Abstract
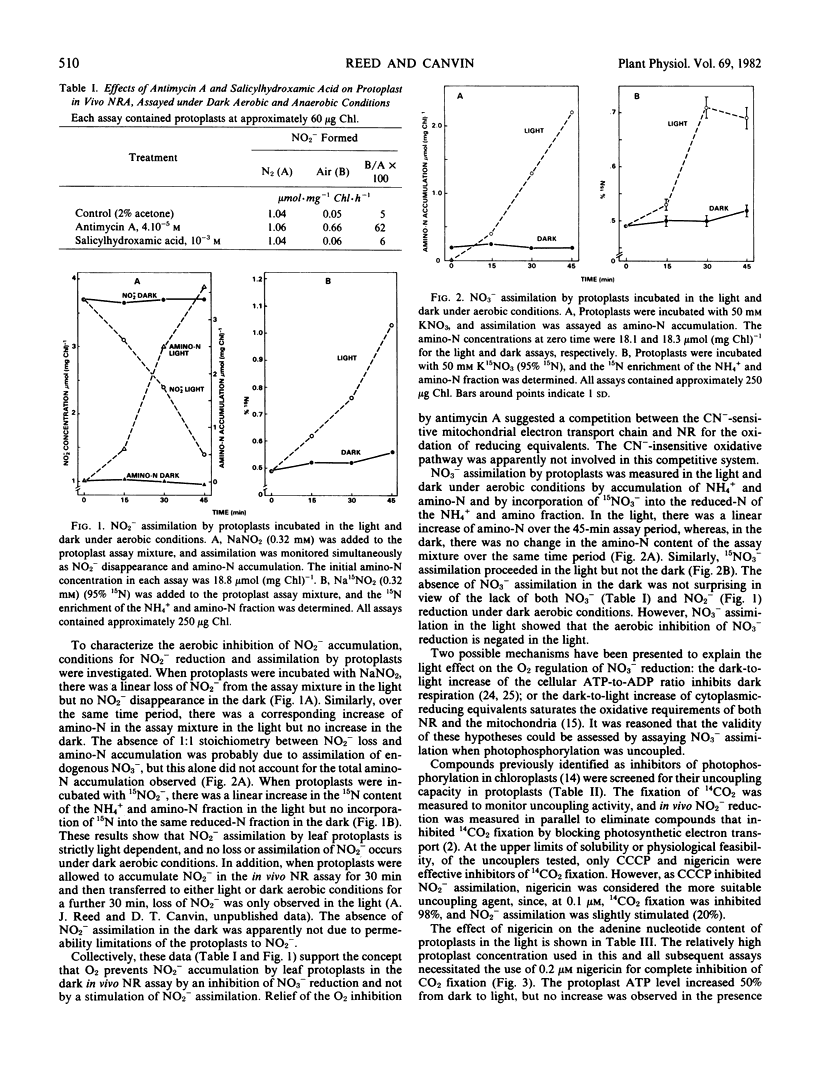
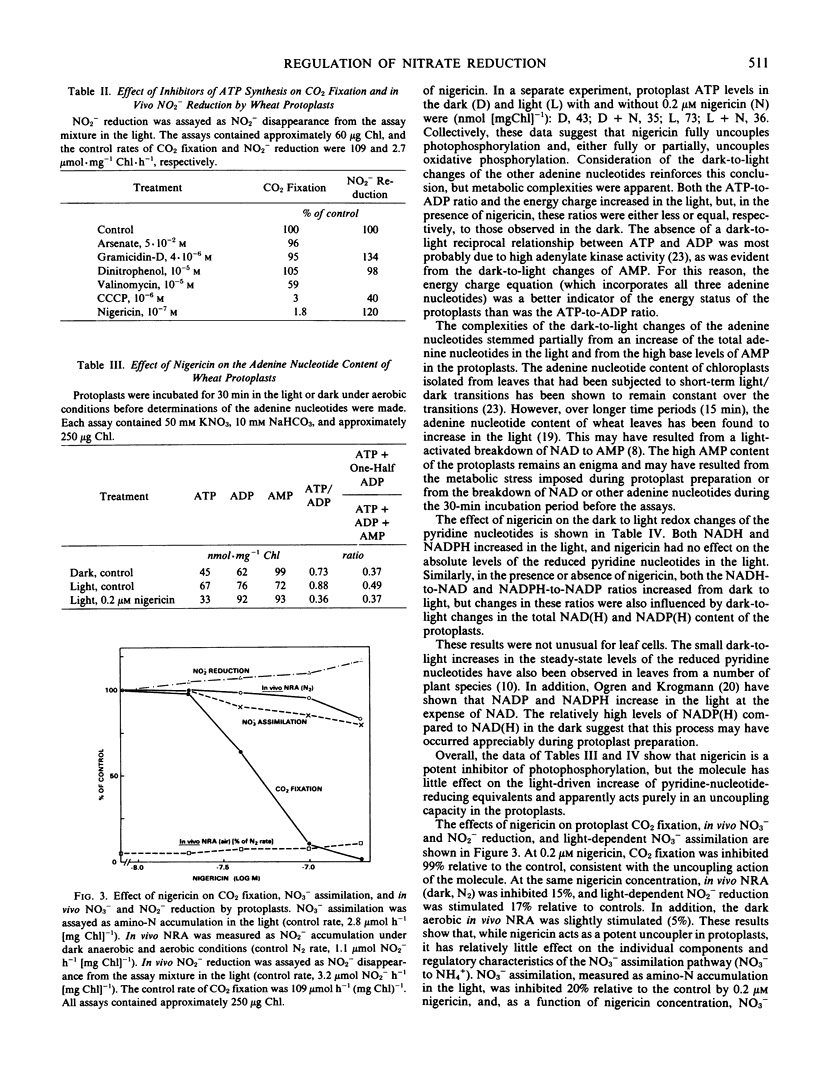
Protoplasts were isolated from the leaves of nitrate-cultured wheat (Triticum aestivum L. var. Frederick) seedlings. When incubated in the dark, protoplasts accumulated nitrite under anaerobic, but not under aerobic, conditions. The assimilation of [15N]nitrite by protoplasts was strictly light-dependent, and no loss of nitrite from the assay medium was observed under dark aerobic conditions. Therefore, the absence of nitrite accumulation under dark aerobic conditions was the result of an O2 inhibition of nitrate reduction and not a stimulation of nitrite reduction. In the presence of antimycin A, protoplasts accumulated nitrite under dark aerobic conditions. The oxygen inhibition of nitrate reduction was apparently due to a competition between nitrate reduction and dark respiration for cytoplasmic-reducing equivalents.
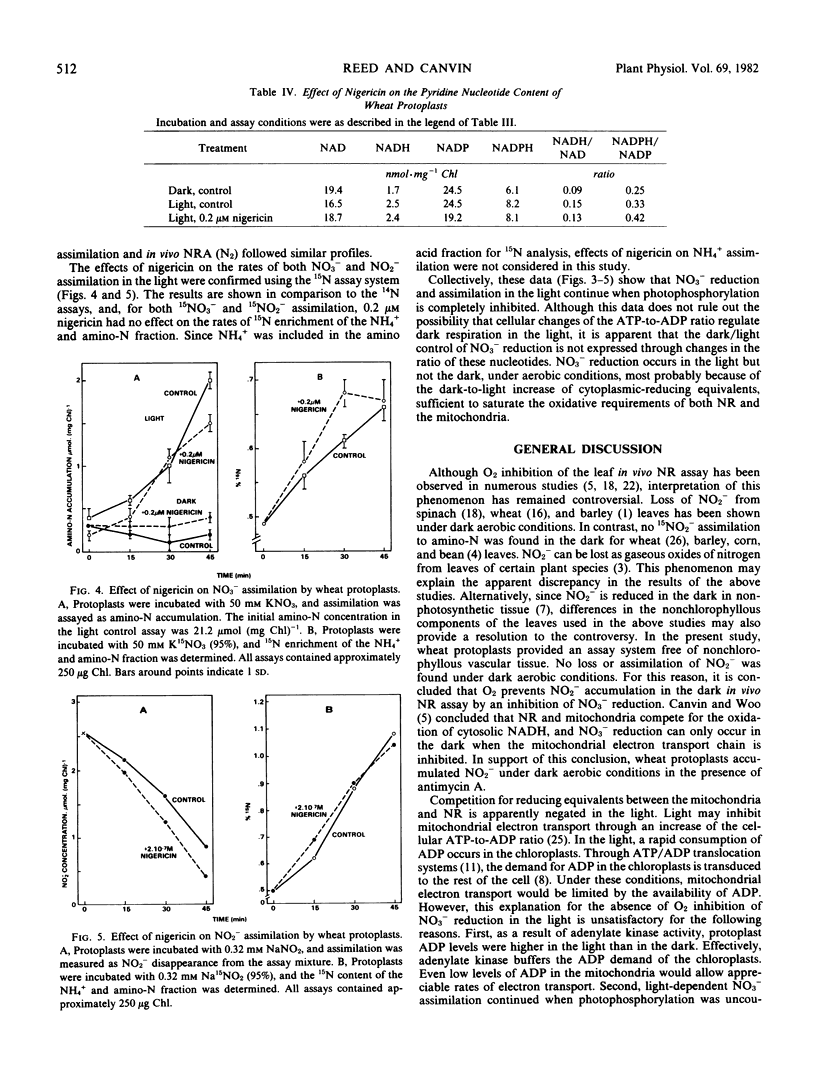
Oxygen control of nitrate reduction was not observed in the light, since protoplasts assimilated [15N]nitrate to amino-N under light aerobic conditions. It has been proposed that the increase of the ATP-to-ADP ratio in the light inhibits dark respiration and allows nitrate reduction to occur under aerobic conditions. To test this hypothesis, protoplast N and C assimilation was assayed in the presence of nigericin, an uncoupler of photophosphorylation. The dark to light increase of the protoplast energy charge was not observed in the presence of nigericin, and CO2 fixation was completely inhibited by the uncoupler. In contrast, rates of in vivo nitrate reduction (N2 and air) and nitrite reduction were relatively unaffected by nigericin, and light-driven nitrate assimilation was inhibited by only 20%. Nigericin had no effect on the dark-to-light increase of protoplast NADH and NADPH levels. It is proposed that the light-induced increase of cytoplasmic-reducing equivalents suppresses the competition between nitrate reduction and dark respiration and allows nitrate reduction to occur under aerobic conditions. Dark-to-light changes of the ATP-to-ADP ratio apparently are not critical to the regulation of nitrate reduction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Huffaker R. C., Rains D. W., Rao K. P. Influence of light and ambient carbon dioxide concentration on nitrate assimilation by intact barley seedlings. Plant Physiol. 1979 Jun;63(6):1205–1209. doi: 10.1104/pp.63.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Varner J. E. Intact tissue assay for nitrite reductase in barley aleurone layers. Plant Physiol. 1971 Jun;47(6):790–794. doi: 10.1104/pp.47.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- Jordan B. R., Givan C. V. Effects of Light and Inhibitors on Glutamate Metabolism in Leaf Discs of Vicia faba L: Sources of ATP for Glutamine Synthesis and Photoregulation of Tricarboxylic Acid Cycle Metabolism. Plant Physiol. 1979 Dec;64(6):1043–1047. doi: 10.1104/pp.64.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren W. L., Krogmann D. W. Studies on pyridine nucleotides in photosynthetic tissue. Concentrations, interconversions, and distribution. J Biol Chem. 1965 Dec;240(12):4603–4608. [PubMed] [Google Scholar]
- Rathnam C. K. Malate and Dihydroxyacetone Phosphate-dependent Nitrate Reduction in Spinach Leaf Protoplasts. Plant Physiol. 1978 Aug;62(2):220–223. doi: 10.1104/pp.62.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Sawhney S. K., Naik M. S., Nicholas D. J. Regulation of NADH supply for nitrate reduction in green plants via photosynthesis and mitochondrial respiration. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1209–1216. doi: 10.1016/0006-291x(78)91265-2. [DOI] [PubMed] [Google Scholar]
- Vanecko S., Varner J. E. Studies on Nitrite Metabolism in Higher Plants. Plant Physiol. 1955 Jul;30(4):388–390. doi: 10.1104/pp.30.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]


