Abstract
Cucumber (Cucumis sativus L.) cotyledons were sensitive to the diphenyl ether herbicide acifluorfen-methyl (AFM); methyl 5-[2-chloro-4-(trifluoro-methyl)phenoxyl-2-nitrobenzoate. Injury was detected by monitoring the efflux of 86Rb+ from treated tissues after exposure to light (600 micro einsteins per meter2 per second; photosynthetically active radiation).
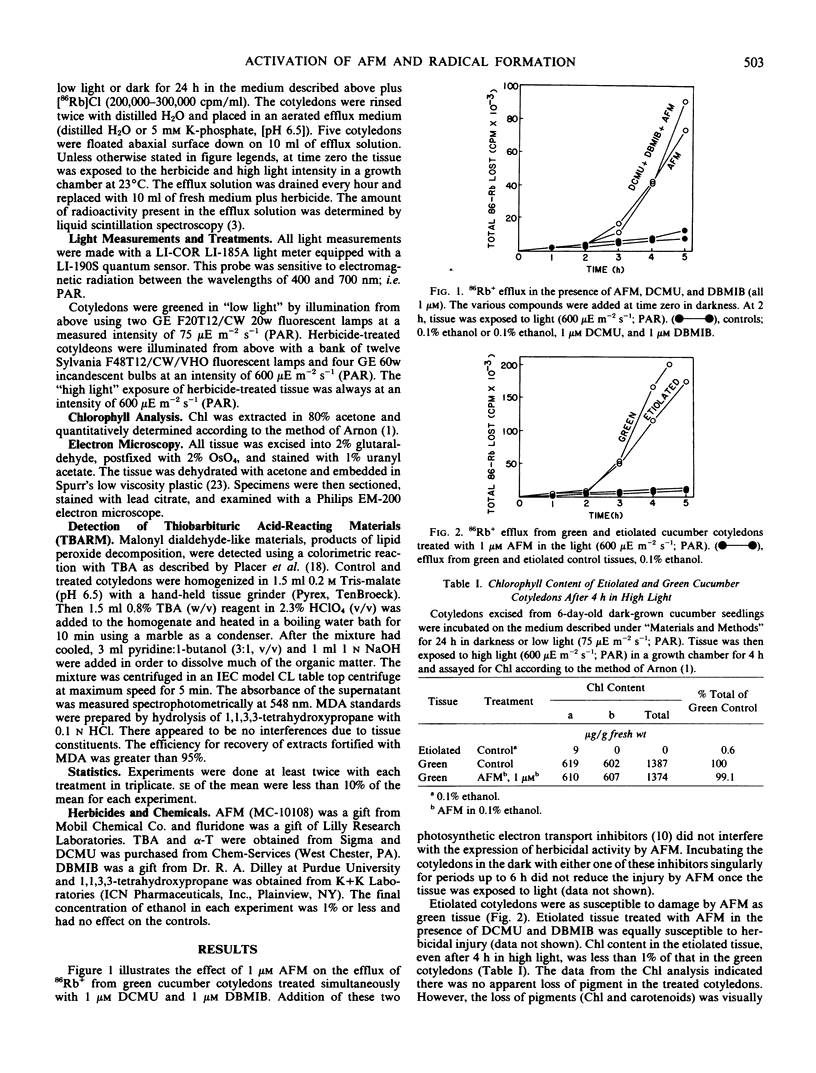
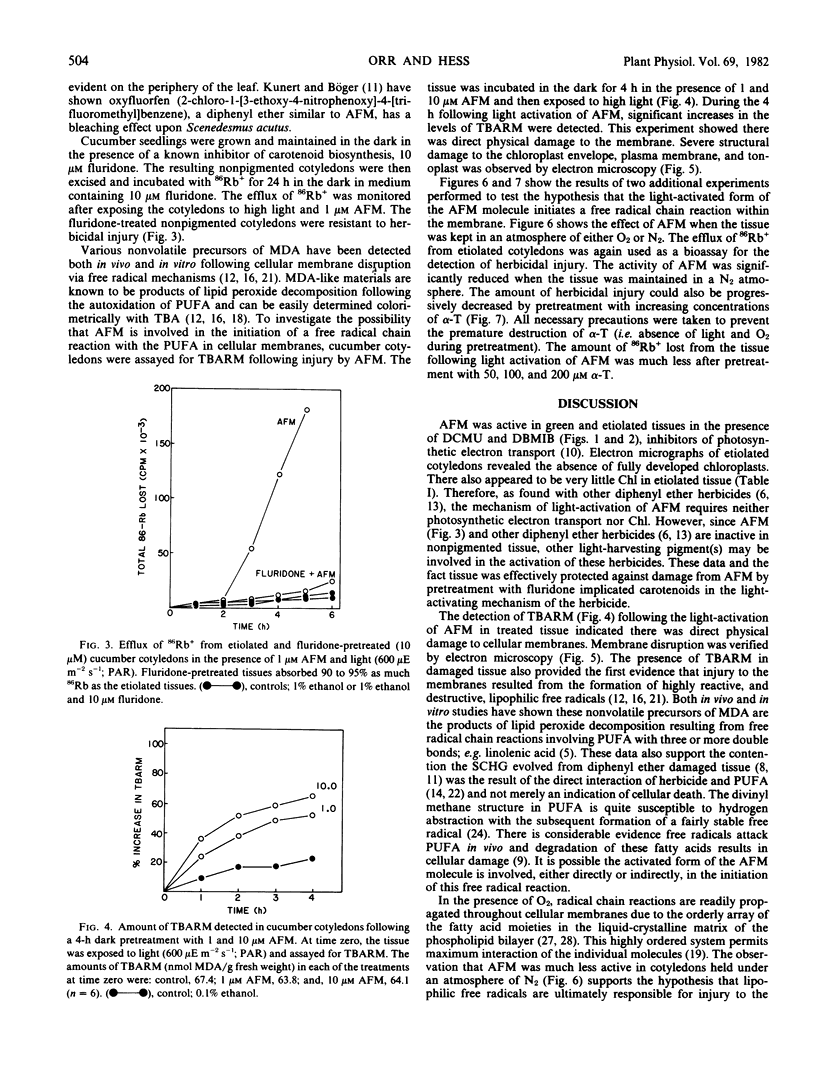
AFM exhibited activity in green and etiolated tissues in the presence of both 1 micromolar 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and 1 micromolar 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB), inhibitors of photosynthetic electron transport. Protection against injury could be obtained by pretreating the seedlings with a carotenoid biosynthesis inhibitor, 10 micromolar fluridone {1-methyl-3-phenyl-5-[3-(trifluoromethyl)phenyl]-4 (H)-pyridinone}.
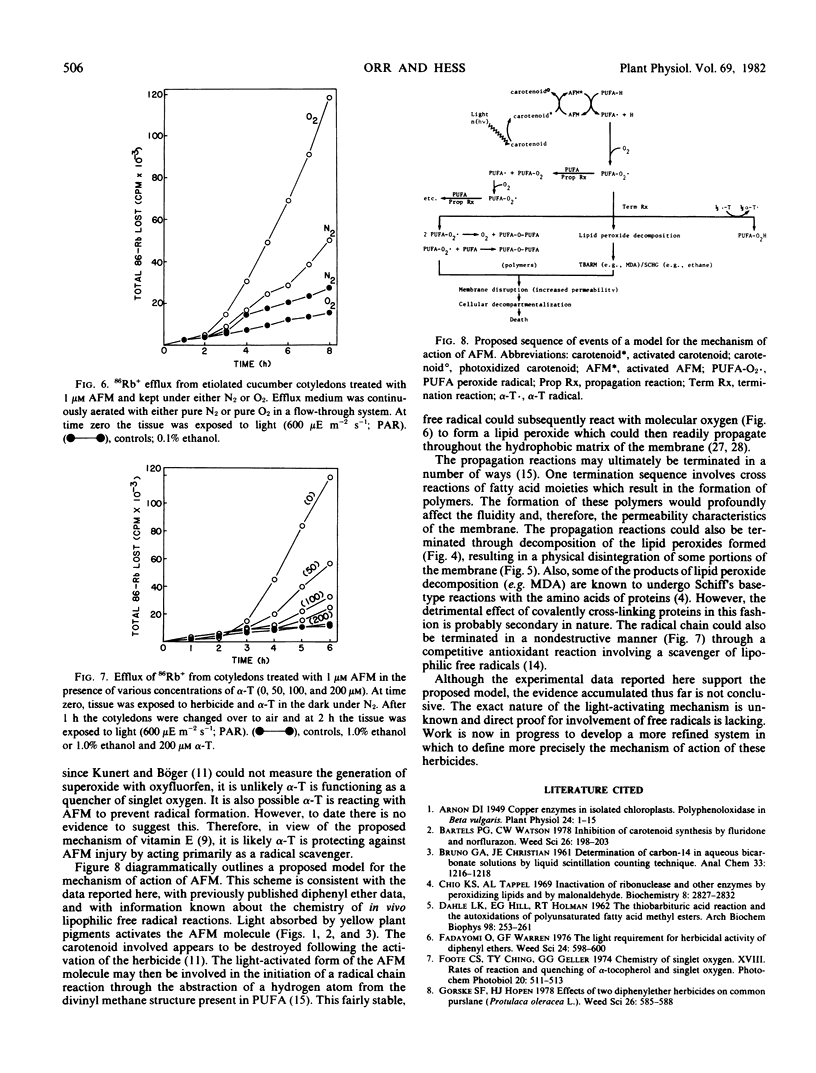
After a 4-hour dark pretreatment with 1 and 10 micromolar AFM, cotyledons were exposed to light (600 micro einsteins per meter2 per second; photosynthetically active radiation). Within 1 to 2 hours after light treatment, significant increases in the level of thiobarbituric acid-reacting materials could be detected. Electron microscopic observations of treated tissues revealed significant structural damage to the chloroplast envelope, tonoplast, and plasma membrane. Etiolated cucumber cotyledons treated with 1 micromolar AFM and exposed to light were less susceptible to injury when maintained in an O2-deficient atmosphere. Protection against injury could be obtained with 50 micromolar α-tocopherol.
These results suggest AFM is activated in light by yellow plant pigments and then is involved in the initiation of a free radical chain reaction with polyunsaturated fatty acid moieties of phospholipid molecules making up cellular membranes. The perturbations that follow result in a loss of the membrane's selective permeability characteristics, thereby leading to cellular death.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Inactivation of ribonuclease and other enzymes by peroxidizing lipids and by malonaldehyde. Biochemistry. 1969 Jul;8(7):2827–2832. doi: 10.1021/bi00835a020. [DOI] [PubMed] [Google Scholar]
- Foote C. S., Ching T. Y., Geller G. G. Chemistry of singlet oxygen. XVIII. Rates of reaction and quenching of alpha-tocopherol and singlet oxygen. Photochem Photobiol. 1974 Dec;20(6):511–513. doi: 10.1111/j.1751-1097.1974.tb06611.x. [DOI] [PubMed] [Google Scholar]
- McCay P. B., Pfeifer P. M., Stipe W. H. Vitamin E protection of membrane lipids during electron transport functions. Ann N Y Acad Sci. 1972 Dec 18;203:62–73. doi: 10.1111/j.1749-6632.1972.tb27858.x. [DOI] [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968 Oct 17;6(1):126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Placer Z. A., Cushman L. L., Johnson B. C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966 Aug;16(2):359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Stanley J. P. Letter: A suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975 Nov 28;40(24):3615–3617. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- WILLS E. D. MECHANISMS OF LIPID PEROXIDE FORMATION IN TISSUES. ROLE OF METALS AND HAEMATIN PROTEINS IN THE CATALYSIS OF THE OXIDATION UNSATURATED FATTY ACIDS. Biochim Biophys Acta. 1965 Apr 5;98:238–251. doi: 10.1016/0005-2760(65)90118-9. [DOI] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. General considerations. Biochem J. 1969 Jun;113(2):315–324. doi: 10.1042/bj1130315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yih R. Y., Swithenbank C. New potent diphenyl ether herbicides. J Agric Food Chem. 1975 May-Jun;23(3):592–593. doi: 10.1021/jf60199a062. [DOI] [PubMed] [Google Scholar]



