Abstract
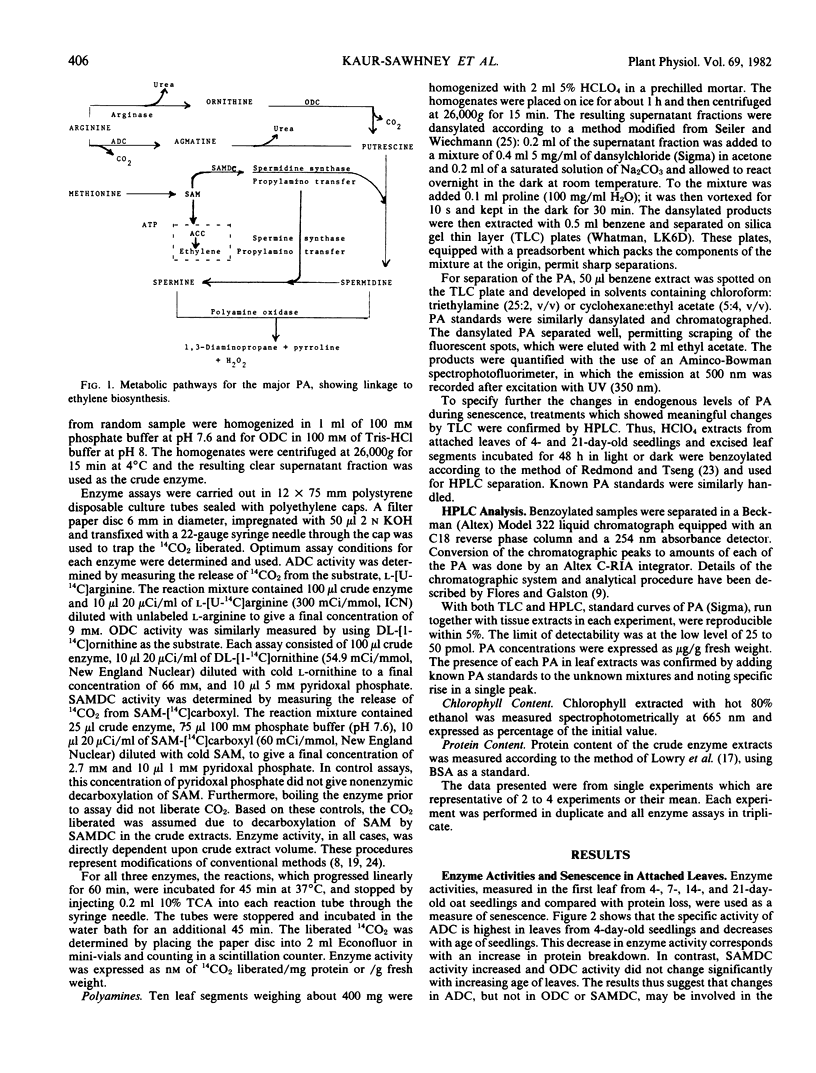
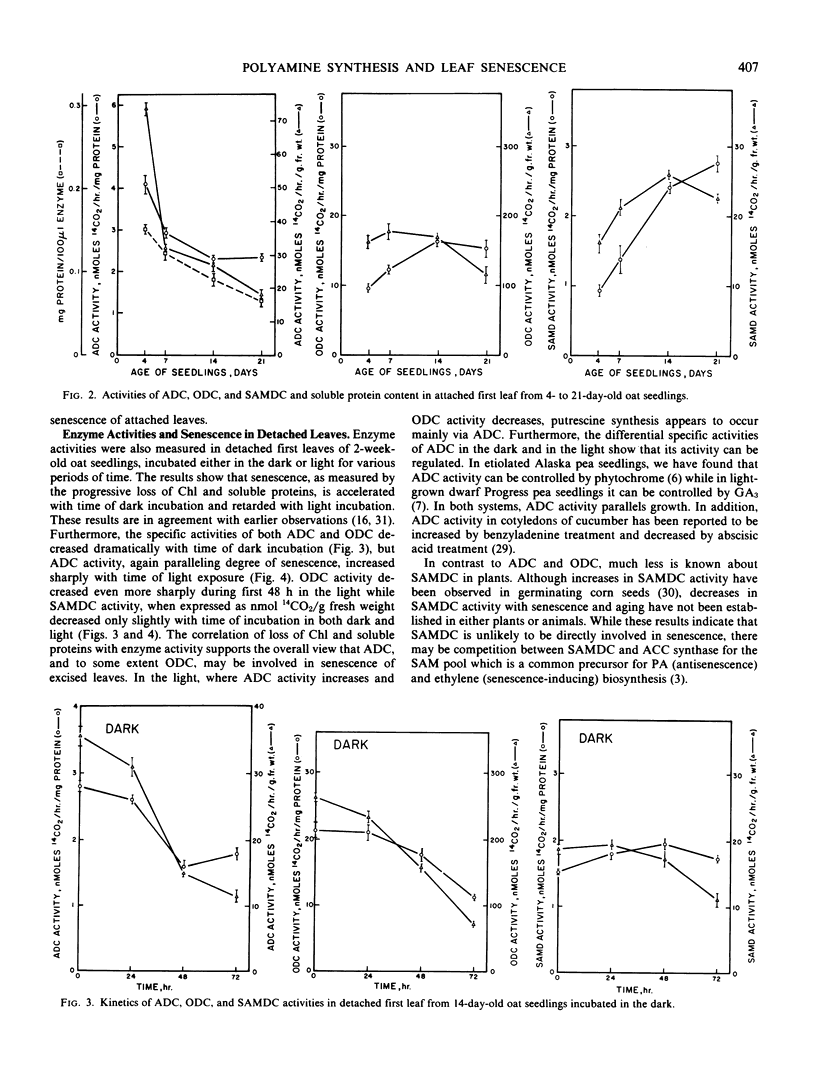
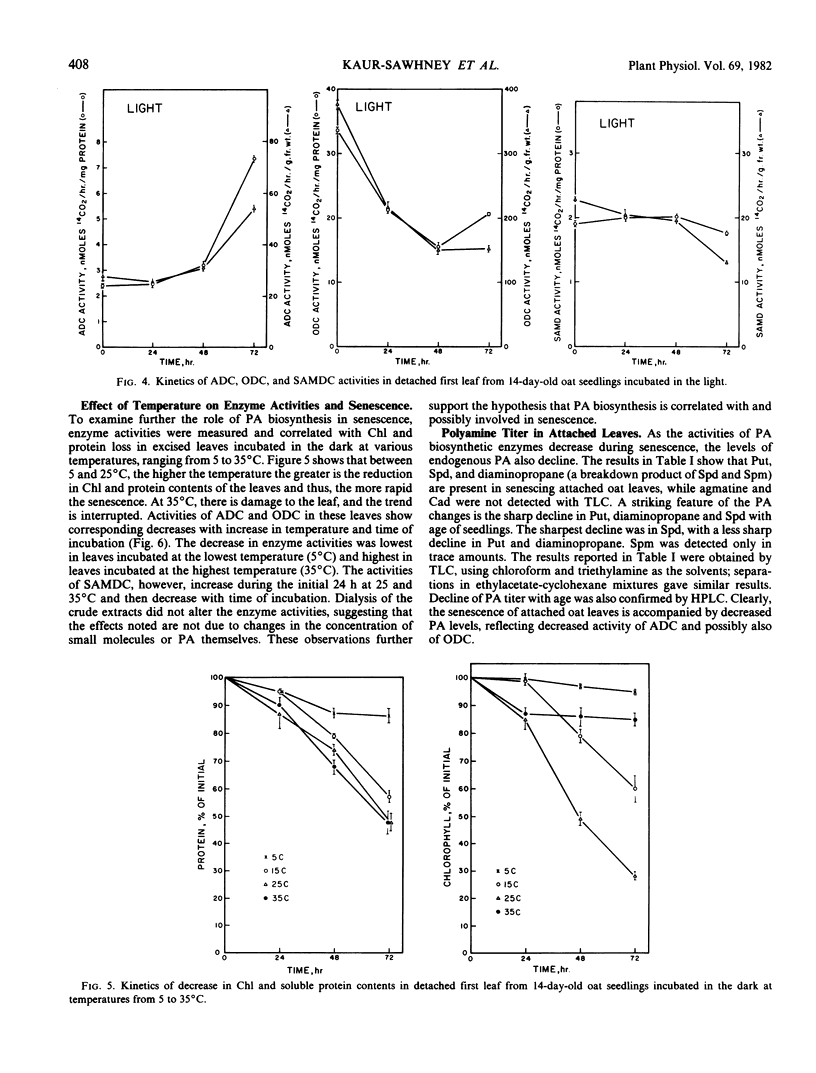
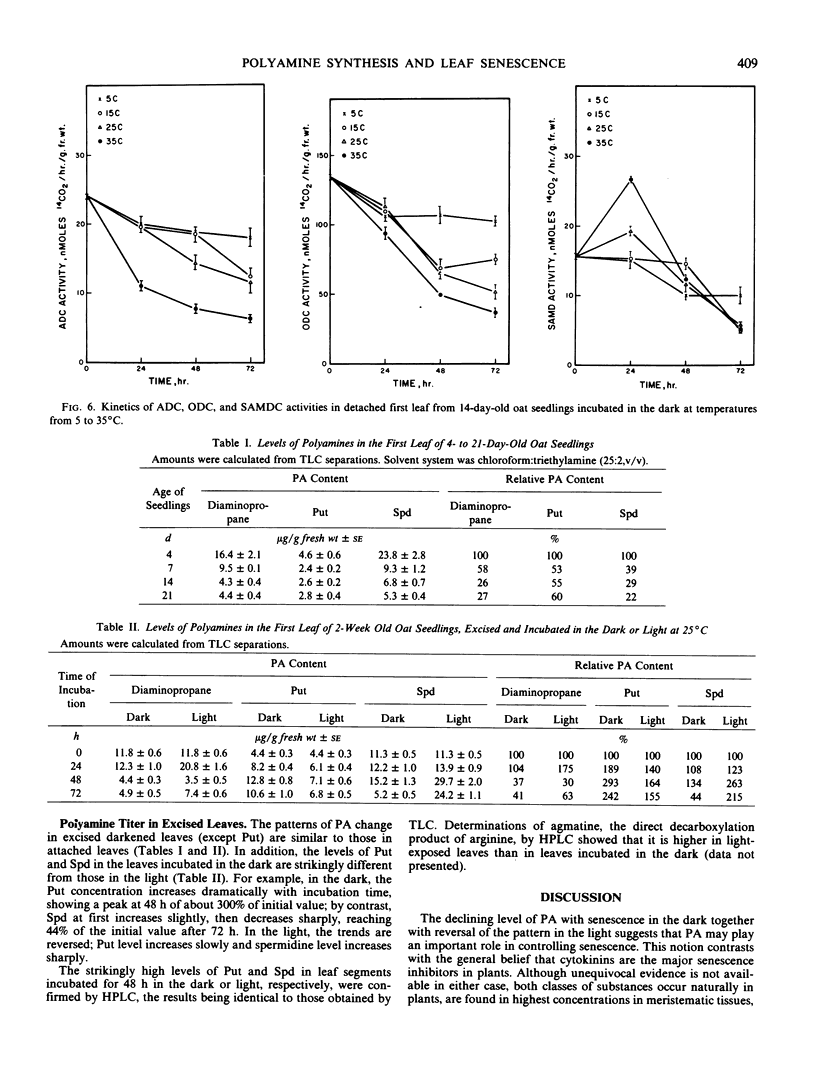
Polyamine biosynthesis in senescing leaves of Avena sativa L. was measured by determining the activities of arginine decarboxylase (EC 4.1.1.19), ornithine decarboxylase (EC 4.1.1.17) and S-adenosyl-l-methionine decarboxylase (EC 4.1.1.50). Polyamine content was also estimated by thin layer chromatography and high performance liquid chromatography. Arginine decarboxylase activity decreases progressively in aging attached first leaves and in senescing excised leaves in the dark. Conversely, it increases during light exposure of excised leaves, which retards senescence. Ornithine decarboxylase activity is high and constant in the attached leaf, irrespective of age; it decreases in excised leaves kept in the dark and in the light, irrespective of senescence. S-Adenosyl-l-methionine decarboxylase shows no correlation with age or senescence. Levels of putrescine, diaminopropane, agmatine, and spermidine are high in young leaves and decline with age. The best single indicator of senescence is usually spermidine, which decreases in excised leaves incubated in the dark, but increases in such leaves with time of light exposure. Spermidine generally has a reciprocal relationship with putrescine, indicating that spermidine synthase, which converts putrescine to spermidine, may exert important physiological control. These data support the view that polyamines play an important role in the regulation of plant development.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Burgoon A. C., Anderson J. D., Lieberman M. Polyamines inhibit biosynthesis of ethylene in higher plant tissue and fruit protoplasts. Plant Physiol. 1981 Aug;68(2):453–456. doi: 10.1104/pp.68.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y. R., Galston A. W. Simultaneous Phytochrome-controlled Promotion and Inhibition of Arginine Decarboxylase Activity in Buds and Epicotyls of Etiolated Peas. Plant Physiol. 1981 Feb;67(2):266–269. doi: 10.1104/pp.67.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y. R., Kaur-Sawhney R., Galston A. W. Promotion by gibberellic Acid of polyamine biosynthesis in internodes of light-grown dwarf peas. Plant Physiol. 1982 Jan;69(1):103–106. doi: 10.1104/pp.69.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M. J., Levy C. C., Russell D. H. Purification and characterization of S-adenosyl-L-methionine decarboxylase from rat liver. Biochemistry. 1972 Feb 29;11(5):671–677. doi: 10.1021/bi00755a002. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Mizrahi Y., Bachrach U. Ornithine decarboxylase activity in rapidly proliferating plant cells. FEBS Lett. 1979 Aug 1;104(1):146–148. doi: 10.1016/0014-5793(79)81102-3. [DOI] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Flores H. E., Galston A. W. Polyamine oxidase in oat leaves: a cell wall-localized enzyme. Plant Physiol. 1981 Aug;68(2):494–498. doi: 10.1104/pp.68.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Flores H. E., Galston A. W. Polyamine-induced DNA Synthesis and Mitosis in Oat Leaf Protoplasts. Plant Physiol. 1980 Feb;65(2):368–371. doi: 10.1104/pp.65.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Montague M. J., Armstrong T. A., Jaworski E. G. Polyamine Metabolism in Embryogenic Cells of Daucus carota: II. Changes in Arginine Decarboxylase Activity. Plant Physiol. 1979 Feb;63(2):341–345. doi: 10.1104/pp.63.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna S., Adiga P. R. Arginine decarboxylase from Lathyrus sativus seedlings. Purification and properites. Eur J Biochem. 1975 Nov 15;59(2):377–386. doi: 10.1111/j.1432-1033.1975.tb02465.x. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Wiechmann M. Die Mikrobestimmung von Spermin und Spermidin als 1-Dimethylamino-naphthalin-5-sulfonsäure-Derivate. Hoppe Seylers Z Physiol Chem. 1967 Oct;348(10):1285–1290. [PubMed] [Google Scholar]
- Suzuki Y., Hirasawa E. S-adenosylmethionine decarboxylase of corn seedlings. Plant Physiol. 1980 Dec;66(6):1091–1094. doi: 10.1104/pp.66.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]


