Nat. Protoc. 2007, 2, 3247–3256. [DOI] [PubMed] [Google Scholar]
- Wang L.Polycationic water soluble copolymer and method for transferring polyanionic macromolecules across biological barriers. U.S. Patent 07060498, June 13, 2006.
- Knop K.; Hoogenboom R.; Fischer D.; Schubert U. S. Angew. Chem., Int. Ed. 2010, 49, 6288–6308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.