Abstract
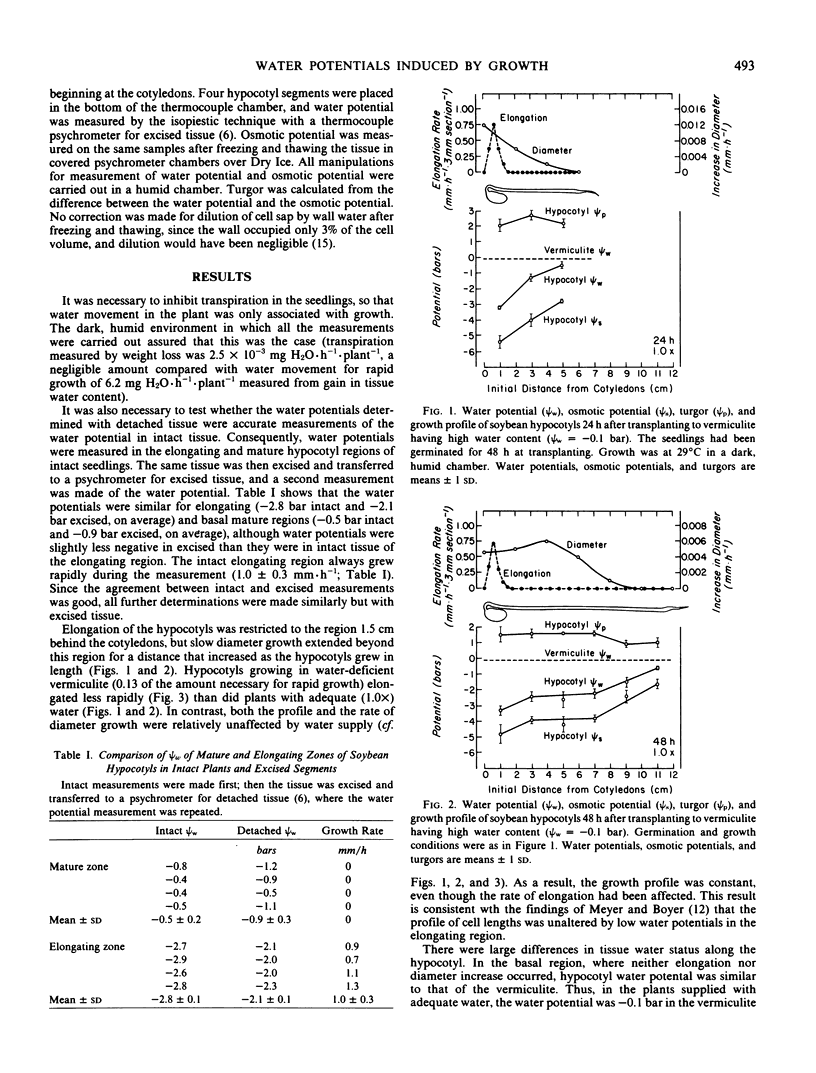
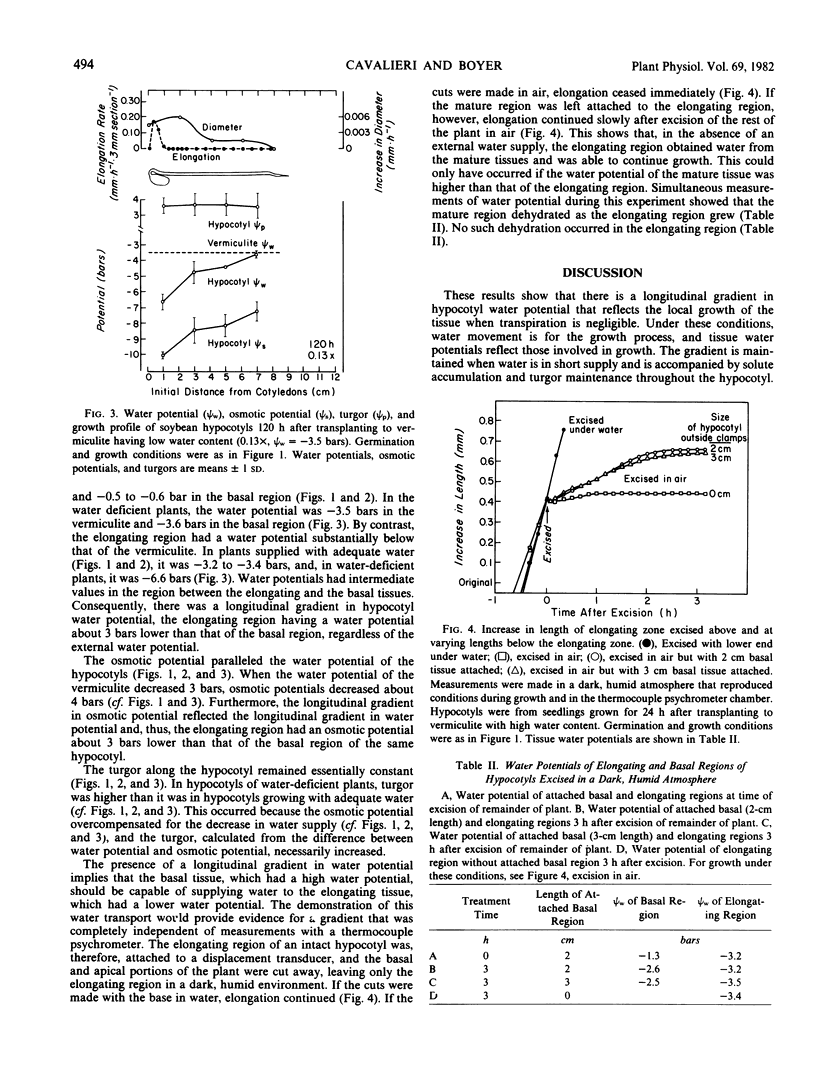
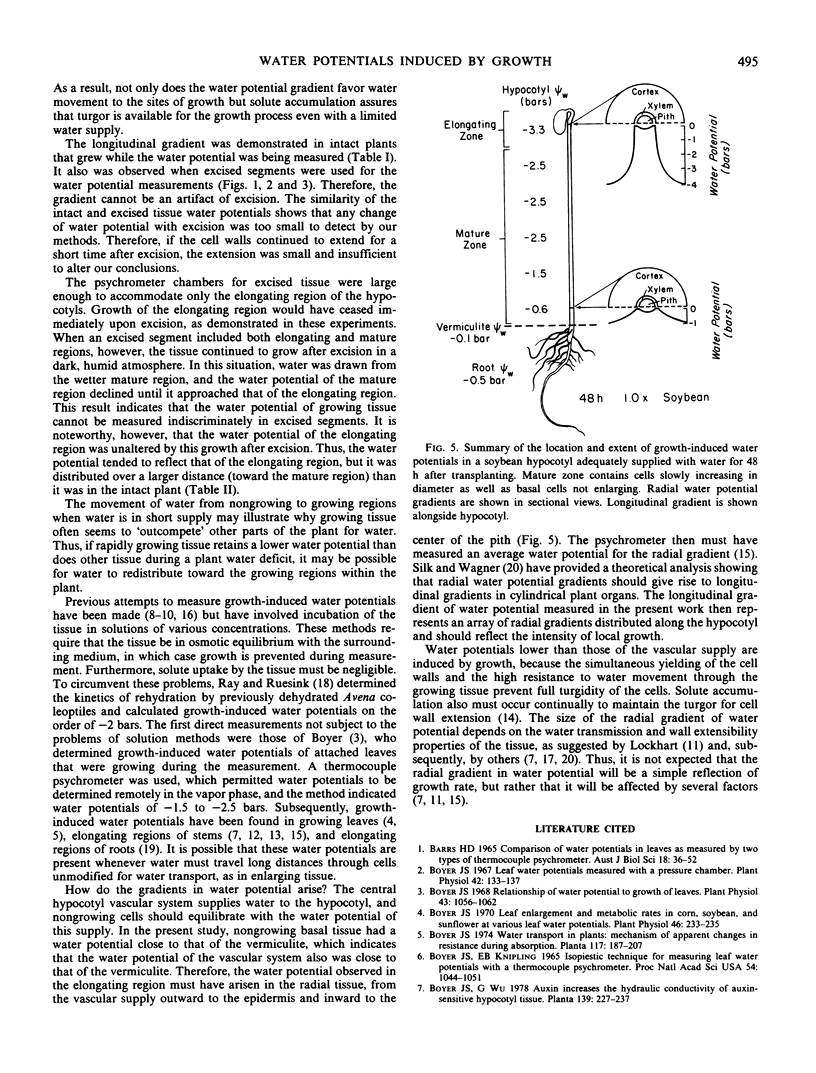
Gradients in water potential form the driving force for the movement of water for cell enlargement. In stems, they are oriented radially around the vascular system but should also be present along the stem. To test this possibility, growth, water potential, osmotic potential, and turgor were determined at intervals along the length of dark-grown soybean (Glycine max L. Merr., cv. Wayne) hypocotyls. Transpiration was negligible in the dark, humid conditions, so that all water uptake was for growth. Elongation occurred in the terminal 1.5 centimeters of the hypocotyl. Water potential was −3.5 bars in the elongating region but −0.5 bar in the mature region, both in intact plants and detached tissue. There was a gradual transition between these values that was related to the growth profile along the hypocotyl. Tissue osmotic potentials generally paralleled tissue water potentials, so that turgor was the same throughout the length of the hypocotyl. If the elongating zone was excised, growth ceased immediately. If the elongating zone was excised along with mature tissue, however, growth continued, which confirmed the presence of a water-potential gradient that caused longitudinal water movement from the mature zone to the elongating zone. When the plants were grown in vermiculite having low water potentials, tissue water potentials and osmotic potentials both decreased, so that water potential gradients and turgor remained undiminished. It is concluded that growth-induced water potentials reflect the local activity for cell enlargement and are supported by appropriate osmotic potentials.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. S. Leaf enlargement and metabolic rates in corn, soybean, and sunflower at various leaf water potentials. Plant Physiol. 1970 Aug;46(2):233–235. doi: 10.1104/pp.46.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Leaf water potentials measured with a pressure chamber. Plant Physiol. 1967 Jan;42(1):133–137. doi: 10.1104/pp.42.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Relationship of water potential to growth of leaves. Plant Physiol. 1968 Jul;43(7):1056–1062. doi: 10.1104/pp.43.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J. A. An analysis of irreversible plant cell elongation. J Theor Biol. 1965 Mar;8(2):264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- Molz F. J. Growth-induced Water Potentials in Plant Cells and Tissues. Plant Physiol. 1978 Sep;62(3):423–429. doi: 10.1104/pp.62.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordin L., Applewhite T. H., Bonner J. Auxin-Induced Water Uptake by Avena Coleoptile Sections. Plant Physiol. 1956 Jan;31(1):44–53. doi: 10.1104/pp.31.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk W. K., Wagner K. K. Growth-sustaining Water Potential Distributions in the Primary Corn Root: A NONCOMPARTMENTED CONTINUUM MODEL. Plant Physiol. 1980 Nov;66(5):859–863. doi: 10.1104/pp.66.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]


