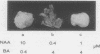
Abstract
The endogenous levels of auxin and cytokinin in teratoma and unorganized tobacco (Nicotiana tabacum L. var Wisconsin #38) crown gall tumor tissues were determined. Teratoma tissues contain levels of auxin and cytokinin favorable for shoot formation, whereas unorganized tumors contain levels of auxin that suppress shoot formation. This conclusion is based upon the observation that when levels of auxin and cytokinin similar to those found in a teratoma were added to the growth medium of nontumorous tobacco tissue, shoot formation resulted; when levels similar to those found in unorganized tumors were added, the normal tissue grew as unorganized callus.
Full text
PDF
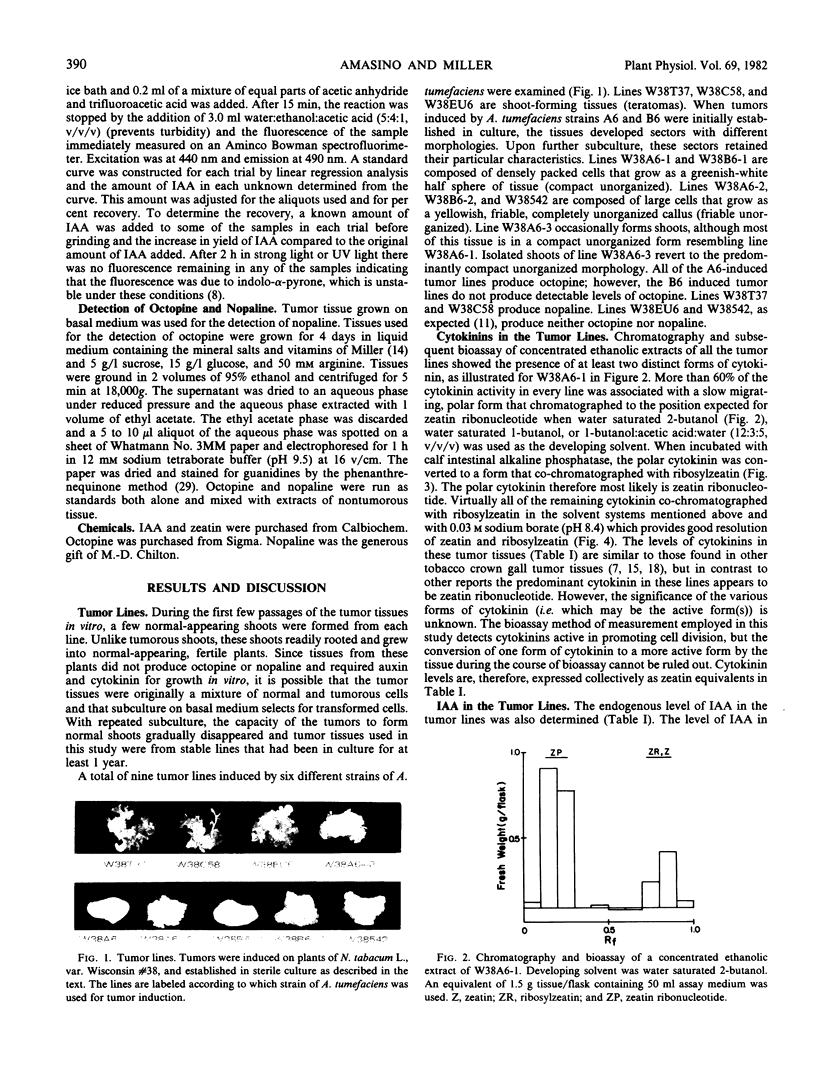
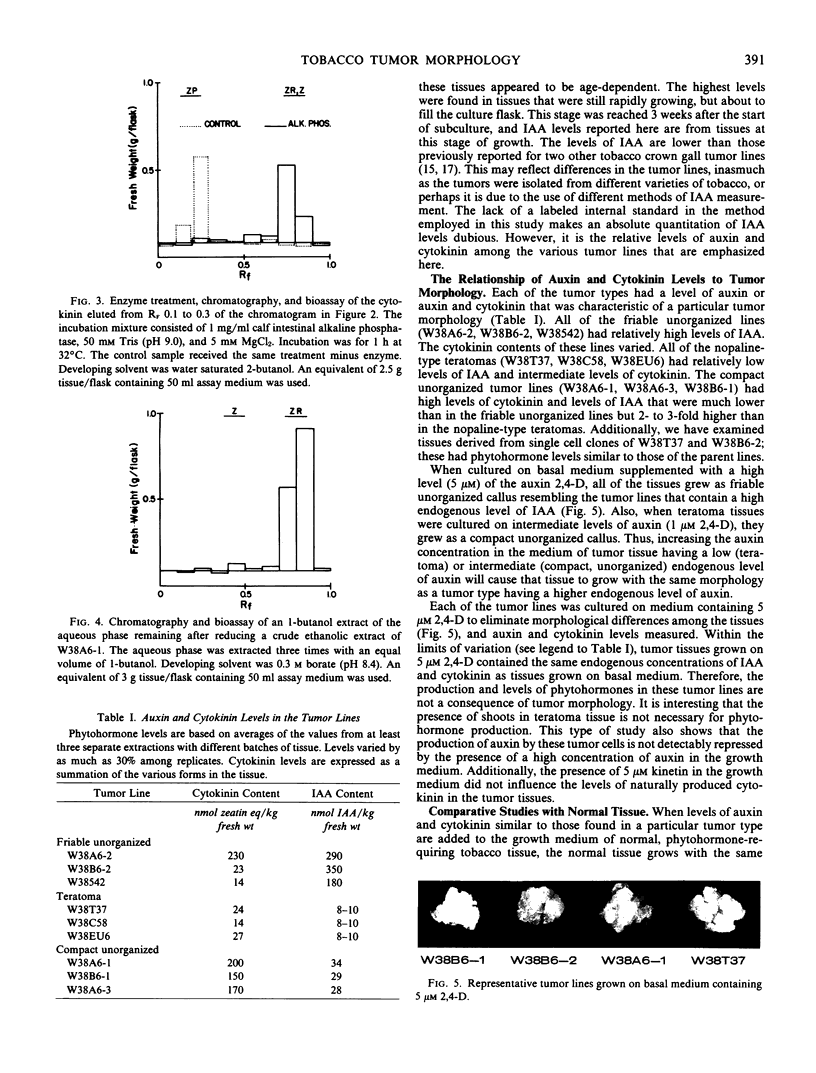

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Braun A. C. A Physiological Basis for Autonomous Growth of the Crown-Gall Tumor Cell. Proc Natl Acad Sci U S A. 1958 Apr;44(4):344–349. doi: 10.1073/pnas.44.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Saiki R. K., Yadav N., Gordon M. P., Quetier F. T-DNA from Agrobacterium Ti plasmid is in the nuclear DNA fraction of crown gall tumor cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4060–4064. doi: 10.1073/pnas.77.7.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einset J. W. Cytokinins in tobacco crown gall tumors. Biochem Biophys Res Commun. 1980 Mar 28;93(2):510–515. doi: 10.1016/0006-291x(80)91106-7. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresshoff P. M., Skotnicki M. L., Rolfe B. G. Crown gall teratoma formation is plasmid and plant controlled. J Bacteriol. 1979 Feb;137(2):1020–1021. doi: 10.1128/jb.137.2.1020-1021.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon P., Chilton M. D., Petit A., Tempé J. Agropine in "null-type" crown gall tumors: Evidence for generality of the opine concept. Proc Natl Acad Sci U S A. 1980 May;77(5):2693–2697. doi: 10.1073/pnas.77.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. Ribosyl-trans-Zeatin, A Major Cytokinin Produced by Crown Gall Tumor Tissue. Proc Natl Acad Sci U S A. 1974 Feb;71(2):334–338. doi: 10.1073/pnas.71.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G., Klapwijk P. M., Poulis J. A., Schilperoort R. A. Characterization of Tn904 insertions in octopine Ti plasmid mutants of Agrobacterium tumefaciens. J Bacteriol. 1980 Oct;144(1):82–91. doi: 10.1128/jb.144.1.82-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Smith E. F., Townsend C. O. A PLANT-TUMOR OF BACTERIAL ORIGIN. Science. 1907 Apr 26;25(643):671–673. doi: 10.1126/science.25.643.671. [DOI] [PubMed] [Google Scholar]
- Stoessl A., Venis M. A. Determination of submicrogram levels of indole-3-acetic acid: a new, highly specific method. Anal Biochem. 1970 Apr;34(2):344–351. doi: 10.1016/0003-2697(70)90118-1. [DOI] [PubMed] [Google Scholar]
- Tegley J. R., Witham F. H., Krasnuk M. Chromatographic analysis of a cytokinin from tissue cultures of crown-gall. Plant Physiol. 1971 Apr;47(4):581–585. doi: 10.1104/pp.47.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Van Montagu M., Holsters M., Zambryski P., Hernalsteens J. P., Depicker A., De Beuckeleer M., Engler G., Lemmers M., Willmitzer L., Schell J. The interaction of Agrobacterium Ti-plasmid DNA and plant cells. Proc R Soc Lond B Biol Sci. 1980 Nov 19;210(1180):351–365. doi: 10.1098/rspb.1980.0139. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]