Abstract
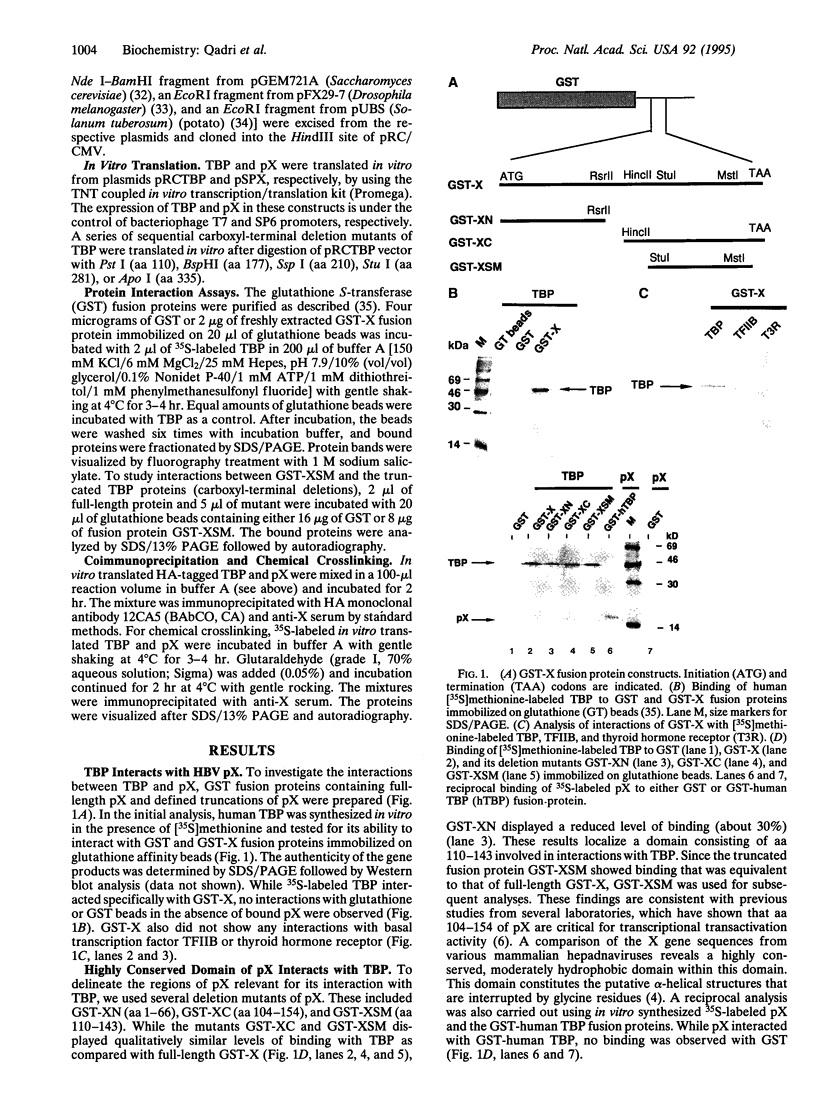
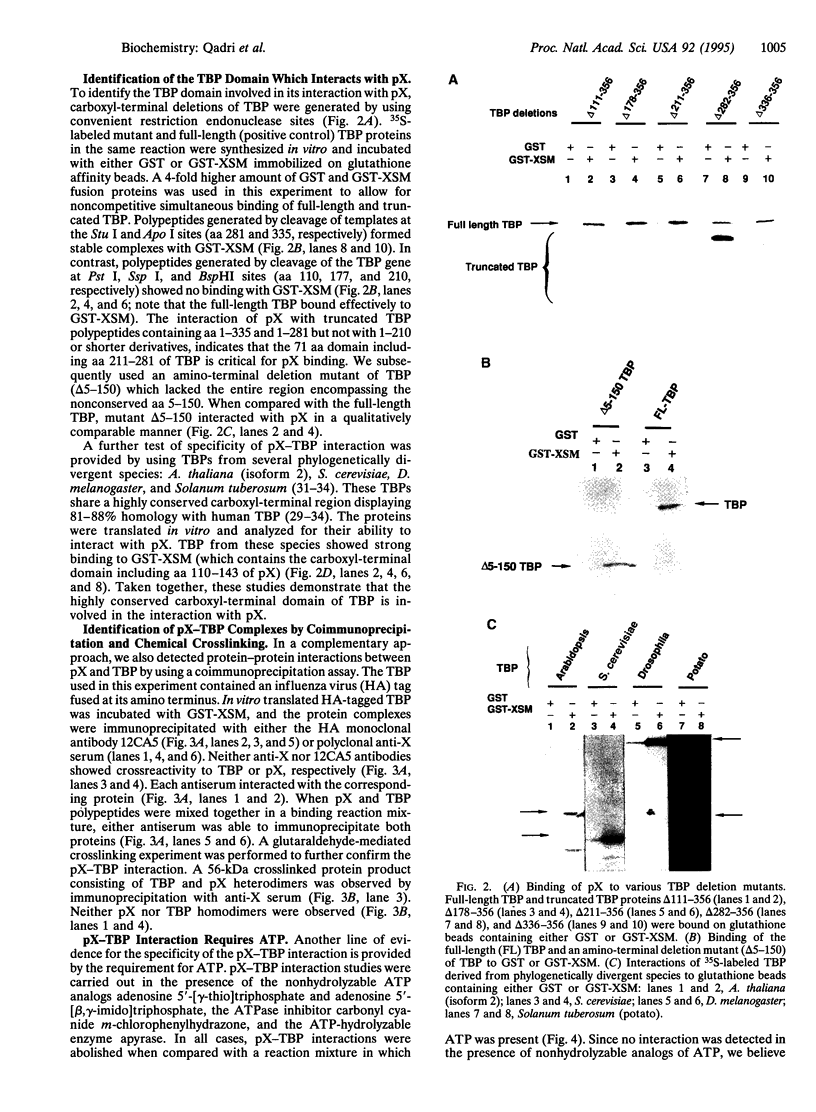
Several viral transcriptional activators have been shown to interact with the basal transcription factor TATA-binding protein (TBP). These associations have been implicated in facilitating the assembly of the transcriptional preinitiation complex. We report here that the hepatitis B virus protein X (pX) specifically binds to TBP in vitro. While truncations of the highly conserved carboxyl terminus of TBP abolished this binding, amino-terminal deletions had no effect. Deletion analysis suggests that a domain consisting of 71 aa in the highly conserved carboxyl-terminal region of TBP is necessary for its interaction with pX. The minimal region in pX sufficient for its interaction with TBP includes aa 110-143. Furthermore, TBP from phylogenetically distinct species including Arabidopsis thaliana, Saccharomyces cerevisiae, Drosophila melanogaster, and Solanum tuberosum (potato) bound to pX. The pX-TBP interaction was inhibited in the presence of nonhydrolyzable analogs of ATP, suggesting a requirement for ATP. These results provide an explanation for the promiscuous behavior of pX in the transactivation of a large repertoire of cellular promoters. This study further implicates a fundamental role for pX in modulating transcriptional regulatory pathways by interacting with the basal transcription factor TBP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balsano C., Avantaggiati M. L., Natoli G., De Marzio E., Will H., Perricaudet M., Levrero M. Full-length and truncated versions of the hepatitis B virus (HBV) X protein (pX) transactivate the cmyc protooncogene at the transcriptional level. Biochem Biophys Res Commun. 1991 May 15;176(3):985–992. doi: 10.1016/0006-291x(91)90379-l. [DOI] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Caron C., Rousset R., Béraud C., Moncollin V., Egly J. M., Jalinot P. Functional and biochemical interaction of the HTLV-I Tax1 transactivator with TBP. EMBO J. 1993 Nov;12(11):4269–4278. doi: 10.1002/j.1460-2075.1993.tb06111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Farmer G., Zhu H., Prywes R., Prives C. Cooperative DNA binding of p53 with TFIID (TBP): a possible mechanism for transcriptional activation. Genes Dev. 1993 Oct;7(10):1837–1849. doi: 10.1101/gad.7.10.1837. [DOI] [PubMed] [Google Scholar]
- Cross J. C., Wen P., Rutter W. J. Transactivation by hepatitis B virus X protein is promiscuous and dependent on mitogen-activated cellular serine/threonine kinases. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8078–8082. doi: 10.1073/pnas.90.17.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch A., Hoffmann A., Horikoshi M., Roeder R. G., Chua N. H. Arabidopsis thaliana contains two genes for TFIID. Nature. 1990 Jul 26;346(6282):390–394. doi: 10.1038/346390a0. [DOI] [PubMed] [Google Scholar]
- Hagemeier C., Cook A., Kouzarides T. The retinoblastoma protein binds E2F residues required for activation in vivo and TBP binding in vitro. Nucleic Acids Res. 1993 Nov 11;21(22):4998–5004. doi: 10.1093/nar/21.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeier C., Walker S., Caswell R., Kouzarides T., Sinclair J. The human cytomegalovirus 80-kilodalton but not the 72-kilodalton immediate-early protein transactivates heterologous promoters in a TATA box-dependent mechanism and interacts directly with TFIID. J Virol. 1992 Jul;66(7):4452–4456. doi: 10.1128/jvi.66.7.4452-4456.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez N. TBP, a universal eukaryotic transcription factor? Genes Dev. 1993 Jul;7(7B):1291–1308. doi: 10.1101/gad.7.7b.1291. [DOI] [PubMed] [Google Scholar]
- Hoey T., Dynlacht B. D., Peterson M. G., Pugh B. F., Tjian R. Isolation and characterization of the Drosophila gene encoding the TATA box binding protein, TFIID. Cell. 1990 Jun 29;61(7):1179–1186. doi: 10.1016/0092-8674(90)90682-5. [DOI] [PubMed] [Google Scholar]
- Holdsworth M. J., Grierson C., Schuch W., Bevan M. DNA-binding properties of cloned TATA-binding protein from potato tubers. Plant Mol Biol. 1992 Jun;19(3):455–464. doi: 10.1007/BF00023393. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Wang C. K., Fujii H., Cromlish J. A., Weil P. A., Roeder R. G. Cloning and structure of a yeast gene encoding a general transcription initiation factor TFIID that binds to the TATA box. Nature. 1989 Sep 28;341(6240):299–303. doi: 10.1038/341299a0. [DOI] [PubMed] [Google Scholar]
- Hu K. Q., Vierling J. M., Siddiqui A. Trans-activation of HLA-DR gene by hepatitis B virus X gene product. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7140–7144. doi: 10.1073/pnas.87.18.7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K. Q., Yu C. H., Vierling J. M. Up-regulation of intercellular adhesion molecule 1 transcription by hepatitis B virus X protein. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11441–11445. doi: 10.1073/pnas.89.23.11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashanchi F., Piras G., Radonovich M. F., Duvall J. F., Fattaey A., Chiang C. M., Roeder R. G., Brady J. N. Direct interaction of human TFIID with the HIV-1 transactivator tat. Nature. 1994 Jan 20;367(6460):295–299. doi: 10.1038/367295a0. [DOI] [PubMed] [Google Scholar]
- Kekulé A. S., Lauer U., Weiss L., Luber B., Hofschneider P. H. Hepatitis B virus transactivator HBx uses a tumour promoter signalling pathway. Nature. 1993 Feb 25;361(6414):742–745. doi: 10.1038/361742a0. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Ransone L. J., Wamsley P., Schmitt M. J., Boyer T. G., Zhou Q., Berk A. J., Verma I. M. Association between proto-oncoprotein Rel and TATA-binding protein mediates transcriptional activation by NF-kappa B. Nature. 1993 Sep 30;365(6445):412–419. doi: 10.1038/365412a0. [DOI] [PubMed] [Google Scholar]
- Kim Y., Geiger J. H., Hahn S., Sigler P. B. Crystal structure of a yeast TBP/TATA-box complex. Nature. 1993 Oct 7;365(6446):512–520. doi: 10.1038/365512a0. [DOI] [PubMed] [Google Scholar]
- Lee W. S., Kao C. C., Bryant G. O., Liu X., Berk A. J. Adenovirus E1A activation domain binds the basic repeat in the TATA box transcription factor. Cell. 1991 Oct 18;67(2):365–376. doi: 10.1016/0092-8674(91)90188-5. [DOI] [PubMed] [Google Scholar]
- Li F. Q., Ueda H., Hirose S. Mediators of activation of fushi tarazu gene transcription by BmFTZ-F1. Mol Cell Biol. 1994 May;14(5):3013–3021. doi: 10.1128/mcb.14.5.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., Berk A. J. The Zta trans-activator protein stabilizes TFIID association with promoter DNA by direct protein-protein interaction. Genes Dev. 1991 Dec;5(12B):2441–2454. doi: 10.1101/gad.5.12b.2441. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Transcription activation by the adenovirus E1a protein. Nature. 1989 Mar 2;338(6210):39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- Lukac D. M., Manuppello J. R., Alwine J. C. Transcriptional activation by the human cytomegalovirus immediate-early proteins: requirements for simple promoter structures and interactions with multiple components of the transcription complex. J Virol. 1994 Aug;68(8):5184–5193. doi: 10.1128/jvi.68.8.5184-5193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire H. F., Hoeffler J. P., Siddiqui A. HBV X protein alters the DNA binding specificity of CREB and ATF-2 by protein-protein interactions. Science. 1991 May 10;252(5007):842–844. doi: 10.1126/science.1827531. [DOI] [PubMed] [Google Scholar]
- Mahé Y., Mukaida N., Kuno K., Akiyama M., Ikeda N., Matsushima K., Murakami S. Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor kB and CCAAT/enhancer-binding protein-like cis-elements. J Biol Chem. 1991 Jul 25;266(21):13759–13763. [PubMed] [Google Scholar]
- Metz R., Bannister A. J., Sutherland J. A., Hagemeier C., O'Rourke E. C., Cook A., Bravo R., Kouzarides T. c-Fos-induced activation of a TATA-box-containing promoter involves direct contact with TATA-box-binding protein. Mol Cell Biol. 1994 Sep;14(9):6021–6029. doi: 10.1128/mcb.14.9.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolov D. B., Hu S. H., Lin J., Gasch A., Hoffmann A., Horikoshi M., Chua N. H., Roeder R. G., Burley S. K. Crystal structure of TFIID TATA-box binding protein. Nature. 1992 Nov 5;360(6399):40–46. doi: 10.1038/360040a0. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Tanese N., Pugh B. F., Tjian R. Functional domains and upstream activation properties of cloned human TATA binding protein. Science. 1990 Jun 29;248(4963):1625–1630. doi: 10.1126/science.2363050. [DOI] [PubMed] [Google Scholar]
- Rossner M. T. Review: hepatitis B virus X-gene product: a promiscuous transcriptional activator. J Med Virol. 1992 Feb;36(2):101–117. doi: 10.1002/jmv.1890360207. [DOI] [PubMed] [Google Scholar]
- Siddiqui A., Gaynor R., Srinivasan A., Mapoles J., Farr R. W. trans-activation of viral enhancers including long terminal repeat of the human immunodeficiency virus by the hepatitis B virus X protein. Virology. 1989 Apr;169(2):479–484. doi: 10.1016/0042-6822(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Siddiqui A., Jameel S., Mapoles J. Expression of the hepatitis B virus X gene in mammalian cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2513–2517. doi: 10.1073/pnas.84.8.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Spandau D. F., Lee C. H. trans-activation of viral enhancers by the hepatitis B virus X protein. J Virol. 1988 Feb;62(2):427–434. doi: 10.1128/jvi.62.2.427-434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer K. F., Ingles C. J., Greenblatt J. Direct and selective binding of an acidic transcriptional activation domain to the TATA-box factor TFIID. Nature. 1990 Jun 28;345(6278):783–786. doi: 10.1038/345783a0. [DOI] [PubMed] [Google Scholar]
- Twu J. S., Chu K., Robinson W. S. Hepatitis B virus X gene activates kappa B-like enhancer sequences in the long terminal repeat of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5168–5172. doi: 10.1073/pnas.86.13.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Schloemer R. H. Transcriptional trans-activating function of hepatitis B virus. J Virol. 1987 Nov;61(11):3448–3453. doi: 10.1128/jvi.61.11.3448-3453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. W., Forrester K., Yeh H., Feitelson M. A., Gu J. R., Harris C. C. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2230–2234. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Prorock C., Ishikawa H., Maldonado E., Ito Y., Gélinas C. Functional interaction of the v-Rel and c-Rel oncoproteins with the TATA-binding protein and association with transcription factor IIB. Mol Cell Biol. 1993 Nov;13(11):6733–6741. doi: 10.1128/mcb.13.11.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm P., Hofschneider P. H., Koshy R. The HBV X-ORF encodes a transactivator: a potential factor in viral hepatocarcinogenesis. Oncogene. 1988 Aug;3(2):169–177. [PubMed] [Google Scholar]
- Zhou D. X., Taraboulos A., Ou J. H., Yen T. S. Activation of class I major histocompatibility complex gene expression by hepatitis B virus. J Virol. 1990 Aug;64(8):4025–4028. doi: 10.1128/jvi.64.8.4025-4028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de-Medina T., Haviv I., Noiman S., Shaul Y. The X protein of hepatitis B virus has a ribo/deoxy ATPase activity. Virology. 1994 Jul;202(1):401–407. doi: 10.1006/viro.1994.1356. [DOI] [PubMed] [Google Scholar]