Abstract
Objective
The role of endothelial GRK2 was investigated in mice with selective deletion of the kinase in the endothelium (Tie2-CRE/GRK2fl/fl).
Approach and Results
Aortas from Tie2-CRE/GRK2fl/fl presented functional and structural alterations as compared to control GRK2fl/fl mice. In particular, vasoconstriction was blunted to different agonists, and collagen and elastic rearrangement and macrophage infiltration were observed. In primary cultured endothelial cells deficient for GRK2, mitochondrial reactive oxygen species (ROS) was increased, leading to expression of cytokines. Chronic treatment with a ROS scavenger in mice corrected the vascular phenotype by recovering vasoconstriction, structural abnormalities and reducing macrophage infiltration.
Conclusions
These results demonstrate that GRK2 removal compromises vascular phenotype and integrity by increasing endothelial ROS production.
Keywords: Vascular Biology, G protein coupled receptor kinases, endothelial cell, mitochondria
Introduction
Endothelium is a cellular monolayer separating blood from parenchymal tissue. Despite this anatomic simplicity, its physiological complexity allows the control of several vascular functions including permeability, tone and angiogenesis, by integrating the multiple stimuli from the bloodstream and elaborating a response through release of specific factors. Adrenergic mechanisms controlling endothelial function have been studied during these years culminating in the observation that endothelium produces catecholamines1 through which regulate specific functions, such as vascular tone and angiogenesis2, eNOS activity3 and intracellular specific pathways by activation of β adrenergic receptor (βAR) signaling4, 5. βAR signaling is in turn heavily regulated, as verified in other tissues, and, in particular, βARs undergo G protein coupled receptor kinase (GRK) mediated desensitization6-8. Interestingly, the G protein coupled receptor kinase 2 (GRK2), has also been demonstrated to regulate eNOS phosphorylation in AKT dependent manner9. The role of GRK2 in physiopathology has been mainly studied at cardiac level, where, both biochemical and transgenic studies, have demonstrated that during congestive heart failure10-12 its increased levels contribute to the progression of the disease. Recent studies have also shown that GRK2 increased levels contribute to the endothelial dysfunction and defective vasorelaxation observed in transgenic model of type II diabetes13. Incoming evidence, however, indicate that GRK2 is able to regulate different molecules and cellular functions, such as inflammation, cellular proliferation, glucose uptake and metabolism, by kinase dependent and independent mechanisms14-16, raising questions about the multiple roles played in the cell. In particular, a recent observation shows that GRK2 localizes at mitochondria level, where it plays a protective role during hypoxic/ischemic conditions by promoting mitochondria biogenesis and increasing ATP production17. Given the importance of mitochondria increased oxygen reactive species (ROS) and ATP production in endothelial dysfunction18, 19, the mitochondrial effects of GRK2 for endothelium appear to be particularly relevant. Hereby, to ascertain the function of endothelial GRK2, we used transgenic mice with selective deletion of GRK2 gene in the endothelium. Our results propose a protective role of GRK2 in the endothelium with effects on inflammation and macrophages and lipids infiltration in the vascular wall.
Results
GRK2 endothelial removal affects aorta's receptor dependent and independent vasoconstriction
First of all, we confirmed by RT-PCR that Tie2CRE-GRK2fl/fl aortas present selective endothelial GRK2 removal. As shown in Supplemental Fig IA, GRK2 expression is reduced in Tie2CRE-GRK2fl/fl aorta compared to GRK2fl/fl which is indicative of GRK2 deletion in the endothelium and presence in the other layers (media and adventitia). This result was confirmed by the finding of no difference in GRK2 expression, after removing endothelium from both GRK2fl/fl and Tie2CRE-GRK2fl/fl.
In our previous studies, to evaluate vasorelaxation to the different agonists, aortas were pre-constricted with increasing concentration of the α1 adrenergic agonist Phenylephrine. However, this was the case only for GRK2fl/fl, while Tie2CRE-GRK2fl/fl showed an impaired response to this drug (Fig 1A). To further evaluate this result, we employed other different compounds known to increase vascular tone as Ser, Oxy and KCl, this last allows the evaluation of the receptor independent vasoconstriction. As shown Fig 1B-D, all these agonists present a defective vasoconstriction as compared to GRK2fl/fl mice. To find an explanation for these results, in isolated GRK2fl/fl ECs, we evaluated the possibility that GRK2 may co-immunoprecipitate with Akt or eNOS as suggested by previous studies9,13 that indicate the ability of GRK2 to affect the Akt/eNOS axle. Therefore GRK2 removal may potentially enhance vasodilation through increased eNOS activation and inhibit vasoconstriction. As shown in supplemental fig. IB, none among Akt and/or eNOS appear to directly or indirectly interact with GRK2. Moreover, GRK2 removal by ADCRE infection (Suppl. Fig IC) does not modify levels of eNOS phosphorylation (Suppl. Fig ID). Finally, we also evaluated the effects of endothelial removal on vasoconstriction. Effective endothelial removal was confirmed by observing paradox vasoconstriction to Ach (data not shown). With this maneuver, aortas displayed a partial recovery of vasoconstriction which, however, was not completely restored as compared to GRK2fl/fl.
Figure 1. Effect of selective endothelial GRK2 deletion on aortic vasoconstriction.

Vasocontriction was tested in response α1 adrenergic agonist Phenylepherine (Phe, 10-8 to 10-6 M), 5HT1 agonist Serotonine (Ser, 10-8 to 10-6 M), Oxytocin (Oxy, 10-7 to 10-5 M) and to Potassium Chloride (KCl, 12.5 mM). Defective vasoconstriction can be observed to the all above agonists in Tie2-CRE/GRK2fl/fl (*, !, #, *! vs GRK2fl/fl, p<0.05, 2-way Anova). Endothelium removal from Tie2CRE-GRK2fl/fl aortas (Tie2CRE-GRK2fl/fl-end) produces partial and not significant recover after endothelium removal of vasoconstriction to the above drugs (vs Tie2CRE-GRK2fl/fl, ns)
All together, these observations indicate that the defective vasoconstriction observed in Tie2CRE/GRK2fl/fl mice is not related to a functional alterations involving eNOS activation but rather to structural abnormalities, leading to the need of histological studies on aortas sections.
Increased vascular inflammation and tissue degeneration in Tie2CRE-GRK2fl/fl mice
The tunica media of the aorta consists of regular concentric elastic lamellae, between which are smooth muscle cells, collagen and elastic fibers. Histological studies by Masson trichrome showed that aorta from GRK2fl/fl mice, used as control, has a normal tunica media with intact elastic lamellae characterized by their peculiar “wavy” look (Fig. 2A, blue arrows). Instead, Tie2CRE-GRK2fl/fl aorta is collapsed and the internal elastic lamina and the elastic lamellae within the tunica media are mostly stretched (Fig 2A, black arrows) (Fig 2A). Also, matrix between elastic lamellae is increased (black stars), indicating an altered deposition of collagen (Fig 2B). To deeply investigate the modifications in Tie2CRE-GRK2fl/fl aorta, transmission electron microscopy (TEM) analysis was performed. Control GRK2fl/fl aorta has a preserved media structure which is composed of smooth muscle cells (SMC) with normal appearance and parallel orientation respect to the thick and compact elastic lamina and lamellae (EL), which confers the ability to equally transmit contractile force to the entire vessel. Moreover, complex bundles of collagen fibers (CF) tightly adhere to the lamellae (Fig 2C, blue arrows). In Tie2CRE-GRK2fl/fl aortas, instead, we observed presence of matrix vesicles and degeneration and altered deposition of collagen fibers (Fig 2C, blue arrows), which appear fragmented and mostly detached from the elastic lamellae. These last also are thinner and less compact than in controls (Fig 2C, yellow arrows). Noteworthy, SMCs display a pyknotic nucleus with marked chromatin condensation and peripheral margination, indicating presence of an apoptotic process. This morphology prompted us to evaluate presence of inflammation and in particular of macrophage infiltration in to the vessels. Immunoistochemistry revealed an extensive infiltration of macrophages in Tie2CRE-GRK2fl/flaortas respect to controls (Fig 2D, black arrows) which may be responsible for the observed alterations of the media. Indeed, we found increased mRNA and protein expression of MMP2 and MMP9, which are produced by macrophages and known to be involved in collagen matrix degradation (Fig 3A-B). Furthermore, also the macrophage chemo attractant factor (MCP-1) resulted to be increased in Tie2CRE-GRK2fl/fl respect to control (Fig 3A-B). Since the alterations observed in vascular endothelium of the Tie2CRE-GRK2fl/fl may be extended to other organ and tissues, we performed histological analysis by Masson and Immunoistochemistry on lungs from Tie2CRE-GRK2fl/fland control mice. We found collagen degradation (Supplemental fig IIIA, black arrows) and macrophage infiltration (Supplemental fig IIIB, blue arrows) in Tie2CRE-GRK2fl/fl mice but not in GRK2fl/fl.
Figure 2. GRK2 endothelial deletion induces an inflammatory phenotype in the aorta of transgenic mice.

A) Masson Trichrome staining of thoracic aorta from GRK2fl/fl and Tie2CRE-GRK2fl/fl. GRK2fl/fl aorta shows conserved morphology with wavy elastic lamina and lamellae (blue arrows). This last appears collapsed with straight elastic lamina and lamellae (black arrows) and increased interlaminae matrix deposition (black star) which is quantified in B as % of total aortic wall (* vs GRK2fl/fl, p<0.05). C) Transmission Electron Microscopy (TEM) on GRK2fl/fl and Tie2CRE-GRK2fl/fl aortas. In GRK2fl/fl aorta, Elastic lamina (EL) is thick and compact with tightly adhering bundles of collagen fibers (CF). Matrix between laminae is mostly occupied by smooth muscle cells (SMCs). Tie2CRE-GRK2fl/fl has a thinner EL (yellow arrows) with detached CF that appear mostly fragmented (blue arrows). Pycnotic SMC nucleus and presence of matrix vesicles can be also noted. D) Immunoistochemistry analysis performed on thoracic aorta. Detection of the F4/80 macrophage fragment reveals an extensive macrophage infiltration in Tie2CRE-GRK2fl/fl (Black arrows) aorta as compared to GRK2fl/fl aorta.
Figure 3. GRK2 endothelial deletion increases mRNA expression and level of inflammatory proteins.
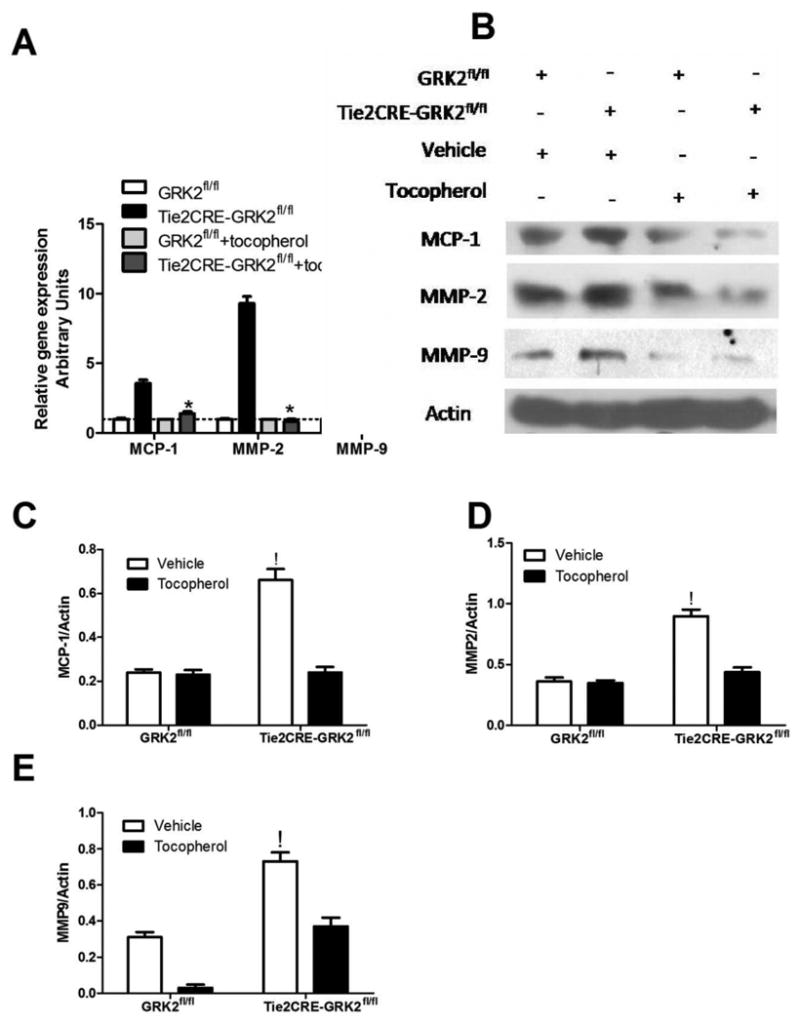
A) mRNA levels of the cytokine MCP1 and metalloproteinase 2 and 9 evaluated by RT-PCR on thoracic aorta samples. Increased expression of MCP1, MMP2 and 9 can be observed in Tie2CRE-GRK2fl/fl as compared to GRK2fl/fl samples (* p<0.01 vs GRK2fl/fl) which are significantly reduced by tocopherol chronic treatment. Data are expressed as relative gene expression. B-E) Protein levels of MCP1, MMP2 and 9 were evaluated by western blot on total aortas lysates. Increased protein levels of MCP1, MMP2 and 9 are observed in Tie2CRE-GRK2fl/fl as compared to GRK2fl/fl samples (! p<0.01 vs GRK2fl/fl) which are significantly reduced by tocopherol as shown by representative images in B and densitometry in C-E.
Endothelial cell and macrophages have the same myeloid origin, which means that, during embryogenesis, the Tie2 promoter could be activated in both cell lines. To ascertain that GRK2 gene is not removed also in leukocytes, we isolated macrophages of Tie2CRE-GRK2fl/fland GRK2fl/fl mice from peritoneum and performed western blot analysis to measure GRK2 protein expression. We did not observe any significant difference in GRK2 expression in macrophages from the two groups of animals (data not shown).
GRK2 gene deletion from endothelial cells induces increased ROS production
The above data indicate that selective knock out of GRK2 in the endothelium produces a dramatic modification of the phenotype of these transgenic mice, characterized by vascular inflammation and defective vascular reactivity. To identify the specific alteration of the endothelial cells, we performed in vitro studies, using intact mitochondria isolated from GRK2fl/fl EC and BAEC, respectively treated with AD-CRE and si-RNA for bovine GRK2 to induce GRK2 knock down (Supplemental figure IC and IE). In particular, we evaluated mitochondrial ROS production basing on two evidence: the recent discover of GRK2 localization in mitochondria, where it exerts a positive effect on ATP production and biogenesis17, and the known role of endothelial ROS in physiopathology of vascular diseases20. GRK2 removal form EC by ADCRE infection produces a typical senescent morphology of the cell (Supplemental fig. IIC) which may be related to an altered ROS production. Indeed, we observed that both in GRK2fl/fl EC and BAEC, GRK2 reduced level by AD-CRE and siRNA significantly increases ROS production (Fig 4A-B). Increased ROS production was also observed in the aorta from Tie2CRE-GRK2fl/fl mice (Supplemental fig. IID) stained with DCHF. To pin down the role of ROS in the above described modifications, we treated endothelial GRK2fl/fl cells and BAEC with the anti-oxidant α-tocopherol (Vitamin-E), which significantly attenuated ROS production when GRK2 gene was deleted (Fig 4A-B).
Figure 4. Endothelial GRK2 reduced level increases ROS production and cytokines expression.

A) ROS production was evaluated in isolated mitochondria from GRK2fl/fl ECs infected with ADCRE and ADLacZ, with or without tocopherol treatment. ROS production has been evaluated by incubation of mitochondria with DCFH as described in method. ADCRE increases ROS production as compared to ADLacZ (* vs ADLacZ, p<0.05) but is significantly attenuated by anti-oxidant treatment (! vs ADCRE+vehicle, p<0.05). Data are expressed as total fluorescence intensity. B) Similarly to A, ROS production were evaluated in BAEC transfected with siRNA for GRK2 and Scramble, in presence or not of tocopherol treatment. GRK2 knock down, enhances mitochondrial ROS production (# vs Scramble, p<0.05) but is reduced by tocopherol treatment (*! Vs siRNA-GRK2+vehicle, p<0.05). C) mRNA levels of MCP-1, IL-1 and 10 were evaluated on GRK2fl/fl endothelial cells, with or without tocopherol treatment. GRK2fl/fl ECs were infected with ADCRE, to induce GRK2 gene deletion, and ADLacZ used as control. ADCRE+vehicle increases MCP-1, IL-1 and 10 gene expression as compared to ADLacZ (** vs ADLacZ+vehicle, p<0.05). Tocopherol treatment significantly reduces cytokines expression (*** vs ADCRE+vehicle, p<0.05). D) Cytokines production were evaluated in culture medium from GRK2fl/fl EC infected with ADLacZ or ADCRE. ADCRE infection significantly increases MCP1, IL1 and 10 protein levels (## vs ADLacZ+vehicle) but they are decreased by tocopherol treatment (### vs ADCRE+vehicle). Data are expressed as pixel density. E) As above, mRNA levels of MCP-1, IL-1 and 10 were evaluated on BAEC, transfected with bovine siRNA for GRK2 or scramble, in presence of tocopherol or vehicle treatment. siRNAGRK2 produces increased cytochines gene expression (!! vs Scramble+vehicle, p<0.05) which is then significantly reduced by tocopherol treatment (!!! vs siRNA-GRK2+vehicle, p<0.05).
Moreover, GRK2 knock-out in GRK2fl/fl cells increased the expression of IL1, IL10 and MCP1, as evaluated by RT-PCR and ELISA assay, indicating that endothelial cells are directly involved in leukocytes migration (Fig 4C-D) and the same result was obtained in BAEC treated with GRK2 siRNA (Fig 4E). However, α-tocopherol treatment significantly reduced cytokine expression in both cell lines with GRK2 removal (Fig 4C-E), indicating potential therapeutic effects of α-tocopherol on vascular inflammation.
Chronic treatment with α-tocopherol ameliorates vascular inflammation and restores aortic vasoconstriction
Since the anti-oxidant α-tocopherol has shown the ability to cut down mitochondrial ROS production in vitro, we tested if the same treatment could ameliorate the inflammation affecting aorta and lung of Tie2CRE-GRK2fl/fl mice. First of all, we evaluated the effects of α-tocopherol chronic treatment on in vivo inflammation by measuring nitrate production, which is expression of increased iNOS and leukocytes activity, in blood and urine samples from GRK2fl/fl and Tie2CRE-GRK2fl/fl mice. As expected, Tie2CRE-GRK2fl/fl mice showed increased nitrate production in blood and urine samples, which were reduced by α-tocopherol treatment (Supplemental Fig IIA-B). Thus, we evaluated the possible ability of the anti-oxidant to attenuate or recover vascular damage induced by increased endothelial ROS production. α-tocopherol treatment significantly blunted the increased expression of MCP-1 and MMP2-9 in the aorta Tie2CRE-GRK2fl/fl mice which was no longer different as compared to that of GRK2fl/fl aorta (Fig 3A-E).
Immunoistochemistry on aortas revealed that the robust macrophages infiltration in Tie2CRE-GRK2fl/fl (Fig 5A-B, black arrows) was reduced by α-tocopherol treatment and morphologic analysis with Masson showed that elastic lamellae were newly folded, recovering the typical “wavy” look (Fig 5C, black arrows), while matrix between lamaelle was reduced (Fig 5C, black star) indicating regression of collagen deposition, as also quantified in figure 5D. Similarly, in lungs we observed absence of macrophage infiltration after α-tocopherol treatment (Supplemental Fig IIIC-D). Inflammatory modifications observed in the aortas of Tie2CRE-GRK2fl/fl are typical of several vascular disease including atherosclerosis. Since vascular inflammation and atherosclerosis are both related to the presence of endothelial dysfunction and loss of vascular integrity, we evaluated lipid deposition in thoracic aorta wall by Red Oil staining. Tie2CRE-GRK2fl/fl shows a significant increase in lipid deposition respect to GRK2fl/fl aorta (Fig 5E). Also, Aortas cross sections (Supplemental fig IIE) shows that lipid accumulation is not organized in an atherosclerotic plaque that indicates presence of an early atherosclerotic lesion. Indeed proteoglycan accumulation, which is a typical alteration of advanced stage of atherosclerosis, was not observed in both aortas samples from GRK2fl/fl and Tie2CRE-GRK2fl/fl mice (data not shown). Of note, aorta red oil staining showed a significant reduction in lipid deposition after tocopherol versus not treated animals samples as evaluated in both gross morphology (Fig 5E) and cross sections of the aorta (Supplemental Fig IIE). GRK2fl/fl and Tie2CRE-GRK2fl/fl do not show significant difference in serum total cholesterol level (Supplemental fig IIF), indicating that the above observations are not due to differences in lipid profile between the two group of animals.
Figure 5. Effect of α-tocopherol chronic treatment on vascular inflammation.

A-B) Immunoistochemistry analysis performed on thoracic aorta. Detection of the F4/80 macrophage fragment reveals an extensive macrophage infiltration in Tie2CRE-GRK2fl/fl (Black arrows) aorta as compared to GRK2fl/fl aorta which is reduced by tocopherol chronic treatment as shown in the representative image in A and in B by quantitative analysis with ImageJ. Data are expressed as % of brown spots respect to total aortic area C-D) Masson Trichrome staining of thoracic aorta from GRK2fl/fl and Tie2CRE-GRK2fl/fl. GRK2fl/fl has a preserved morphology which is modified in particular at tunica media level as above described. Here, chronic tocopherol treatment mostly recovers typical aortic appearance, with folded elastic lamina and lamellae (black arrows) and reduced matrix deposition (black stars), as also quantified in D (* vs Tie2CRE-GRK2fl/fl+vehicle, p<0.05). E) Red Oil O staining of thoracic aortas. Tie2CRE-GRK2fl/fl shows increased lipid deposition respect to GRK2fl/fl as quantified in the bar graph as percent of total aortic area (# vs GRK2fl/fl, p < 0.05). However, treatment with α-tocopherol dramatically reduced lipid deposition in the aorta (*! vs Tie2CRE-GRK2fl/fl+vehicle, p<0.01).
Finally, we evaluated if the beneficial effects brought by α-tocopherol at structural and biochemical level can be also extended to the vasomotor responses. As evidenced in Fig. 6, α-tocopherol administration significantly restored receptor dependent and independent vasoconstriction to the different compounds used in this study (Fig 6A-D) and, notably, at same levels of GRK2fl/fl mice. Nonetheless restored vasoconstriction is also accompanied by receptor dependent (Ach and Iso) and independent (NP) vasodilation as shown in Fig 7A-C
Figure 6. Effect of α-tocopherol treatment on vasomotor responses.
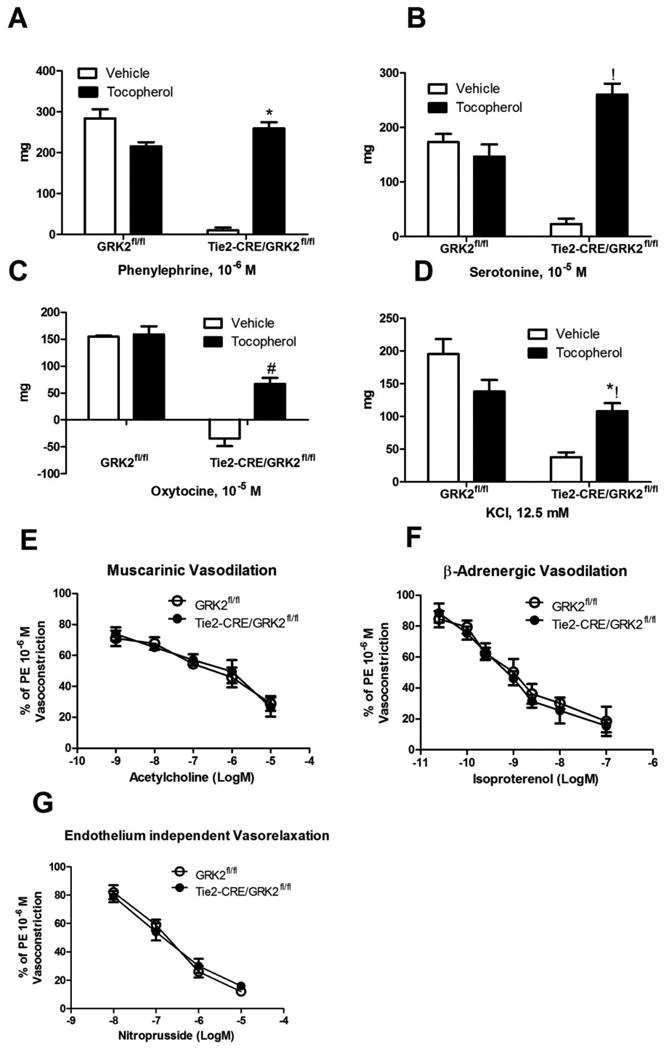
A-D) Vasocontriction was evaluated as above to the maximal concentrations tested: Phenylepherine (Phe, 10-6 M), Serotonine (Ser, 10-6 M), to Oxytocin (Oxy, 10-5 M) and to Potassium Chloride (KCl, 12.5 mM). Significant recover of vasoconstriction can be observed in Tie2-CRE/GRK2fl/fl treated with the antioxidant respect to vehicle (*, !, #, *! Vs Tie2-CRE/GRK2fl/fl + vehicle, p<0.05, 2-way Anova). E-G) Vasodilation was evaluated in GRK2fl/fl and Tie2-CRE/GRK2fl/fl from mice treated with α-tocopherol in response to Acetylcholine (Ach, 10-9–10-5 M) and Isoproterenol (Iso, 3*10-10-10-7 M) for endothelial dependent vasorelaxation, and Sodium Nitroprusside (NP, 10-9-10-5 M) for endothelial independent vasorelaxation, after maximal vasoconstriction induced by Phe 10-6 M. No significant differences were found among the two groups (2-way Anova).
All together, these indicate that inflammatory modifications induced by GRK2 endothelial removal can be significantly attenuated by a ROS scavanger.
Discussion
Vascular integrity and function are preserved and regulated by endothelium. This accepted knowledge underlies complex molecular mechanisms which allow the endothelium to regulate functions such as cellular migrations and metabolites passages. Our data for the first time show that the presence of GRK2 in the endothelial cell is necessary for the proper function of the endothelium. GRK2 removal from the endothelium associates to an inflammatory state of the vessel wall, as evidenced by elastic and collagen fibers degeneration, defective vasoconstriction, and increased lipid deposition into the aortic wall of Tie2CRE-GRK2fl/fl mice. This feature is generalized since inflammation can be found also in other tissues rich in endothelium such as lung. The underlying mechanism appears to be the increased endothelial ROS production since chronic treatment of Tie2CRE-GRK2fl/fl with tocopherol, a ROS scavenger, reduces aorta and lung inflammation and restores aortic receptor dependent and independent vasoconstriction. These observations unveil the crucial role played by GRK2 in the vascular homeostasis, indicating that its role is fundamental not only for the embryonic development as previously described21, but also for the adult life. Our previous report has described the ability of GRK2 to localize at the mitochondria level, in particular, when the cells are exposed to hypoxia. This phenomenon has favorable effect on cell metabolism, by preserving ATP production and mitochondrial biogenesis. In pathological models, as hyperglycemia or hypertension, mitochondrial ROS generation is increased and targeting ROS by antioxidants such as α-tocopherol20 improve endothelium function22 and reduce expression of adhesion molecules, such as VCAM1, on endothelial surface22, 23. In this study we have observed that GRK2 removal from cell increases mitochondrial ROS production that can be cut down using a lipophilic anti-oxidant such as Vit-E. This mechanism appears quite specific for the transgenic model used in this study since we did not observe influence of GRK2 removal on eNOS activity, which is a known regulator of vascular responses. Indeed, previous studies have identified in GRK2 as an important regulator of eNOS phosphorylation and activity9. Specifically, GRK2 appears able to bind and sequester Akt rendering it unavailable for eNOS activation. Then, lacking of GRK2 would remove a potential brake on Akt activity and leading to eNOS over-activation and vasodilation, which may explain the defective vasoconstriction in Tie2GRK2fl/fl aortas. However this was not the case in our system, where GRK2 does not show ability to interact either with Akt and/or eNOS and also GRK2 removal from EC does not produce any significant modification in eNOS phosphorylation and activation.
In conclusion, our data add a new knowledge along the way to explore GRK2 role in mitochondria, concluding that it participates to the mitochondrial REDOX reactions and contributes to the physiological functioning of the cell. Even if this finding is not supported by a complete explanation of the mechanisms through which GRK2 interfere with the mitochondrial functions, however, basing on our previous finding that GRK2 localizes in mitochondria and promotes mitogenesis, we can hypothesize that GRK2 deals with mitochondrial integrity and regeneration. In particular GRK2 may favor mitochondrial localization of important protein belonging to the Mitochondrial Complexes and its absence will then reduce ATP production, as previous demonstrated, and increase ROS production as here evidenced. Nonetheless, we here demonstrate that GRK2 is not only fundamental for cardiovascular development during embryogenesis but it is also important for cellular integrity and function during adult life by regulating a mitochondrial function as ROS production, which in turn are important for development of several vascular disease.
Indeed, GRK2 has been associated to cardiovascular diseases, such as congestive heart failure and hypertension10, 11 but, through the years, this kinase has been related also to other complex diseases like Type II Diabetes, Alzheimer, Rheumatoid Arthritis and Atherosclerosis 24-26. Of note, these conditions share the common feature of being initiated and/or perpetrated by an inflammatory process, which, as known, involves release of cytokines and migration of leukocytes and lymphocytes from the bloodstream to the parenchymal tissue. Here we observe a similar phenotype, since removing GRK2 from endothelium induces cytokines production and macrophages migration in to the vascular media, where they release metalloproteinase such as MMP2 and 9 corrupting the elastic and collagen fibers of the vessel. Moreover, the physical interlinkages between components of the tunica media, made by collagen fibers which extend through fenestration of the parallel elastic lamellae are important in maintaining structural integrity and physicomechanical properties of the aortic wall27 and their disruptions, such as those observed in Tie2CRE-GRK2fl/fl mice, are implicated in disease processes such as atherosclerosis28, 29, aneurysm formation30 and in ageing31. In our study we were able to rescue these vascular alterations and recover defective vasoconstriction in Tie2CRE-GRK2fl/fl by chronic administration of Vit-E which reduces endothelial ROS production from mitochondria. Besides the specific cellular lineage used in this study, this and our previous studies raise the importance for GRK2 in mitochondrial regulation, indicating that the optimal therapeutic strategy for treatment of chronic inflammatory diseases would be to restore and/or increase GRK2 localization at mitochondria level, to attenuate the increased ROS production observed during chronic inflammation. F
Apparently, our study contradicts the knowledge that accumulating GRK2 is deleterious for the cell and it may appear provocative as it shows that GRK2 is protective in the vasculature. On the contrary, our data are in agreement with previous observations that GRK2 gene deletion is detrimental for embryonic cardiac development21 and, in adult life, cardiac selective GRK2 removal alters the cardiac hypertrophic response to chronic β adrenergic receptor stimulation, leading to an eccentric dilatation of the heart similar to that observed in intermediate-advanced phases of heart failure32. To reconcile these apparently contradictive evidence, we need to take in account two findings: first, our is a gene deletion model, which cannot be considered the reciprocal of GRK2 accumulation observed in pathological conditions; second, GRK2 is a complex molecule which has demonstrated to interact and regulate several substrates through its kinase activity, RGS and PH domains, giving it the ability to have multiple regulatory roles in to the cells33, 34. Moreover, the only effective therapeutic strategy aimed to counteract GRK2 mediated receptor desensitization at plasma membrane level, for example on beta adrenergic and insulin signaling, has employed βARKct, whose mechanisms in animal models of cardiovascular diseases are not completely unveiled. This molecule represents the carboxyl terminal portion of GRK2, which retains the ability to localize at plasmamembrane binding Gβγ subunit through the PH domain, but lack the kinase activity35, 36. βARKct displaces from plasmamembrane, but does not inhibits GRK2 activity, which is then theoretically free to move in other compartment like mitochondria where can accomplishes its protective role.
Therefore, an integrative and fascinating hypothesis would provide that GRK2 plays different roles into the cell according to its subcellular localization or compartmentalization, furnishing also a complete explanation for the positive effects of βARKct for cell functioning during disease.
Of course, this hypothesis needs to be confirmed with further studies, by evaluating the roles of GRK2 in the different cellular compartment, but it is already becoming evident that we need to move to a different way to target GRK2 in a therapeutic strategy. Indeed if increased levels of GRK2 are negative for cell function, on the other side a complete reduction or inhibition of the kinase level or activity are dangerous as well. Thus, a new perspective therapy includes a tight modulation of GRK2 activity and/or level in to the cell, taking also in to account the specific role played by GRK2 in the different cellular compartment.
Methods
Generation of endothelial cell-specific GRK2 knock-out mice
Conditional mice bearing floxed GRK2 alleles (GRK2fl/fl) were bred with Tie2CRE transgenic mice to generate Tie2CRE-GRK2fl/fl mice 1,2. For this study, 8 weeks old Tie2CRE-GRK2fl/fl and GRK2fl/fl, used as control group, fed with a normal diet, were sacrificed to perform vascular reactivity and histological studies. A group of them was also chronically treated with the anti-oxidant α-tocopherol for 14 consecutive days. Mice in the vitamin E group were injected I.P. with vegetable oil containing 20% ethyl alcohol, 1% benzyl alcohol and α-tocopherol (670 mg/kg, Sigma), while mice in the placebo group were injected I.P. with vegetable oil containing 20% ethyl alcohol, 1% benzyl alcohol and 0 mg of α-tocopherol (vehicle)3.
Vascular reactivity determined on thoracic aortas
After isolation, mice thoracic aortas were suspended in isolated tissue baths filled with 25 mL Krebs solution (in mmol/L: NaCl 118.3, KCl4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, and glucose5.6) continuously bubbled with a mixture of 5% CO2 and 95% O2 (pH 7.37 to 7.42) at 37°C. One end of the vessel was secured to a tissue holder and the other to an isometric force transducer connected to a data acquisition system (Power Lab, ADI Instruments). Aortas (1 cm length) were pre-tensioned to 0.5 g. Vasoconstrictions to phenylephrine (Phe), Serotonin (Ser), Oxytocin (Oxy) and potassium chloride (KCl) and were assessed by generating concentration response curves (10-9 to 10-6mol/ and 16 mM for KCl). Vasorelaxation was assessed in vessel preconstricted with phe (10-6mol/L) in response to the muscarinic agonist acetylcholine (Ach 10-9 to 10-5mol/L). Drug concentrations are reported as the final molar concentration in the organ bath. Drugs were prepared daily in distilled water.
Immunoistochemistry
Aortas and lungs from Tie2CRE-GRK2fl/fl and GRK2fl/fl were fixed in formaline, embedded in paraffin and sectioned at 5 μm with a rotary microtome. Sections were dewaxed, rehydrated and incubated with anti-F4/80 primary antibody (SantaCruz) at a 1:50 dilution at room temperature for two hours. Specific secondary antibody (Santacruz) at a 1:100 dilution was incubated at room temperature for 1 hour. The peroxidase was revealed in presence of 0,03% hydrogen peroxide and of an electron donor, 2,5% diaminobenzidine, which becomes visible as a brown precipitate. Sections were then counterstained with hematoxylin, then dehydradated, cleared with histolemon and coverslipped. For Masson's trichrome staining of collagen fibers, slides were processed as previously described4. Briefly, sections were stained with Weigert hematoxylin (Sigma-Aldrich) for 10 minutes, Biebrich scarlet-acid fuchsin (Sigma-Aldrich) for 5 minutes, phosphomolybdic/phosphotungstic acid solution (Sigma-Aldrich) for 5 minutes, and then stained with light green (Sigma-Aldrich) for 5 minutes. Images were taken by using an Eclipse E1000 Fluorescence Microscope (Nikon) and acquired by using Sigma Scan Pro software (Jandel). Images were optimized for contrast in Adobe PhotoShop, but no further manipulations were made. Quantification of collagen fibers was made using appropriate software (Image J) by dr. Sorriento.
Red Oil Staining
Aortas from GRK2 fl/fl and GRK2 fl/Cre mice were fixed in 4% paraformaldehyde and then frozen in Optimum Cutting Temperature (OCT) compound. Consecutive frozen aortic sections (10 μm thickness) were prepared and stained with Oil Red O (SigmaAldrich) for 10 min and counterstained with hematoxylin at room temperature. Images were taken at Eclipse E1000 Fluorescence Microscope (Nikon) and acquired using Sigma Scan Pro software (Jandel).
Transmission Electron Microscopy (TEM)
TEM was performed as previously described5. Briefly, aortas were fixed with 2.5% glutaraldehyde, washed in PBS, fixed in osmium tetroxide, dehydrated in an ethanol series, embedded in epoxy resin, and then examined under a transmission electron microscope (JEM-2000EX), at the Federico II facility for advanced imaging (CISME). Three different sections from n=3 aortas samples for each group were analyzed.
Real Time RT-PCR
Total RNA was isolated using Trizol reagent (Invitrogen) and cDNA was synthetized by means of Thermo-Script real-time quantitative polymerase chain reaction (RT-PCR) (Invitrogen), following the manufacturer instruction. After reverse transcription reaction, RT-PCR was performed with the SYBR Green Real Time PCR master mix kit (Applied Biosystems). The reaction was visualized by SYBR Green Analysis (Applied Biosystem) on StepOne instrument (Applied Biosystem).
Primers for gene expression analysis were as follows:
| 18S FOR | 5′-GTAACCCGTTGAACCCCATT-3′ |
| 18S REV | 5′- CCATCCAATCGGTAGTAGCG -3′ |
| IL-1 FOR | 5′- GCCTTGGGCCTCAAAGGAAAGAATC -3′ |
| IL-1 REV | 5′- GGAAGACACAGATTCCATGGTGAAG -3′ |
| IL-10 FOR | 5′- GTGAAGACTTTCTTTCAAACAAAG -3′ |
| IL-10 REV | 5′- CTGCTCCACTGCCTTGCTCTTATT -3′ |
| MCP-1 FOR | 5′- GGAAAAATGGATCCACACCTTGC -3′ |
| MCP-1 REV | 5′- TCTCTTCCTCCACCACCATGCAG -3′ |
| MMP2 FOR | 5′- GAAACCGTGGATGATGCTTT -3′ |
| MMP2 REV | 5′- CCATCAGCGTTCCCATACTT -3′ |
| MMP-9 FOR | 5′- CGTCGTGATCCCCACTTACT -3′ |
| MMP-9 REV | 5′- AACACACAGGGTTTGCCTTC -3′ |
| GRK2 FOR | 5′- CCCTCTCACCATCTCTGAGC -3′ |
| GRK2 REV | 5′- CGGTTGGGGAACAAGTAGAA -3′ |
All values obtained were normalized with the 18S primers (endogenous control) and relative to the reference sample (basal control) using the formula 2-ΔΔCt. Results are expressed as relative gene expression.
Isolation and culture of endothelial cells
Endothelial cells (EC) were isolated from aortas of GRK2fl/fl mice as previously described6. These last and Bovine aorta EC (BAEC, Lonza) were cultured in Dulbecco's MEM (DMEM) supplemented with 10% foetal bovine serum (FBS) at 37°C in 95% air and 5% CO2. In some experiments GRK2fl/fl cells were treated with adenovirus encoding for CRE recombinase (ADCRE) or control virus (ADLacZ). BAEC were instead transfected with siRNA for bovine GRK2 (5′-AAGAAAUACGAGAAGCUGGAG-3′) or Scramble.
Western Blot
Proteins expression were evaluated by western blot on entire whole aorta lysate as previously described7. Briefly, we pooled together at least three vessels and pulverized them in a liquid nitrogen chilled inox mortar and homogenated them in RIPA/SDS buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0, 25% deoxycholate, 9,4 mg/50 ml sodium orthovanadate, 20% SDS]. Protein concentration was determined by using BCA assay kit (Pierce). Total extracts were electrophoresed by SDS/PAGE and transferred to nitrocellulose. MCP-1, MMP2 and 9 were visualized using specific primary antibodies (Santa Cruz) and standard chemiluminescence (Pierce). Also, macrophages were isolated from the peritoneum Tie2CRE-GRK2fl/fl and GRK2fl/fl mice after three days from thioglycolate injection. They were lysed in RIPA/SDS and electrophoresed by SDS/PAGE as above. Endogenous GRK2 and Actin (Santacruz) were visualized by specific antibodies, anti-rabbit HRP conjugated secondary antibody (Santa Cruz) and standard chemiluminescence (Pierce). Densitometry analysis of digitalized autoradiographies was performed using a dedicated software (Image QuaNT Molecular Diagnostic).
Cytokines production in GRK2fl/fl ECs
To evaluate inflammatory cytokines production, we performed an array assay on medium collected from cultered GRK2fl/fl EC treated with ADLacZ or ADCRE. Medium samples were used to perform cytokines analysis using the “Cytokine array panel A” kit (R&D Systems) accordingly to manufacturer's instruction.
Measurement of mitochondrial ROS
Cells were treated with alpha-tocopherol (35 μM/ml) or vehicle for 12 hours and mitochondria were isolated from GRK2fl/fl EC and BAEC with a previously validated protocol5 (1). Reactive oxygen species were measured by DCFH method. Briefly, Mitochondria were resuspended in phosphate buffer (PBS) and incubated with DCFH-DA (10μM/ml - Invitrogen) at 37°C for 45 min (2). After washing with PBS, DCFH-fluorescence of the mitochondria was measured in a fluorescence microplate reader (TECAN infinite F200 pro) at an excitation wavelength of 485 + 20 nm and emission at 535 + 25 nm. The intensity of fluorescence reflects the extent of oxidative stress. To measure ROS production in vivo, frozen sections of aortas from GRK2 fl/fl and Tie2CRE-GRK2fl/fl mice were incubated with DCFH-DA at room temperature for 45 min. Fluorescence images were taken at Eclipse E1000 Fluorescence Microscope (Nikon) and acquired using Sigma Scan Pro software (Jandel).
Statistical Analysis
All experiments were performed at least in triplicate. The results were expressed as mean±SEM. We used Prism 4 and SPSS 17 to perform statistical analysis with a 2-way and repeated measures ANOVA with a Bonferroni posthoc test for all the studies.
Supplementary Material
Significance.
Maintenance of endothelium integrity is fundamental in preventing development of vascular disease such as atherosclerosis. Increased reactive oxygen species are often associated to the loss of endothelial homeostasis, however mechanisms that lead to the alteration of the cellular REDOX reactions are poorly understood. Here we demonstrated that GRK2 is important for the physiological functioning of the cell, since its deletion induces increased mitochondrial ROS and a dramatic alteration of the vascular phenotype and function. We here add new knowledge on the complex role played by GRK2 in to the cell, which can be different, detrimental or protective, according to its subcellular localization.
Acknowledgments
We thank Dr A. Ninni for his technical support in measuring cholesterol level in mice.
Sources of Funding: This work was in part supported by a grant from Italian Ministry of Research (PRIN 2009, to Prof Iaccarino), University of Salerno Funds for Basic Research (FARB) and Italian Society of Hypertension (SIIA).
Abbreviations
- ROS
Reactive Oxygen Species
- GRK2
G protein coupled receptor kinase 2
Footnotes
Disclosure: None
References
- 1.Sorriento D, Santulli G, Del Giudice C, Anastasio A, Trimarco B, Iaccarino G. Endothelial cells are able to synthesize and release catecholamines both in vitro and in vivo. Hypertension. 2012;60:129–136. doi: 10.1161/HYPERTENSIONAHA.111.189605. [DOI] [PubMed] [Google Scholar]
- 2.Iaccarino G, Ciccarelli M, Sorriento D, et al. Ischemic neoangiogenesis enhanced by beta2-adrenergic receptor overexpression: a novel role for the endothelial adrenergic system. Circ Res. 2005;97:1182–1189. doi: 10.1161/01.RES.0000191541.06788.bb. [DOI] [PubMed] [Google Scholar]
- 3.Iaccarino G, Cipolletta E, Fiorillo A, Annecchiarico M, Ciccarelli M, Cimini V, Koch WJ, Trimarco B. Beta(2)-adrenergic receptor gene delivery to the endothelium corrects impaired adrenergic vasorelaxation in hypertension. Circulation. 2002;106:349–355. doi: 10.1161/01.cir.0000022690.55143.56. [DOI] [PubMed] [Google Scholar]
- 4.Ciccarelli M, Cipolletta E, Santulli G, Campanile A, Pumiglia K, Cervero P, Pastore L, Astone D, Trimarco B, Iaccarino G. Endothelial beta2 adrenergic signaling to AKT: role of Gi and SRC. Cell Signal. 2007;19:1949–1955. doi: 10.1016/j.cellsig.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Ciccarelli M, Santulli G, Campanile A, Galasso G, Cervero P, Altobelli GG, Cimini V, Pastore L, Piscione F, Trimarco B, Iaccarino G. Endothelial alpha1-adrenoceptors regulate neo-angiogenesis. Br J Pharmacol. 2008;153:936–946. doi: 10.1038/sj.bjp.0707637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 7.Hausdorff WP, Hnatowich M, O'Dowd BF, Caron MG, Lefkowitz RJ. A mutation of the beta 2-adrenergic receptor impairs agonist activation of adenylyl cyclase without affecting high affinity agonist binding. Distinct molecular determinants of the receptor are involved in physical coupling to and functional activation of Gs. J Biol Chem. 1990;265:1388–1393. [PubMed] [Google Scholar]
- 8.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005;11:952–958. doi: 10.1038/nm1289. [DOI] [PubMed] [Google Scholar]
- 10.Izzo R, Cipolletta E, Ciccarelli M, Campanile A, Santulli G, Palumbo G, Vasta A, Formisano S, Trimarco B, Iaccarino G. Enhanced GRK2 expression and desensitization of betaAR vasodilatation in hypertensive patients. Clin Transl Sci. 2008;1:215–220. doi: 10.1111/j.1752-8062.2008.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J. 2005;26:1752–1758. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- 12.Napolitano R, Campanile A, Sarno L, Anastasio A, Maruotti GM, Morlando M, Trimarco B, Martinelli P, Iaccarino G. GRK2 levels in umbilical arteries of pregnancies complicated by gestational hypertension and preeclampsia. Am J Hypertens. 2011;25:366–371. doi: 10.1038/ajh.2011.211. [DOI] [PubMed] [Google Scholar]
- 13.Taguchi K, Kobayashi T, Matsumoto T, Kamata K. Dysfunction of endothelium-dependent relaxation to insulin via PKC-mediated GRK2/Akt activation in aortas of ob/ob mice. Am J Physiol Heart Circ Physiol. 2011;301:H571–583. doi: 10.1152/ajpheart.01189.2010. [DOI] [PubMed] [Google Scholar]
- 14.Cipolletta E, Campanile A, Santulli G, Sanzari E, Leosco D, Campiglia P, Trimarco B, Iaccarino G. The G protein coupled receptor kinase 2 plays an essential role in beta-adrenergic receptor-induced insulin resistance. Cardiovasc Res. 2009;84:407–415. doi: 10.1093/cvr/cvp252. [DOI] [PubMed] [Google Scholar]
- 15.Ciccarelli M, Chuprun JK, Rengo G, Gao E, Wei Z, Peroutka RJ, Gold JI, Gumpert A, Chen M, Otis NJ, Dorn GW, 2nd, Trimarco B, Iaccarino G, Koch WJ. G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation. 2011;123:1953–1962. doi: 10.1161/CIRCULATIONAHA.110.988642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Usui I, Imamura T, Babendure JL, Satoh H, Lu JC, Hupfeld CJ, Olefsky JM. G protein-coupled receptor kinase 2 mediates endothelin-1-induced insulin resistance via the inhibition of both Galphaq/11 and insulin receptor substrate-1 pathways in 3T3-L1 adipocytes. Mol Endocrinol. 2005;19:2760–2768. doi: 10.1210/me.2004-0429. [DOI] [PubMed] [Google Scholar]
- 17.Fusco A, Santulli G, Sorriento D, Cipolletta E, Garbi C, Dorn GW, 2nd, Trimarco B, Feliciello A, Iaccarino G. Mitochondrial localization unveils a novel role for GRK2 in organelle biogenesis. Cell Signal. 2012;24:468–475. doi: 10.1016/j.cellsig.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Luo Z, Wang X, Jose PA, Falck JR, Welch WJ, Aslam S, Teerlink T, Wilcox CS. Impaired endothelial function and microvascular asymmetrical dimethylarginine in angiotensin II-infused rats: effects of tempol. Hypertension. 2010;56:950–955. doi: 10.1161/HYPERTENSIONAHA.110.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lass A, Sohal RS. Effect of coenzyme Q(10) and alpha-tocopherol content of mitochondria on the production of superoxide anion radicals. Faseb J. 2000;14:87–94. doi: 10.1096/fasebj.14.1.87. [DOI] [PubMed] [Google Scholar]
- 21.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci U S A. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E isoforms differentially regulate intercellular adhesion molecule-1 activation of PKCalpha in human microvascular endothelial cells. PLoS One. 2012;7:e41054. doi: 10.1371/journal.pone.0041054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalan U, Fernandez-Castillejo S, Pons L, Heras M, Aragones G, Angles N, Morello JR, Sola R. Alpha-tocopherol and BAY 11-7082 reduce vascular cell adhesion molecule in human aortic endothelial cells. J Vasc Res. 2012;49:319–328. doi: 10.1159/000337466. [DOI] [PubMed] [Google Scholar]
- 24.Vroon A, Kavelaars A, Limmroth V, Lombardi MS, Goebel MU, Van Dam AM, Caron MG, Schedlowski M, Heijnen CJ. G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Immunol. 2005;174:4400–4406. doi: 10.4049/jimmunol.174.7.4400. [DOI] [PubMed] [Google Scholar]
- 25.Lombardi MS, Kavelaars A, Schedlowski M, Bijlsma JW, Okihara KL, Van de Pol M, Ochsmann S, Pawlak C, Schmidt RE, Heijnen CJ. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. Faseb J. 1999;13:715–725. doi: 10.1096/fasebj.13.6.715. [DOI] [PubMed] [Google Scholar]
- 26.Otten JJ, de Jager SC, Kavelaars A, Seijkens T, Bot I, Wijnands E, Beckers L, Westra MM, Bot M, Busch M, Bermudez B, van Berkel TJ, Heijnen CJ, Biessen EA. Hematopoietic G-protein-coupled receptor kinase 2 deficiency decreases atherosclerotic lesion formation in LDL receptor-knockout mice. Faseb J. 2013;27:265–276. doi: 10.1096/fj.12-205351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogeng'o JA, Malek AA, Kiama SG. Structural organisation of tunica intima in the aorta of the goat. Folia Morphol (Warsz) 2010;69:164–169. [PubMed] [Google Scholar]
- 28.Cimmino G, Ragni M, Cirillo P, Petrillo G, Loffredo F, Chiariello M, Gresele P, Falcinelli E, Golino P. C-reactive protein induces expression of matrix metalloproteinase-9: A possible link between inflammation and plaque rupture. Int J Cardiol. 2012;12:415–420. doi: 10.1016/j.ijcard.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Lavezzi AM, Ottaviani G, Matturri L. Biology of the smooth muscle cells in human atherosclerosis. Apmis. 2005;113:112–121. doi: 10.1111/j.1600-0463.2005.apm1130204.x. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi T, Morishita E, Ohtake H, Oda Y, Asakura H, Nakao S. Expression of annexin II in experimental abdominal aortic aneurysms. Int J Hematol. 2009;90:336–342. doi: 10.1007/s12185-009-0410-6. [DOI] [PubMed] [Google Scholar]
- 31.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- 32.Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW., 2nd Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 33.Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Ciccarelli M, Cipolletta E, Iaccarino G. GRK2 at the control shaft of cellular metabolism. Curr Pharm Des. 2012;18:121–127. doi: 10.2174/138161212799040493. [DOI] [PubMed] [Google Scholar]
- 35.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 36.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 2.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci U S A. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huey KA, Fiscus G, Richwine AF, Johnson RW, Meador BM. In vivo vitamin E administration attenuates interleukin-6 and interleukin-1beta responses to an acute inflammatory insult in mouse skeletal and cardiac muscle. Exp Physiol. 2008;93:1263–1272. doi: 10.1113/expphysiol.2008.043190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorriento D, Santulli G, Fusco A, Anastasio A, Trimarco B, Iaccarino G. Intracardiac injection of AdGRK5-NT reduces left ventricular hypertrophy by inhibiting NF-kappaB-dependent hypertrophic gene expression. Hypertension. 2010;56:696–704. doi: 10.1161/HYPERTENSIONAHA.110.155960. [DOI] [PubMed] [Google Scholar]
- 5.Fusco A, Santulli G, Sorriento D, Cipolletta E, Garbi C, Dorn GW, 2nd, Trimarco B, Feliciello A, Iaccarino G. Mitochondrial localization unveils a novel role for GRK2 in organelle biogenesis. Cell Signal. 2012;24:468–475. doi: 10.1016/j.cellsig.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iaccarino G, Cipolletta E, Fiorillo A, Annecchiarico M, Ciccarelli M, Cimini V, Koch WJ, Trimarco B. Beta(2)-adrenergic receptor gene delivery to the endothelium corrects impaired adrenergic vasorelaxation in hypertension. Circulation. 2002;106:349–355. doi: 10.1161/01.cir.0000022690.55143.56. [DOI] [PubMed] [Google Scholar]
- 7.Ciccarelli M, Cipolletta E, Santulli G, Campanile A, Pumiglia K, Cervero P, Pastore L, Astone D, Trimarco B, Iaccarino G. Endothelial beta2 adrenergic signaling to AKT: role of Gi and SRC. Cell Signal. 2007;19:1949–1955. doi: 10.1016/j.cellsig.2007.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


