Abstract
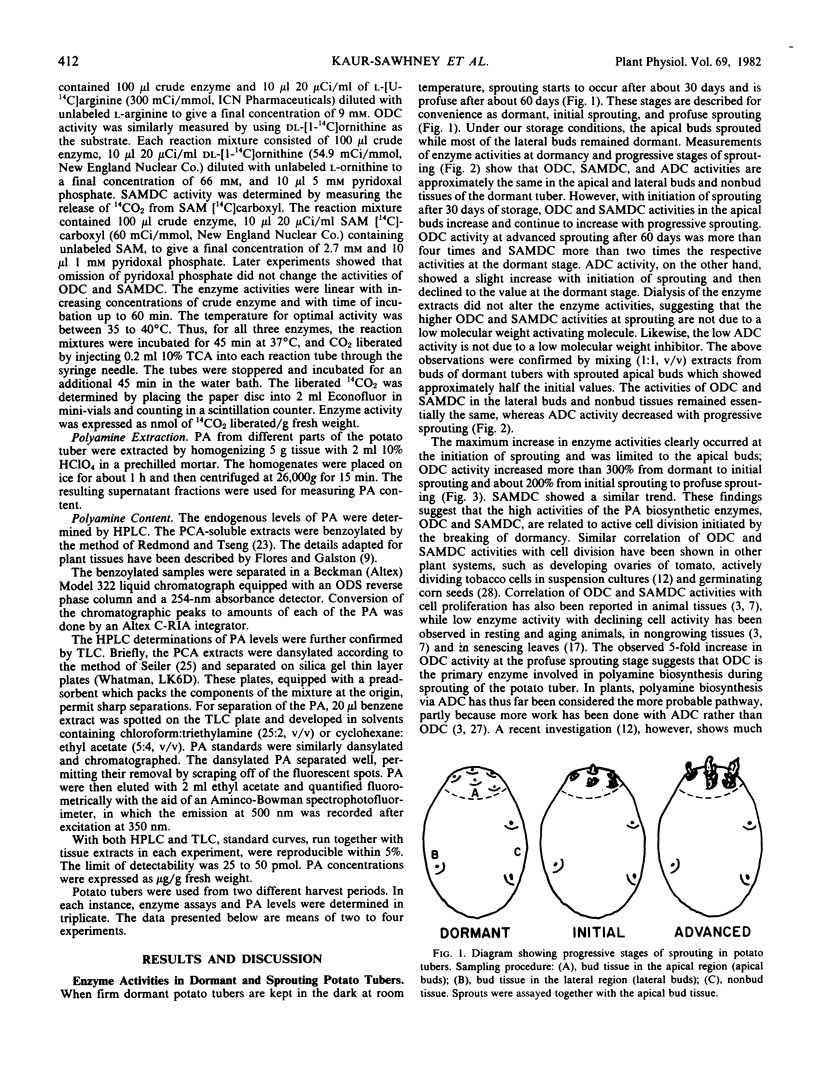
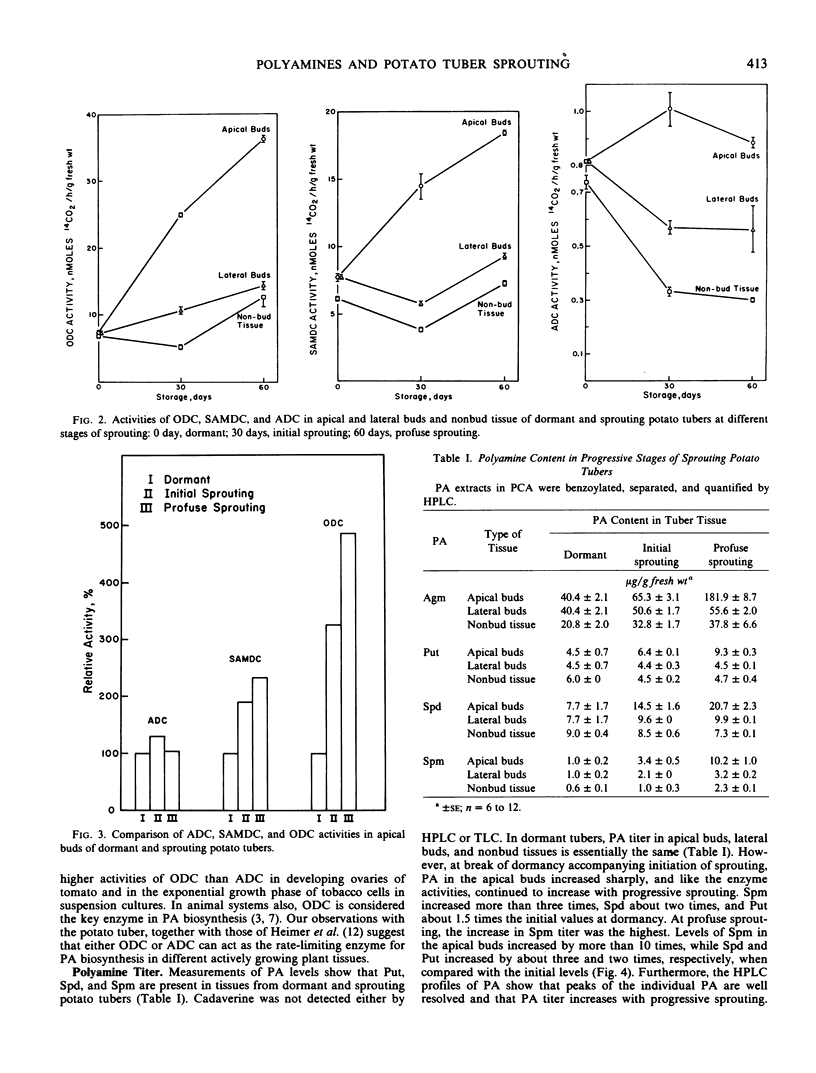
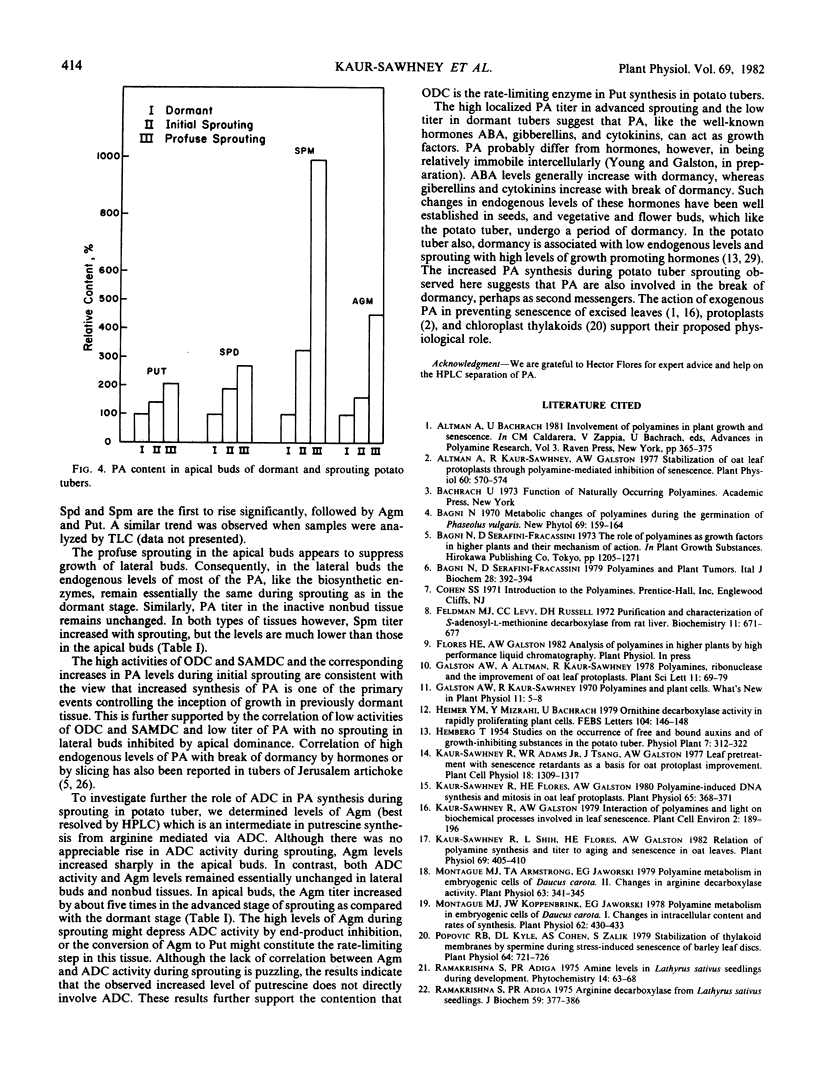
The polyamines putrescine, spermidine, and spermine and their biosynthetic enzymes arginine decarboxylase, ornithine decarboxylase and S-adenosyl-l-methionine decarboxylase are present in all parts of dormant potato (Solanum tuberosum L.) tubers. They are equally distributed among the buds of apical and lateral regions and in nonbud tissues. However, the breaking of dormancy and initiation of sprouting in the apical bud region are accompanied by a rapid increase in ornithine decarboxylase and S-adenosyl-l-methionine decarboxylase activities, as well as by higher levels of putrescine, spermidine, and spermine in the apical buds. In contrast, the polyamine biosynthetic enzyme activities and titer remain practically unchanged in the dormant lateral buds and in the nonbud tissues. The rapid rise in ornithine decarboxylase, but not arginine decarboxylase activity, with initiation of sprouting suggests that ornithine decarboxylase is the rate-limiting enzyme in polyamine biosynthesis. The low level of polyamine synthesis during dormancy and its dramatic increase in buds in the apical region at break of dormancy suggest that polyamine synthesis is linked to sprouting, perhaps causally.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M. J., Levy C. C., Russell D. H. Purification and characterization of S-adenosyl-L-methionine decarboxylase from rat liver. Biochemistry. 1972 Feb 29;11(5):671–677. doi: 10.1021/bi00755a002. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Mizrahi Y., Bachrach U. Ornithine decarboxylase activity in rapidly proliferating plant cells. FEBS Lett. 1979 Aug 1;104(1):146–148. doi: 10.1016/0014-5793(79)81102-3. [DOI] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Flores H. E., Galston A. W. Polyamine-induced DNA Synthesis and Mitosis in Oat Leaf Protoplasts. Plant Physiol. 1980 Feb;65(2):368–371. doi: 10.1104/pp.65.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague M. J., Armstrong T. A., Jaworski E. G. Polyamine Metabolism in Embryogenic Cells of Daucus carota: II. Changes in Arginine Decarboxylase Activity. Plant Physiol. 1979 Feb;63(2):341–345. doi: 10.1104/pp.63.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague M. J., Koppenbrink J. W., Jaworski E. G. Polyamine Metabolism in Embryogenic Cells of Daucus carota: I. Changes in Intracellular Content and Rates of Synthesis. Plant Physiol. 1978 Sep;62(3):430–433. doi: 10.1104/pp.62.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic R. B., Kyle D. J., Cohen A. S., Zalik S. Stabilization of Thylakoid Membranes by Spermine during Stress-induced Senescence of Barley Leaf Discs. Plant Physiol. 1979 Nov;64(5):721–726. doi: 10.1104/pp.64.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna S., Adiga P. R. Arginine decarboxylase from Lathyrus sativus seedlings. Purification and properites. Eur J Biochem. 1975 Nov 15;59(2):377–386. doi: 10.1111/j.1432-1033.1975.tb02465.x. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Wiechmann M. Die Mikrobestimmung von Spermin und Spermidin als 1-Dimethylamino-naphthalin-5-sulfonsäure-Derivate. Hoppe Seylers Z Physiol Chem. 1967 Oct;348(10):1285–1290. [PubMed] [Google Scholar]
- Suzuki Y., Hirasawa E. S-adenosylmethionine decarboxylase of corn seedlings. Plant Physiol. 1980 Dec;66(6):1091–1094. doi: 10.1104/pp.66.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]


