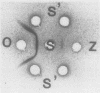
Abstract
Antiserum was prepared against proteolytically undegraded phytochrome obtained from etiolated zucchini squash (Cucurbita pepo L., cv. Black Beauty). The antiserum was prepared by injecting into a rabbit immunoprecipitates between zucchini phytochrome and specific antiserum against undegraded oat (Avena sativa L., cv. Garry) phytochrome. Specific antiphytochrome immunoglobulins were purified from this crude serum by an affinity column consisting of conventionally purified undegraded pea phytochrome covalently linked to cyanogen bromide-activated agarose. These purified immunoglobulins were also linked to cyanogen bromide-activated agarose and were used to immunopurify zucchini, pea (Pisum sativum L., cv. Alaska), and lettuce (Lactuca sativa L., cv. Grand Rapids) phytochrome. All three dicotyledonous phytochromes exhibited a monomer size near 120,000 daltons by sodium dodecyl sulfate, polyacrylamide gel electrophoresis. Absorbance spectra of immunopurified zucchini phytochrome indicated that the ratio of visible to ultraviolet absorbance for purified zucchini phytochrome is lower than that observed for oat phytochrome. The isoelectric point of zucchini phytochrome, which was observed to be heterogeneous by this criterion, was found to be in the range of 6.5 to 7.0, higher than that observed for oat phytochrome. The electrophoretic mobility of zucchini phytochrome was found to be similar to that observed for oat and pea phytochrome under conditions that were nondenaturing and did not involve any molecular sieving effect. The amino acid analysis of zucchini phytochrome is similar to that reported previously for oat and rye (Secale cereale L., cv. Balbo) phytochrome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Cundiff S. C., Pratt L. H. Immunological and physical characterization of the products of phytochrome proteolysis. Plant Physiol. 1975 Feb;55(2):212–217. doi: 10.1104/pp.55.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundiff S. C., Pratt L. H. Phytochrome Characterization by Rabbit Antiserum against High Molecular Weight Phytochrome. Plant Physiol. 1975 Feb;55(2):207–211. doi: 10.1104/pp.55.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner G., Pike C. S., Rice H. V., Briggs W. R. "Disaggregation" of phytochrome in vitro-a consequence of proteolysis. Plant Physiol. 1971 Dec;48(6):686–693. doi: 10.1104/pp.48.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Partial characterization of undegraded oat phytochrome. Biochemistry. 1980 Jan 22;19(2):390–394. doi: 10.1021/bi00543a022. [DOI] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Phytochrome immunoaffinity purification. Plant Physiol. 1979 Aug;64(2):332–336. doi: 10.1104/pp.64.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H. Comparative immunochemistry of phytochrome. Plant Physiol. 1973 Jan;51(1):203–209. doi: 10.1104/pp.51.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Cundiff S. C. Spectral characterization of high-molecular-weight phytochrome. Photochem Photobiol. 1975 Feb;21(2):91–97. doi: 10.1111/j.1751-1097.1975.tb06634.x. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Schäfer E., Marmé D. De novo synthesis of phytochrome in pumpkin hooks. Plant Physiol. 1973 Aug;52(2):124–127. doi: 10.1104/pp.52.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail P. H., Schäfer E., Marmé D. Turnover of phytochrome in pumpkin cotyledons. Plant Physiol. 1973 Aug;52(2):128–131. doi: 10.1104/pp.52.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail P. Interaction of phytochrome with other cellular components. Photochem Photobiol. 1975 Dec;22(6):299–301. doi: 10.1111/j.1751-1097.1975.tb06755.x. [DOI] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R. Immunochemistry of phytochrome. Plant Physiol. 1973 May;51(5):939–945. doi: 10.1104/pp.51.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R., Jackson-White C. J. Purification of oat and rye phytochrome. Plant Physiol. 1973 May;51(5):917–926. doi: 10.1104/pp.51.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R. Partial characterization of oat and rye phytochrome. Plant Physiol. 1973 May;51(5):927–938. doi: 10.1104/pp.51.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti P., Drysdale J. W. Isoelectric focusing in polyacrylamide gels. Biochim Biophys Acta. 1971 Apr 27;236(1):17–28. doi: 10.1016/0005-2795(71)90144-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]