Abstract
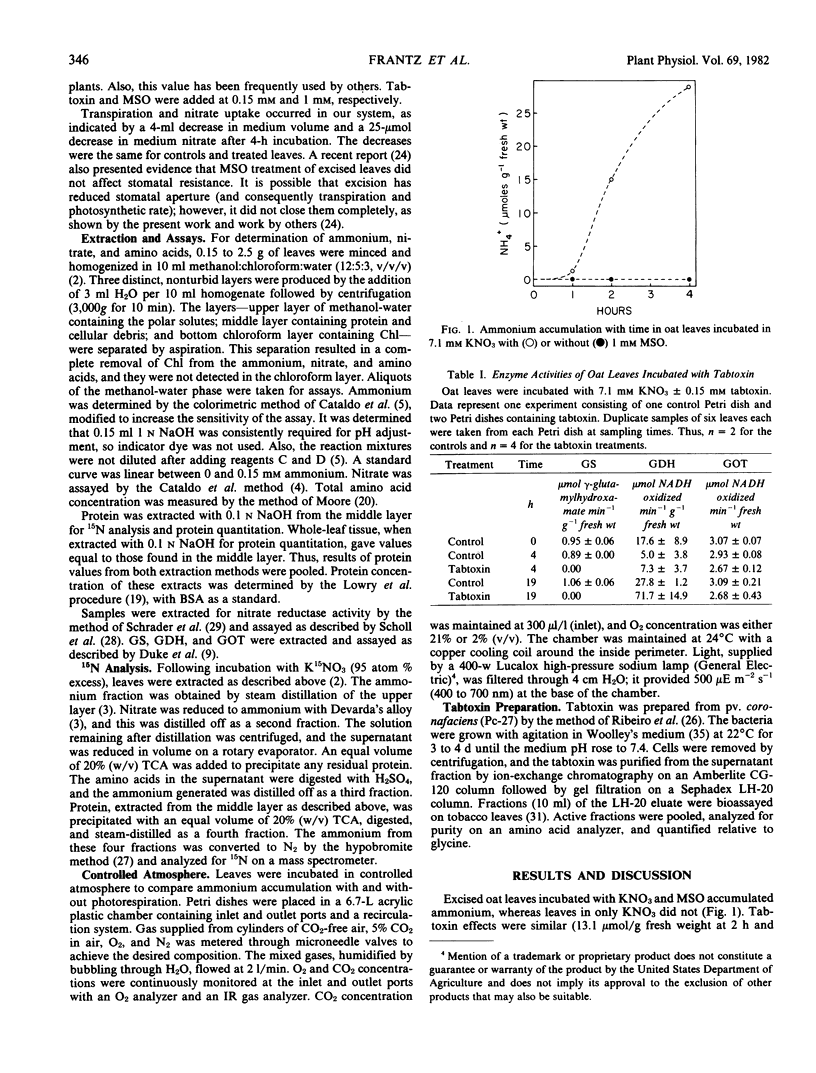
Excised 7-day-old oat (Avena sativa L. cv. Jaycee) leaves were incubated in media containing 7.1 millimolar KNO3 and 0.15 millimolar tabtoxin or 1 millimolar methionine sulfoximine (MSO) to investigate the sources of the observed ammonium accumulated. Tabtoxin and MSO are known inhibitors of glutamine synthetase, the first enzyme in the primary pathway of ammonium assimilation. During a 4- to 6-hour incubation, there was little net change in protein or total amino acid concentration. Alanine, aspartate/asparagine, and glutamate/glutamine decreased markedly under these treatments, whereas several other amino acids increased. Exogenous 15N from K15NO3 was taken up and incorporated into the nitrate and ammonium fractions of leaves treated with tabtoxin or MSO. This result and the high in vitro activities of nitrate reductase indicated that reduction of nitrate was one source of the accumulated ammonium. Leaves incubated under 2% O2 to reduce photorespiration accumulated only about 13% as much ammonium as did those under normal atmospheres. We conclude that most of the tabtoxin- or MSO-induced ammonium came from photo-respiration, and the remainder was from nitrate reduction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barash I., Sadon T., Mor H. Induction of a specific isoenzyme of glutamate dehydrogenase by ammonia in oat leaves. Nat New Biol. 1973 Aug 1;244(135):150–152. doi: 10.1038/newbio244150a0. [DOI] [PubMed] [Google Scholar]
- Bieleski R. L., Turner N. A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal Biochem. 1966 Nov;17(2):278–293. doi: 10.1016/0003-2697(66)90206-5. [DOI] [PubMed] [Google Scholar]
- Dougall D. K. Evidence for the presence of glutamate synthase in extracts of carrot cell cultures. Biochem Biophys Res Commun. 1974 Jun 4;58(3):639–646. doi: 10.1016/s0006-291x(74)80466-3. [DOI] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Miller M. G. Low Temperature Effects on Soybean (Glycine max [L.] Merr. cv. Wells) Free Amino Acid Pools during Germination. Plant Physiol. 1978 Oct;62(4):642–647. doi: 10.1104/pp.62.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler M. W., Jessup W., Sarkissian G. S. Glutamate synthetase type activity in higher plants. FEBS Lett. 1974 Sep 15;46(1):340–342. doi: 10.1016/0014-5793(74)80401-1. [DOI] [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T., Avron M. Uncouplers of Spinach Chloroplast Photosynthetic Phosphorylation. Plant Physiol. 1959 May;34(3):272–277. doi: 10.1104/pp.34.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968 Dec 10;243(23):6281–6283. [PubMed] [Google Scholar]
- Platt S. G., Anthon G. E. Ammonia accumulation and inhibition of photosynthesis in methionine sulfoximine treated spinach. Plant Physiol. 1981 Mar;67(3):509–513. doi: 10.1104/pp.67.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puritch G. S., Barker A. V. Structure and function of tomato leaf chloroplasts during ammonium toxicity. Plant Physiol. 1967 Sep;42(9):1229–1238. doi: 10.1104/pp.42.9.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Cataldo D. A., Peterson D. M. Use of protein in extraction and stabilization of nitrate reductase. Plant Physiol. 1974 May;53(5):688–690. doi: 10.1104/pp.53.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden S. L., Durbin R. D. Glutamine synthetase inhibition: possible mode of action of wildfire toxin from Pseudomonas tabaci. Nature. 1968 Jul 27;219(5152):379–380. doi: 10.1038/219379a0. [DOI] [PubMed] [Google Scholar]
- WOOLLEY D. W., PRINGLE R. B., BRAUN A. C. Isolation of the phytopathogenic toxin of Pseudomonas tabaci, an antagonist of methionine. J Biol Chem. 1952 May;197(1):409–417. [PubMed] [Google Scholar]
- Wedler F. C., Horn B. R. Catalytic mechanisms of glutamine synthetase enzymes. Studies with analogs of possible intermediates and transition states. J Biol Chem. 1976 Dec 10;251(23):7530–7538. [PubMed] [Google Scholar]



