Abstract
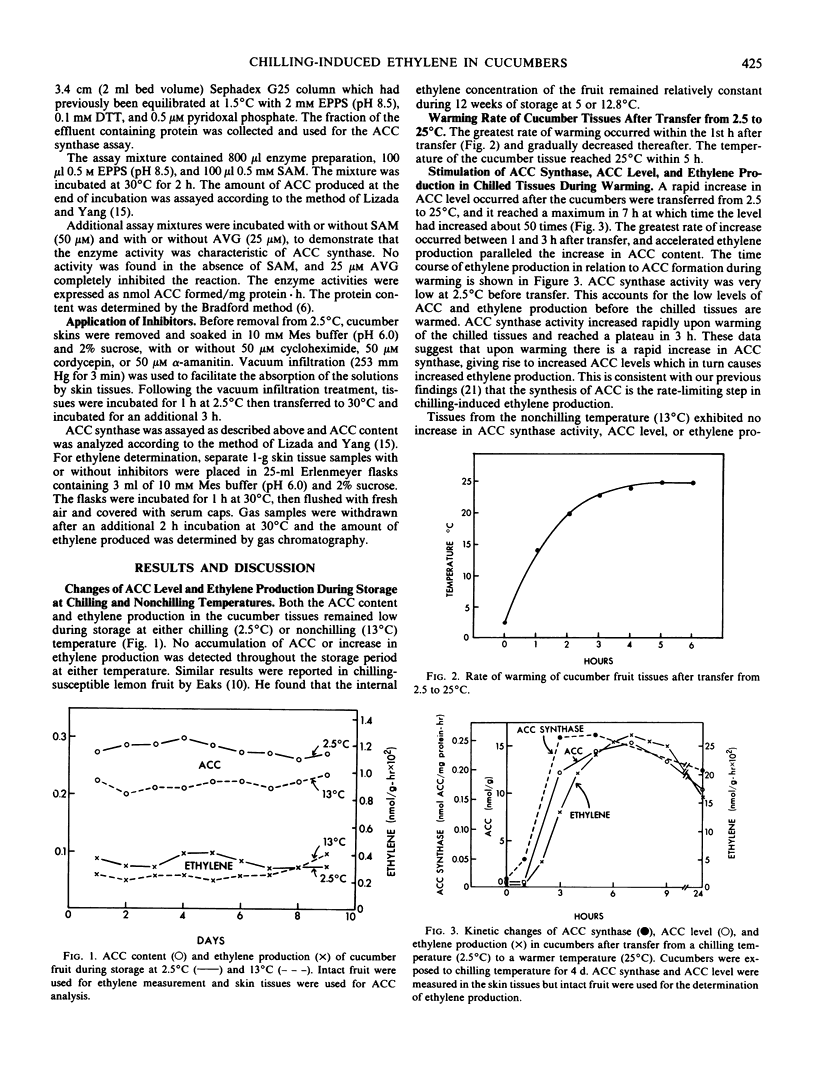
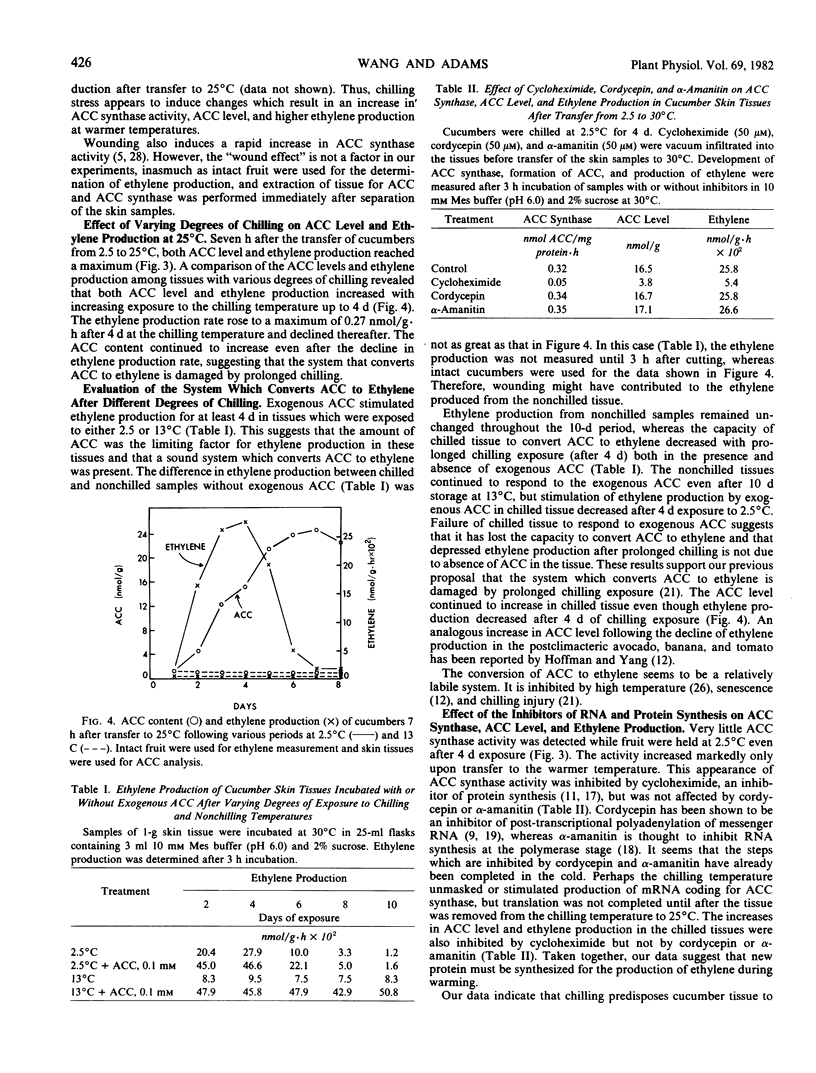
1-Aminocyclopropane-1-carboxylic acid (ACC) level, ACC synthase activity, and ethylene production in cucumbers (Cucumis sativus L.) remain low while the fruit are held at a temperature which causes chilling injury (2.5°C) and increase rapidly only upon transfer to warmer temperatures. The increase in ACC synthase activity during the warming period is inhibited by cycloheximide but not cordycepin or α-amanitin. Our data indicate that the synthesis of ACC synthase, which results in increased ACC levels and accelerated ethylene production, occurs only upon warming, possibly from a message produced or unmasked during the chilling period. Ethylene production by chilled (2.5°C) cucumbers increased very little upon transfer to 25°C if the fruit were chilled for more than 4 days. The fruit held for 4 days or longer showed a large increase in ACC levels but little ethylene production even in the presence of exogenous ACC. This suggests that the system which converts ACC to ethylene is damaged by prolonged exposure to the chilling temperature. Cucumbers stored at a low but nonchilling temperature (13°C) showed very little change in ACC level, ethylene production, or ACC synthase activity even after transfer to 25°C.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cooper W. C., Rasmussen G. K., Waldon E. S. Ethylene evolution stimulated by chilling in citrus and persea sp. Plant Physiol. 1969 Aug;44(8):1194–1196. doi: 10.1104/pp.44.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., Macdonald I. R. Specificity of cycloheximide in higher plant systems. Plant Physiol. 1970 Aug;46(2):227–232. doi: 10.1104/pp.46.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Strain G. C., Mullinix K. P., Bogorad L. RNA polymerases of maize: nuclear RNA polymerases. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2647–2651. doi: 10.1073/pnas.68.11.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Adams D. O. Ethylene Production by Chilled Cucumbers (Cucumis sativus L.). Plant Physiol. 1980 Nov;66(5):841–843. doi: 10.1104/pp.66.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. Inhibition of ethylene production by 2,4-dinitrophenol and high temperature. Plant Physiol. 1980 Aug;66(2):286–290. doi: 10.1104/pp.66.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Auxin-induced Ethylene Production and Its Inhibition by Aminoethyoxyvinylglycine and Cobalt Ion. Plant Physiol. 1979 Dec;64(6):1074–1077. doi: 10.1104/pp.64.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Biosynthesis of wound ethylene. Plant Physiol. 1980 Aug;66(2):281–285. doi: 10.1104/pp.66.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]


