Abstract
Background
Because of their geographical location and traditional lifestyle, Canadian Inuit children are highly exposed to polychlorinated biphenyls (PCBs) and lead (Pb), environmental contaminants that are thought to affect fetal and child growth. We examined the associations of these exposures with the fetal and postnatal growth of Inuit children.
Methods
We conducted a prospective cohort study among Inuit from Nunavik (Arctic Québec). Mothers were recruited at their first prenatal visit; children (n = 290) were evaluated at birth and at 8–14 years of age. Concentrations of PCB 153 and Pb were determined in umbilical cord and child blood. Weight, height and head circumference were measured at birth and during childhood.
Results
Cord blood PCB 153 concentrations were not associated with anthropometric measurements at birth or school age, but child blood PCB 153 concentrations were associated with reduced weight, height and head circumference during childhood. There was no association between cord Pb levels and anthropometric outcomes at birth, but cord blood Pb was related to smaller height and a tendency to a smaller head circumference during childhood.
Interpretation
Our results suggest that chronic exposure to PCBs during childhood is negatively associated with skeletal growth and weight, while prenatal Pb exposure is related to reduce growth during childhood. This study is the first to link prenatal Pb exposure to poorer growth in school-age children.
Keywords: Fetal growth, child growth, polychlorinated biphenyls, lead, Inuit
1. Introduction
Several environmental contaminants have been suspected of having effects on the growth of fetuses and children. Polychlorinated biphenyls (PCBs) are a group of persistent, lipophilic contaminants that were used as heat transfer fluids in transformers until the 1970s. They are still detected in children’s blood at low levels and have been identified as endocrine disruptors that could interfere with fetal and postnatal development (Cocchi, Tulipano et al. 2009, Casals-Casas and Desvergne 2011). Children are particularly vulnerable to these contaminants due to their physiological immaturity and the transplacental transfer that occurs during the fetal period (Grandjean 2008). Whereas the effects of prenatal PCB exposure on fetal growth remain controversial (Govarts, Nieuwenhuijsen et al. 2010, El Majidi, Bouchard et al. 2013), no previous study has investigated the relations of pre- and postnatal PCB exposure to the growth of school-age children.
Lead (Pb) intoxication in children has been related to stunted growth in previous studies (Schwartz, Angle et al. 1986, Ballew, Khan et al. 1999). Although Pb concentrations in the environment have been steadily decreasing since the ban of Pb-based paints and leaded fuel (Levin, Brown et al. 2008), children are still exposed to that Pb, particularly in families with low socioeconomic status (Liu, Ai et al. 2012) and in developing countries (Falk 2003). Because Pb is stored in bone, the fetal period is a sensitive window of exposure due to maternal resorption from bone during pregnancy (Tellez-Rojo, Hernandez-Avila et al. 2004). Few studies have investigated the effects of low level postnatal Pb exposure on child growth (Frisancho and Ryan 1991, Kim, Hu et al. 1995, Ignasiak, Slawinska et al. 2006), and only one study have evaluated the relation of postnatal growth to in utero exposure (Gardner, Kippler et al. 2013).
Inuit children in Nunavik are more exposed to PCBs than their southern Quebec counterparts due to their geographical location and consumption of traditional native foods, especially marine mammal fat. Although the Stockholm Convention has banned PCB production and uses internationally since 2001, their persistence and biomagnification through the Arctic marine food chain continue to result in chronic dietary exposure in Inuit children (Muckle, Ayotte et al. 2001). Pb is also a contaminant of concern in this population, with the main source of exposure being the use of lead shots for hunting (Couture, Levesque et al. 2012). Frequent consumption of marine mammals, fish and migratory birds during infancy and childhood is, therefore, an important source of postnatal exposure to these contaminants among the Inuit. An extended duration of breastfeeding is also a significant source of exposure to PCBs. Several health and neurobehavioral effects of pre- and postnatal PCB and Pb exposure have already been reported among Inuit children (Dallaire, Dewailly et al. 2006, Boucher, Muckle et al. 2009, Boucher, Jacobson et al. 2012, Ethier, Muckle et al. 2012). The aim of this study was to evaluate the associations of pre- and postnatal PCB and Pb blood concentrations with fetal and child growth in a relatively highly exposed population.
2. Methods
2.1 Study population
Participants were 290 Inuit children residing in the 14 Inuit communities of Nunavik, a region located north of the 55th parallel, in Arctic Québec. Most participants (n = 233) were initially recruited (1993–1998) as part of a study aimed at monitoring prenatal exposure to environmental contaminants present in the marine food web (Dewailly, Bruneau et al. 1993). The remaining children (n = 57) were recruited (1996–2000) in the three largest communities of the Hudson Bay coast (Puvirnituq, Inukjuak and Kuujjuarapik) as part of a study undertaken to document the effects of prenatal exposure to environmental contaminants on infant development (Muckle, Ayotte et al. 2001, Jacobson, Jacobson et al. 2008). All children were seen at birth. The combined number of participants recruited for the two initial studies was 548. All families came from the same geographical area and should be considered as coming from one population although three of the largest communities were overrepresented.
Between September 2005 and February 2010, the children’s caregivers were contacted by telephone, provided with information about the study protocol, and invited to participate with their school-age children in a follow-up study. Inclusion criteria were age between 8.5 and 14.5 years, birth weight ≥ 2.5 kg, gestation duration ≥ 35 weeks, no major birth defects, neurological or chronic health problems affecting growth (hepatic chronic disease and asthma) and cord blood sample collected at birth (N = 461). Participation rate was 63%. Main reasons for lost to follow-up were; children moved to another village or outside Nunavik, refusal to participate and inability to recontact participants. The principal caregivers (the biological mother in 67.6% of cases) were interviewed to provide information on sociodemographic background, food insecurity, obstetrical and child medical history, as well as maternal lifestyle habits, including smoking, alcohol, and drug use during pregnancy. Written informed consent was obtained from a parent of each participant; oral assent was obtained from each child. The study was endorsed by community stakeholders and public health authorities, approved by Laval University and Wayne State University ethics committees, and conducted in accordance with the ethical standards of the 1983 Declaration of Helsinki.
2.2 Anthropometric parameters
Weight, height and head circumference were measured at birth and during childhood by midwives and our research nurses, respectively. Midwives and nurses were trained to use standard measurement procedures. Newborn and child weights were determined using a digital balance, while head circumference was measured with a measuring tape. Length at birth and height during childhood were measured with an infantometer and a stadiometer, respectively. Two measurements were performed for each parameter, and a third was obtained in case of a discrepancy > 5 g between the two measurements for birth weight, > 500 g for weight during childhood, > 0.3 cm for length and head circumference at birth, and > 0.5 cm for both parameters in childhood. The final value of a given growth parameter was based on the average of the two closest measurements. Additionally, the body mass index (BMI) was calculated using the formula BMI = weight (kg)/ height (m)2.
2.3 Biomarkers of exposure to environmental contaminants
A blood sample (30 mL) obtained from the umbilical cord was used to determine prenatal exposure to PCBs and toxic metals (Pb and Hg), whereas a venous blood sample (20 mL) collected from each child was used to document exposure to these contaminants at the time of testing. In cord blood samples, total Hg concentrations were determined using cold vapor atomic absorption spectrometry (Pharmacia Model 120; Pharmacia, Piscataway, NJ). Pb levels were determined by graphite furnace atomic absorption spectroscopy, using the Zeeman-effect background correction (PerkinElmer model ZL 4100; PerkinElmer, Norwalk, CT). Total Hg and Pb concentrations in children blood samples were determined by inductively coupled plasma mass spectrometry (ICP-MS) using a PerkinElmer Sciex Elan 6000 and a PE DRC II instruments for Pb and Hg determination, respectively. We verified that the tubes used for blood collection and storing of samples were free of analytically significant lead contamination.
The 14 most prevalent PCB congeners (International Union of Pure and Applied Chemistry numbers 28, 52, 99, 101, 105, 118, 128, 138, 153, 156, 170, 180, 183 and 187) were measured in purified cord and child plasma extracts using gas chromatography/mass spectrometry. The system used for the latter was an Agilent 6890 Network gas chromatograph (Wilmington, DE) equipped with an Agilent 7683 series automatic injector and an Agilent 5973 Network mass spectrometer. The gas chromatograph was fitted with an Agilent 60 m DB-XLB column (0.25 mm i.d, 0.25 μm film thickness). The carrier gas was helium and the injection volumes were 3 μL in splitless mode for the first fraction and 2 μL in splitless mode for the second fraction. The mass spectrometer was operated in selected ion monitoring mode, using negative chemical ionization with CH4 (99.97 %) as reagent gas. Limits of detection (LOD) in cord samples were 0.2 μg/L for Hg and Pb, and 0.02 μg/L for PCB congeners. LOD for children samples were 0.1 μg/L for Hg, 0.002 μg/dL for Pb and <0.05 μg/L for all PCB congeners except PCB 52 (0.15 μg/L). These analyses were performed at the Laboratoire de Toxicologie, Institut National de Santé Publique du Québec (Québec, QC, Canada).
Plasma PCB 153 concentration, expressed on a lipid basis, was selected as a single marker for exposure to the mixture of PCBs found in the Arctic environment, because it is the prevailing PCB congener and is highly correlated with the other congeners (Muckle, Ayotte et al. 2001).
2.4 Other laboratory analyses
Cholesterol, triglycerides, phospholipids and n-3 polyunsaturated fatty acids (n-3 PUFAs) were determined in cord plasma samples. In addition, hemoglobin, ferritin, and vitamins A and D were quantified in child plasma samples. Analytical procedures are provided in the Supplemental Material.
2.5 Control variables
The following potential confounding variables were documented: (1) child characteristics: sex, duration of gestation, duration of breastfeeding, age of child at testing, as well as Hg, vitamin and hemoglobin concentrations in child blood; (2) characteristics of biological mothers: pre-pregnancy maternal weight (kg), height (cm), age at delivery, parity; (3) maternal characteristics at child follow-up: education (years), marital status (single vs. married/living with partner), socioeconomic status (SES) (Hollingshead 1975) (the score includes the occupational status of the principal caregiver and, when relevant, the secondary caregiver who was either the child’s biological father or mother’s partner in 77.3% of cases), and food insecurity of the family (yes/no, refers to having experienced at least 1 day without food during the month prior to the interview); (4) cord plasma levels of the omega-3 fatty acid docosahexaenoic acid (DHA); and (5) other prenatal exposures: cord Hg, maternal smoking (yes/no), alcohol use (yes/no), and illicit drug use (yes/no). Prenatal exposure to tobacco, alcohol and illicit drugs as well as breastfeeding duration were retrospectively documented at 11 years of age for most participants (n = 233) and through prenatal interview (for substance use) and 1-month postnatal interviews (for substance use and breastfeeding) for the remaining mothers.
2.6 Statistical analysis
Preliminary multiple regression analysis at each testing time allowed for the identification of relevant confounders for each anthropometric parameter separately at birth and at 11 years. Covariates were initially retained as potential confounders if they were correlated with outcomes with a p ≤ 0.20. All covariates found to be associated with outcomes at a p value ≤ 0.20 were first included together in multiple regression models to assess their confounding influence and then removed using a backward procedure, starting with the least significant association with the outcome. Covariates modifying the β associated with a given contaminant by at least 10% were retained in the final models. Sex and age of child at follow-up as well as food insecurity were included in all models (see the figures’ notes for a complete list of the covariates retained for each outcome). Interaction terms between PCB 153 and Pb, as well as with gender and breastfeeding status were tested in full models. All the interaction terms were not significant.
Contaminant concentrations, weight, BMI, and head circumference at 11-year as well as duration of breastfeeding were all log-transformed to improve normality of distributions. Path analyses were conducted to model the longitudinal relations between exposure variables and growth outcomes in newborns and in children. This method simultaneously estimates all the regression and correlation coefficients specified in models with more than one dependent variable.
Path models were estimated using the Mplus 5.21 software. Missing data were taken into account with the full information maximum likelihood (FIML) estimator, which represents a state-of-the-art method for estimating models with missing data (Graham 2009). The FIML estimator fits the model tested with all data available for each participant so that any participant with at least one valid data point is retained for the analyses. In our sample, the proportion of missing data ranged from 0% to 25% (see Table 1). A 2-tailed p-value < 0.05 was considered statistically significant. The coefficients reported in the figures are standardized regression coefficients (unstandardized estimates are available from the authors).
Table 1.
Sample characteristics
| Characteristic | N | Arithmetic Mean | Geo. Meanb | SD | Range | % |
|---|---|---|---|---|---|---|
| Maternal | ||||||
| Maternal age | 290 | 24.0 | 6 | 14.0 – 42.0 | ||
| Pre-pregnancy maternal weight (kg) | 241 | 59.1 | 10.4 | 41.0 – 108.0 | ||
| Maternal height (cm) | 218 | 154.3 | 4.9 | 143.7 – 170.8 | ||
| Parity | 289 | 1.9 | 1.8 | 0 – 9 | ||
| Smoking during pregnancy (% yes) | 282 | 84.8 | ||||
| Alcohol use during pregnancy (% yes) | 249 | 50.2 | ||||
| Occupational status (11 years) | 289 | |||||
| No occupation | 20.4 | |||||
| Unskilled laborers | 2.8 | |||||
| Semiskilled laborers | 22.4 | |||||
| Skilled craftswomen, clerks, saleswomen | 22.1 | |||||
| Technical jobs, small businesse | 11.4 | |||||
| Professionals | 20.0 | |||||
| Socioeconomic statusa | 290 | 28.4 | 11.7 | 8.0 –66.0 | ||
| Marital status (% married or living with someone) | 73,4% | |||||
| Newborn | ||||||
| Sex (% boys) | 290 | 49.7 | ||||
| Gestational age (weeks) | 290 | 39.2 | 1.5 | 35.0 – 44.0 | ||
| Birth weight (kg) | 288 | 3.50 | 0.45 | 2.50 – 4.74 | ||
| Length (cm) | 281 | 51.2 | 2.4 | 44.0 – 65.0 | ||
| Head circumference (cm) | 269 | 34.6 | 1.6 | 25.0 – 39.0 | ||
| Cord Hg (μg/L) | 278 | 21.8 | 16.11 | 17.5 | 1.0 – 99.3 | 100 d |
| Cord PCB 153 (μg/kg lipids) | 278 | 123.2 | 94.7 | 99.8 | 9.7 – 653.6 | 100 d |
| Cord lead (μg/dL) | 278 | 4.8 | 3.9 | 3.3 | 0.8 – 20.9 | 100 d |
| Breastfeeding (months) | 279 | 11.7 | 5.3 | 16.7 | 0.0 – 108.0 | |
| Children | ||||||
| Age (years) | 290 | 11.3 | 0.8 | 8.5 – 14.3 | ||
| Weight (kg) | 290 | 40.1 | 39.1 | 9.9 | 22.1 – 88.1 | |
| Height (cm) | 289 | 141.4 | 7.4 | 118.9 – 167.8 | ||
| BMI | 289 | 19.85 | 19.63 | 3.17 | 15.08 – 38.88 | |
| Head circumference (cm) | 289 | 54.7 | 54.7 | 1.6 | 50.5 – 60.8 | |
| Hg (μg/L) | 286 | 5.0 | 3.0 | 4.7 | 0.1 – 34.1 | 100 d |
| PCB 153 (μg/kg lipids) | 284 | 73.1 | 47.8 | 82.1 | 3.5 – 809.5 | 100 d |
| Lead (μg/dL) | 286 | 2.7 | 2.2 | 2.1 | 0.4 – 12.8 | 100 d |
| Food insecurity (% yes)c | 288 | 38.9 | ||||
Hollingshead index (1975) for the mean of the two principal caregivers of the children, based on occupational status and education;
Geometric mean is given for log-transformed variables;
Food insecurity refers to having experienced at least 1 day without food during the month prior to the interview.
Percent detected.
3. Results
Descriptive statistics are presented in Table 1. Most women were young at delivery, low in socioeconomic status, and cigarette smokers; half had consumed alcohol at least once during pregnancy. Six percent of the children were born before 37 weeks of gestation. The mean age of children at follow-up was 11 years. At follow-up, the socioeconomic status of the mother continued to be low, and food insecurity was reported by almost 40% of interviewed women. Levels of contaminants were higher at birth than during childhood. A comparison of the children participating to the 11-year follow-up with the children who did not on anthropometrics, exposure and socioeconomic characteristics showed few differences. The children who participated to the follow-up showed slightly longer length at birth (average difference = 0.48 cm, p = 0.02), slightly lower pre-pregnancy maternal weight (average difference = 2.23 kg, p = 0.02), and were more exposed to mercury prenatally (average difference (geometric means) = 2.33 ug/L, p < 0.001).
Figures 1–3 show the path models used to test the longitudinal relations of PCB 153 and Pb with height, head circumference, and weight at birth and 11 years. Each standardized regression coefficient relating PCB exposure to growth outcomes was adjusted for Pb exposure and vice versa. All models fitted the data satisfactorily (likelihood ratio χ2 test p > 0.05, root mean square error of approximation < 0.05, comparative fit index > 0.95), although correlations had to be added to the model initially postulated (see Supplemental Materials).
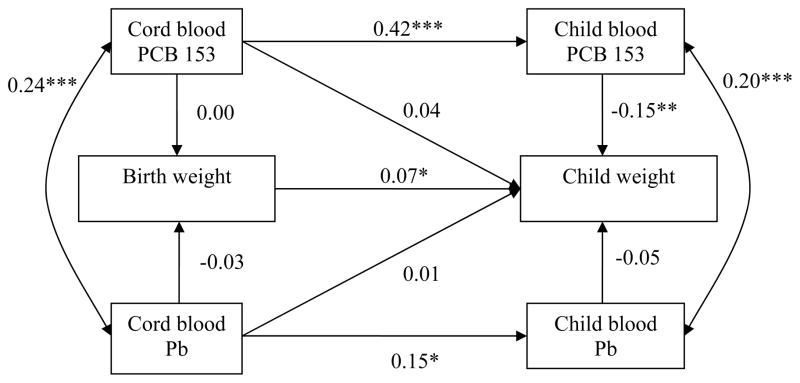
Figure 1.
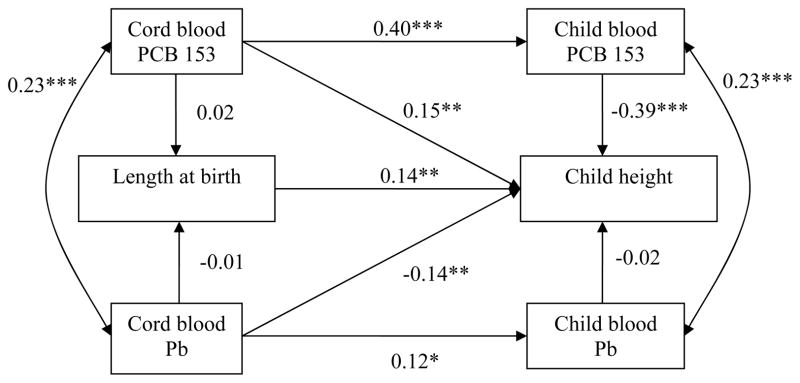
Path model, relating PCB 153 and Pb concentrations in cord and child blood to height at birth and in 8–14 year-old children.
Abbreviations: PCB 153, polychlorinated biphenyl congener 153; Pb, lead.
A single arrow represents a regression coefficient adjusted for the other variables in the model. A double-headed arrow represents a correlation coefficient.
* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
Adjustment for birth length: maternal height, pregnancy smoking and drinking, gestational age, sex; adjustment for child height: maternal height, pregnancy smoking, sex, breastfeeding duration, child age at follow-up, food insecurity.
Fit statistics: χ2 = 31.20 (25), p = 0.18; CFI = 0.99; RMSEA = 0.03, CI: 0.00 – 0.06.
Figure 3.
Path model relating PCB 153 and Pb levels in cord and child blood to weight at birth and in 8 – 14 year-old children.
Abbreviations: PCB 153, polychlorinated biphenyl congener 153; Pb, lead.
A single arrow represents a regression coefficient adjusted for the other variables in the model. A double-headed arrow represents a correlation coefficient.
* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
Adjustment for birth weight: pregnancy smoking and drinking, maternal weight before pregnancy, parity, gestational age, sex; adjustment for child weight: maternal weight before pregnancy, pregnancy smoking, breastfeeding duration, sex, child height, child age at follow-up, food insecurity.
Fit statistics: χ2 = 27.38 (25), p = 0.34; CFI = 0.99; RMSEA = 0.02, CI: 0.00 – 0.05.
Cord plasma PCB 153 concentrations were not related to anthropometric parameters at birth but were positively related to greater child height. The total effects of cord PCB 153 (sum of indirect effect and direct effect) on growth parameters at 11-year were not significant (unstandardized estimates: height = −0.04, p = 0.93; head circumference = 0.00, p = 0.95; weight = −0.00, p = 0.53). With regard to height, the direct cord PCB association was no longer significant when child plasma PCB level was removed from the model (cord PCB 153 association with child height, β = −0.007, p = 0.88), indicating that this association was likely due to collinearity between cord and child PCB 153 concentrations (β = 0.40, p < 0.001). Child blood PCB 153 concentrations were related to reduced child height, head circumference and weight, even after controlling for cord PCB 153. The path model for body mass index (BMI) (β = −0.33, p < 0.001) was not presented as the association was similar to those observed with weight and height (see Supplemental Materials, Table S.1).
Cord blood Pb levels were not associated with any of the fetal growth outcomes in any of the models. Cord Pb concentrations were not associated with child weight (p = 0.70) or BMI (β = −0.07, p = 0.23) but were related to a lower height (p = 0.007) and marginally related to a smaller head circumference (p = 0.07). The total effects of cord Pb on height and head circumference at 11-year were significant (unstandardized estimates: height = − 1.57, p = 0.004; head circumference = −0.005, p = 0.04). The latter relations persisted even when child blood Pb level was removed from the model. Child blood Pb levels were not related to child growth outcomes.
4. Discussion
Prenatal exposure to PCB 153 and Pb were not associated with fetal growth in this longitudinal study. However, chronic postnatal PCB exposure was associated with smaller height and head circumference and lower weight in school-age children. Furthermore, prenatal exposure to Pb, but not childhood Pb exposure, was related to a shorter height and marginally related to smaller head circumference in childhood.
Adverse PCB effects on fetal growth have been reported following the ingestion of PCB-contaminated cooking oil by pregnant women in Taiwan (Yu-cheng disease) and Japan (Yusho disease). Neonates born to Yu-cheng- and Yusho-disease affected mothers were smaller for gestational age than nonexposed newborns (Yamashita and Hayashi 1985, Guo, Lambert et al. 1995). It was later suspected that these effects could be attributed to polychlorinated dibenzo-p-dioxin and polychlorinated dibenzofurans also detected in the contaminated oil. Since those incidents, several studies conducted with populations exposed to background PCB levels have been undertaken to examine the associations between in utero exposure and fetal growth. However, inconsistent results have been reported with regard to prenatal PCB exposure in populations with lower exposure: eight studies reported significant associations with lower birth weight (Fein, Jacobson et al. 1984, Dar, Kanarek et al. 1992, Patandin, Koopman-Esseboom et al. 1998, Rylander, Stromberg et al. 1998, Karmaus and Zhu 2004, Lamb, Taylor et al. 2006, Sonneborn, Park et al. 2008, Murphy, Gollenberg et al. 2010, Wojtyniak, Rabczenko et al. 2010), while nine others failed to detect significant associations with neonate growth (Rogan, Gladen et al. 1987, Vartiainen, Jaakkola et al. 1998, Grandjean, Bjerve et al. 2001, Gladen, Shkiryak-Nyzhnyk et al. 2003, Longnecker, Klebanoff et al. 2005, Givens, Small et al. 2007, Khanjani and Sim 2007, Sagiv, Tolbert et al. 2007, Wolff, Engel et al. 2007). Recently, a meta-analysis involving twelve European birth cohorts concluded that birth weight was reduced in relation with cord PCB 153 concentrations (Govarts, Nieuwenhuijsen et al. 2010).
In our study, in utero exposure to PCB 153 was not related to growth at birth although in another sample from the same population, we previously reported that cord plasma PCB 153 concentrations were related to a shorter gestation duration, which was related to reduced fetal growth (Dallaire, Dewailly et al. 2013). In that study, levels of DHA, an n-3 polyunsatured fatty acid found in fish and marine mammals, were found to mitigate the negative effect of PCBs on fetal growth. PCBs are present in the environment as complex mixtures of different PCB congeners, and the relative proportions of the congeners that comprise these mixtures can differ markedly in different environments. Our failure to replicate effects on fetal growth seen in studies in Europe and elsewhere, assuming that it is not due to methodological differences or residual confounding, suggests that, although the total PCB levels in the population studied here are relatively high, the congeners forming the PCB mixture found in the Arctic might be somewhat less toxic.
In children affected by Yu-cheng disease, growth delay was observed at multiple periods during childhood (Rogan, Gladen et al. 1988, Guo, Lin et al. 1994). In low exposure populations, prenatal PCB exposure was associated with greater weight in children in three studies (Hertz-Picciotto, Charles et al. 2005, Verhulst, Nelen et al. 2009, Valvi, Mendez et al. 2012), with no association observed in one study of toddlers (Mendez, Garcia-Esteban et al. 2011). In moderately to highly exposed populations, prenatal PCB exposure has been found to be related to lower weight during childhood in four studies (Jacobson, Jacobson et al. 1990, Patandin, Koopman-Esseboom et al. 1998, Lamb, Taylor et al. 2006), although not in two (Gladen, Ragan et al. 2000, Jackson, Lynch et al. 2010). In recent years, some papers have considered the role of prenatal exposure to contaminants, including PCBs, in the etiology of obesity (Tang-Peronard, Andersen et al. 2011), but to the contrary of Tang-Peronard et al. (2014) we did not observe a positive correlation between prenatal PCB 153 exposure and weight during childhood. We also observed no clear association of prenatal PCB exposure with skeletal growth, which is consistent with the lack of association between these variables reported in four studies (Jacobson, Jacobson et al. 1990, Gladen, Ragan et al. 2000, Blanck, Marcus et al. 2002, Jackson, Lynch et al. 2010). However, prenatal PCB exposure was found to be related to reduced length and head circumference through 3 months of age among infants (Patandin, Koopman-Esseboom et al. 1998) and to greater height in 5-year-old girls (Hertz-Picciotto, Charles et al. 2005) and 17-year-old male teenagers (Lamb, Taylor et al. 2006).
Few studies have evaluated the relation between childhood PCB exposure and growth. In our study, we found that blood PCB concentrations at 11 years of age were associated with lower weight, shorter height, smaller head circumference, and lower BMI. Consistent with our findings, a study of 8- to 9-year-old boys in Russia reported a significant decrease in BMI, height, and height velocity among the most exposed children (Burns, Williams et al. 2011). A similar association between PCB exposure and reduced BMI but not height, was seen in a less exposed population of Flemish adolescents (Dhooge, Den Hond et al. 2010). Thus, the hypothesis that PCB exposure adversely affects skeletal growth and weight during childhood has received some empirical support from studies with moderately to highly exposed cohorts. One possible mechanism that might account for these associations relates to disruption of thyroid hormone homeostasis or bone formation by PCBs (Zoeller 2001, Cocchi, Tulipano et al. 2009), given that thyroid hormones are involved in fat metabolism and bone growth in humans (Duntas 2002, Combs, Nicholls et al. 2011). Another explanation might be reverse causation related to dilution of PCBs in fat tissue in heavier children since PCBs are lipophilic contaminants. Dilution might contribute to the PCB-weight association but is not likely to explain the relation with skeletal growth.
Consistent with our results, Pb concentration in cord or maternal blood during pregnancy has not been related to anthropometric measurements at birth in most studies (Greene and Ernhart 1991, Gonzalez-Cossio, Peterson et al. 1997, Hernandez-Avila, Peterson et al. 2002, Gundacker, Frohlich et al. 2010), but not all (Osman, Akesson et al. 2000, Zhu, Fitzgerald et al. 2010, Xie, Ding et al. 2013). Also, Pb concentration in maternal urine was not related to birth weigth in newborns from Bangladesh. Some studies have evaluated several biomarkers of Pb exposure in relation to fetal growth. Maternal tibia Pb levels determined at 1 month postpartum, but not cord nor maternal blood concentrations, were associated with a lower birth weight (Gonzalez-Cossio, Peterson et al. 1997), as well as decreased length and head circumference at birth (Hernandez-Avila, Peterson et al. 2002). Given these varying findings, it remains unclear whether prenatal Pb exposure is related to poorer fetal growth.
In our study, prenatal Pb exposure was associated with reduced height and a tendency for smaller head circumference at school age, whereas no associations were found between child blood Pb concentration and child growth parameters. These results suggest that in utero Pb exposure might have more detrimental effects on child growth than Pb exposure during childhood. Few prospective studies have investigated associations between prenatal low-level Pb exposure and postnatal growth, and none have presented data spanning such an extended time period. Three studies have found significant associations between prenatal Pb exposure and lower growth parameters from 6 to 48 months, depending on the variations in blood Pb levels between the pre- and postnatal periods, with higher Pb concentrations having more detrimental effects (Shukla, Dietrich et al. 1991, Rothenberg, Schnaas et al. 1999, Schell, Denham et al. 2009). However, one study failed to report an association between pre- and postnatal exposure to Pb and growth outcomes from 0 to 4 years of age (Greene and Ernhart 1991).
Unlike other studies, our data show that Pb concentrations in Inuit children are not associated with growth parameters at school age. Negative associations have been previously found between child blood Pb concentrations and skeletal growth (Schwartz, Angle et al. 1986, Frisancho and Ryan 1991, Kafourou, Touloumi et al. 1997, Ballew, Khan et al. 1999, Ignasiak, Slawinska et al. 2006, Yang, Huo et al. 2013) in children with higher blood Pb concentrations than those observed in our sample. The association with weight was less clear, two studies having reported lower weight with increasing Pb concentrations (Schwartz, Angle et al. 1986, Ignasiak, Slawinska et al. 2006), whereas another found a positive association between Pb dentin level and BMI (Kim, Hu et al. 1995). One possible explanation for the lack of significant negative associations with growth in our study relates to the timing of our Pb measurements. Because the peak exposure to Pb in children is around 2-years of age (Brody, Pirkle et al. 1994), we might have missed the appropriate postnatal window of exposure to identify adverse effects on the child growth. Several mechanisms have been proposed to explain how Pb may interfere with skeletal growth. Pb has been shown (1) to inhibit the osteoblast-mediated secretion of two proteins involved in bone mineralization, namely osteonectin (Klein and Wiren 1993) and osteocalcin (Long and Rosen 1992), (2) to interfere with binding of Ca2+ by transport proteins (Sauk and Somerman 1991), and 3) to affect growth hormone secretion (Berry, Moriarty et al. 2002).
This study has several strengths. Its longitudinal design allowed us to simultaneously consider prenatal and childhood exposure to ubiquitous contaminants on child growth. Furthermore, we were able to consider the concomitant exposure to contaminants present in the diet, along with nutrients. Although numerous confounders were considered in our analyses, substances used during pregnancy were documented retrospectively for most children, and we controlled for food insecurity of the family rather than the caloric intake and the physical activity of participating children. As a result, residual confounding may have affected our estimates for weight models, but this is less likely to have affected the results that were reported with skeletal growth indicators. Other limitations are absence of data on father characteristics and Tanner stage of the children as well as a single postnatal Pb measurement. Due to the long half-lives of several PCB congeners (4 to 10 years)(Grandjean, Budtz-Jorgensen et al. 2008), a unique measurement of postnatal PCB concentrations was considered adequate.
5. Conclusion
Our study supports findings from two other studies of moderately-exposed children indicating that chronic exposure to PCBs during childhood can adversely affect skeletal growth and body weight. Although the absence of an effect of prenatal PCB exposure on growth suggests that the congener mixture in Arctic Quebec may be less toxic than in other locales, the PCB exposure experienced during breast-feeding (Muckle, Ayotte et al. 2001) and childhood in this population presumably accounts for the effects seen on growth during childhood. In addition, this study is the first to link prenatal Pb exposure to poorer growth in school-age children, providing additional evidence that there is no safe blood Pb level for pregnant women.
Supplementary Material
Figure 2.
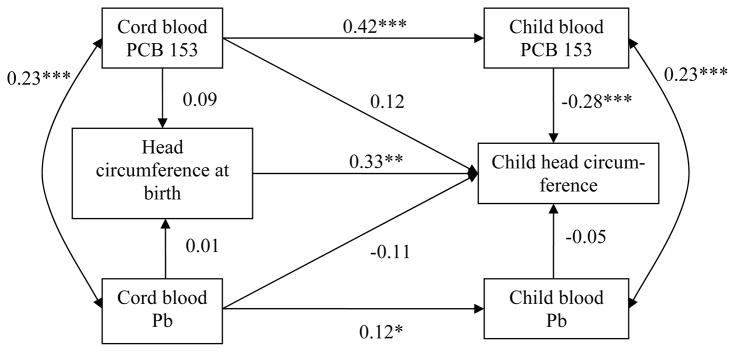
Path model relating PCB 153 and Pb levels in cord and child blood to head circumference at birth and in 8 – 14 year-old children.
Abbreviations: PCB 153, polychlorinated biphenyl congener 153; Pb, lead.
A single arrow represents a regression coefficient adjusted for the other variables in the model. A double-headed arrow represents a correlation coefficient.
* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
Adjustment for newborn head circumference: maternal height, gestational age, sex; adjustment for child head circumference: maternal height, sex, breastfeeding duration, child age at follow-up, food insecurity.
Fit statistics: χ2 = 18.96 (17), p = 0.33; CFI = 0.99; RMSEA = 0.02, CI: 0.00 – 0.06.
Highlights.
Polychlorinated biphenyls and lead are thought to affect fetal and child growth.
We examined their relations with the fetal and postnatal growth of Inuit children.
Exposure to PCBs during childhood is negatively related to growth and weight.
Prenatal Pb exposure is associated with reduced growth during childhood.
Acknowledgments
Funding sources:
This study was funded by grants from the National Institutes of Environmental Health Sciences/NIH (R01-ES007902 to J.L. Jacobson); Northern Contaminant Program, Indian and Northern Affairs Canada (to G. Muckle); and the Joseph Young Sr Fund from the State of Michigan (to S.W. Jacobson). R. Dallaire obtained a postdoctoral fellowship from the Canadian Institutes of Health Research.
We are grateful to the Nunavik population and their organizations for their participation in this research. We also thank Jocelyne Gagnon, Johanne Varin, Line Roy, Alacie Pov, Christine Bouffard, Karine Poitras, Carole Vézina, Germain Lebel, Edna Lachance, Renee Sun, Brenda Tuttle, Neil Dodge and Richard Poulin for their committed involvement in this study.
Abbreviations
- BMI
body mass index
- DHA
docosahexaenoic acid
- FIML
full information maximum likelihood
- n-3 PUFAs
n-3 polyunsaturated fatty acids
- Pb
lead
- PCBs
polychlorinated biphenyls
- SES
socioeconomic status
Footnotes
Ethics:
The participation of human subjects did occur after informed consent was obtained. The research procedures were approved by the community stakeholders and public health authorities, approved by Laval University and Wayne State University ethics committees, and conducted in accordance with the ethical standards of the 1983 Declaration of Helsinki.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballew C, et al. Blood lead concentration and children’s anthropometric dimensions in the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. J Pediatr. 1999;134(5):623–630. doi: 10.1016/s0022-3476(99)70250-7. [DOI] [PubMed] [Google Scholar]
- Berry WD, Jr, et al. Lead attenuation of episodic growth hormone secretion in male rats. Int J Toxicol. 2002;21(2):93–98. doi: 10.1080/10915810252866060. [DOI] [PubMed] [Google Scholar]
- Blanck HM, et al. Growth in girls exposed in utero and postnatally to polybrominated biphenyls and polychlorinated biphenyls. Epidemiology. 2002;13(2):205–210. doi: 10.1097/00001648-200203000-00016. [DOI] [PubMed] [Google Scholar]
- Boucher O, et al. Prenatal Methylmercury, Postnatal Lead Exposure, and Evidence of Attention Deficit/Hyperactivity Disorder among Inuit Children in Arctic Quebec. Environ Health Perspect. 2012;120(10):1456–1461. doi: 10.1289/ehp.1204976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, et al. The relation of lead neurotoxicity to the event-related potential P3b component in Inuit children from arctic Quebec. Neurotoxicology. 2009;30(6):1070–1077. doi: 10.1016/j.neuro.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DJ, et al. Blood lead levels in the US population. Phase 1 of the Third National Health and Nutrition Examination Survey (NHANES III, 1988 to 1991) Jama. 1994;272(4):277–283. doi: 10.1001/jama.272.4.277. [DOI] [PubMed] [Google Scholar]
- Burns JS, et al. Serum dioxins and polychlorinated biphenyls are associated with growth among Russian boys. Pediatrics. 2011;127(1):e59–68. doi: 10.1542/peds.2009-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- Cocchi D, et al. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat: Part 1: Effects on somatic growth, growth hormone-axis activity and bone mass in the offspring. Toxicol Appl Pharmacol. 2009;237(2):127–136. doi: 10.1016/j.taap.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Combs CE, et al. Thyroid hormones and bone development. Minerva Endocrinol. 2011;36(1):71–85. [PubMed] [Google Scholar]
- Couture A, et al. Lead exposure in Nunavik: from research to action. Int J Circumpolar Health. 2012;71:18591. doi: 10.3402/ijch.v71i0.18591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire F, et al. Effect of prenatal exposure to polychlorinated biphenyls on incidence of acute respiratory infections in preschool Inuit children. Environ Health Perspect. 2006;114(8):1301–1305. doi: 10.1289/ehp.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire R, et al. Exposure to organochlorines and mercury through fish and marine mammal consumption: associations with growth and duration of gestation among Inuit newborns. Environ Int. 2013;54:85–91. doi: 10.1016/j.envint.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar E, et al. Fish consumption and reproductive outcomes in Green Bay, Wisconsin. Environ Res. 1992;59(1):189–201. doi: 10.1016/s0013-9351(05)80239-7. [DOI] [PubMed] [Google Scholar]
- Dewailly E, et al. Health status at birth of Inuit newborn prenatally exposed to organochlorines. Chemosphere. 1993;27:359–366. [Google Scholar]
- Dhooge W, et al. Internal exposure to pollutants and body size in Flemish adolescents and adults: associations and dose-response relationships. Environ Int. 2010;36(4):330–337. doi: 10.1016/j.envint.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12(4):287–293. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- El Majidi N, et al. Relationship between prenatal exposure to polychlorinated biphenyls and birth weight: a systematic analysis of published epidemiological studies through a standardization of biomonitoring data. Regul Toxicol Pharmacol. 2013;64(1):161–176. doi: 10.1016/j.yrtph.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Ethier AA, et al. Effects of environmental contaminant exposure on visual brain development: A prospective electrophysiological study in school-aged children. Neurotoxicology. 2012;33(5):1075–1085. doi: 10.1016/j.neuro.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Falk H. International environmental health for the pediatrician: case study of lead poisoning. Pediatrics. 2003;112(1 Pt 2):259–264. [PubMed] [Google Scholar]
- Fein GG, et al. Prenatal exposure to polychlorinated biphenyls: effects on birth size and gestational age. J Pediatr. 1984;105(2):315–320. doi: 10.1016/s0022-3476(84)80139-0. [DOI] [PubMed] [Google Scholar]
- Frisancho AR, Ryan AS. Decreased stature associated with moderate blood lead concentrations in Mexican-American children. Am J Clin Nutr. 1991;54(3):516–519. doi: 10.1093/ajcn/54.3.516. [DOI] [PubMed] [Google Scholar]
- Gardner RM, et al. Environmental exposure to metals and children’s growth to age 5 years: a prospective cohort study. Am J Epidemiol. 2013;177(12):1356–1367. doi: 10.1093/aje/kws437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens ML, et al. Maternal exposure to polybrominated and polychlorinated biphenyls: infant birth weight and gestational age. Chemosphere. 2007;69(8):1295–1304. doi: 10.1016/j.chemosphere.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladen BC, et al. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J Pediatr. 2000;136(4):490–496. doi: 10.1016/s0022-3476(00)90012-x. [DOI] [PubMed] [Google Scholar]
- Gladen BC, et al. Persistent organochlorine compounds and birth weight. Ann Epidemiol. 2003;13(3):151–157. doi: 10.1016/s1047-2797(02)00268-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cossio T, et al. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics. 1997;100(5):856–862. doi: 10.1542/peds.100.5.856. [DOI] [PubMed] [Google Scholar]
- Govarts E, et al. Birth Weight and Prenatal Exposure to Polychlorinated Biphenyls (PCBs) and Dichlorodiphenyldichloroethylene (DDE): A Meta-analysis within 12 European Birth Cohorts. Environ Health Perspect. 2010;120(2):162–170. doi: 10.1289/ehp.1103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Grandjean P. Late insights into early origins of disease. Basic Clin Pharmacol Toxicol. 2008;102(2):94–99. doi: 10.1111/j.1742-7843.2007.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, et al. Birthweight in a fishing community: significance of essential fatty acids and marine food contaminants. Int J Epidemiol. 2001;30(6):1272–1278. doi: 10.1093/ije/30.6.1272. [DOI] [PubMed] [Google Scholar]
- Grandjean P, et al. Elimination half-lives of polychlorinated biphenyl congeners in children. Environ Sci Technol. 2008;42(18):6991–6996. doi: 10.1021/es800778q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene T, Ernhart CB. Prenatal and preschool age lead exposure: relationship with size. Neurotoxicol Teratol. 1991;13(4):417–427. doi: 10.1016/0892-0362(91)90091-a. [DOI] [PubMed] [Google Scholar]
- Gundacker C, et al. Perinatal lead and mercury exposure in Austria. Sci Total Environ. 2010;408(23):5744–5749. doi: 10.1016/j.scitotenv.2010.07.079. [DOI] [PubMed] [Google Scholar]
- Guo YL, et al. Growth abnormalities in the population exposed in utero and early postnatally to polychlorinated biphenyls and dibenzofurans. Environ Health Perspect. 1995;103(Suppl 6):117–122. doi: 10.1289/ehp.95103s6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YL, et al. Musculoskeletal changes in children prenatally exposed to polychlorinated biphenyls and related compounds (Yu-Cheng children) J Toxicol Environ Health. 1994;41(1):83–93. doi: 10.1080/15287399409531828. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila M, et al. Effect of maternal bone lead on length and head circumference of newborns and 1-month-old infants. Arch Environ Health. 2002;57(5):482–488. doi: 10.1080/00039890209601441. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, et al. In utero polychlorinated biphenyl exposures in relation to fetal and early childhood growth. Epidemiology. 2005;16(5):648–656. doi: 10.1097/01.ede.0000173043.85834.f3. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of a social status. Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Ignasiak Z, et al. Lead and growth status of school children living in the copper basin of south-western Poland: differential effects on bone growth. Ann Hum Biol. 2006;33(4):401–414. doi: 10.1080/03014460600730752. [DOI] [PubMed] [Google Scholar]
- Jackson LW, et al. Prenatal and postnatal exposure to polychlorinated biphenyls and child size at 24 months of age. Reprod Toxicol. 2010;29(1):25–31. doi: 10.1016/j.reprotox.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, et al. Effects of exposure to PCBs and related compounds on growth and activity in children. Neurotoxicol Teratol. 1990;12(4):319–326. doi: 10.1016/0892-0362(90)90050-m. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, et al. Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the Inuit of Arctic Quebec. J Pediatr. 2008;152(3):356–364. doi: 10.1016/j.jpeds.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Kafourou A, et al. Effects of lead on the somatic growth of children. Arch Environ Health. 1997;52(5):377–383. doi: 10.1080/00039899709602214. [DOI] [PubMed] [Google Scholar]
- Karmaus W, et al. Childhood growth and exposure to dichlorodiphenyl dichloroethene and polychlorinated biphenyls. J Pediatr. 2002;140(1):33–39. doi: 10.1067/mpd.2002.120764. [DOI] [PubMed] [Google Scholar]
- Karmaus W, Zhu X. Maternal concentration of polychlorinated biphenyls and dichlorodiphenyl dichlorethylene and birth weight in Michigan fish eaters: a cohort study. Environ Health. 2004;3(1):1. doi: 10.1186/1476-069X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanjani N, Sim MR. Maternal contamination with PCBs and reproductive outcomes in an Australian population. J Expo Sci Environ Epidemiol. 2007;17(2):191–195. doi: 10.1038/sj.jes.7500495. [DOI] [PubMed] [Google Scholar]
- Kim R, et al. A Longitudinal-Study of Chronic Lead-Exposure and Physical Growth in Boston Children. Environmental Health Perspectives. 1995;103(10):952–957. doi: 10.1289/ehp.95103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RF, Wiren KM. Regulation of osteoblastic gene expression by lead. Endocrinology. 1993;132(6):2531–2537. doi: 10.1210/endo.132.6.8504755. [DOI] [PubMed] [Google Scholar]
- Lamb MR, et al. Prenatal exposure to polychlorinated biphenyls and postnatal growth: a structural analysis. Environ Health Perspect. 2006;114(5):779–785. doi: 10.1289/ehp.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R, et al. Lead exposures in U.S. Children, 2008: implications for prevention. Environ Health Perspect. 2008;116(10):1285–1293. doi: 10.1289/ehp.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. Blood lead levels and associated sociodemographic factors among preschool children in the South Eastern region of China. Paediatr Perinat Epidemiol. 2012;26(1):61–69. doi: 10.1111/j.1365-3016.2011.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GJ, Rosen JF. Lead perturbs epidermal growth factor (EGF) modulation of intracellular calcium metabolism and collagen synthesis in clonal rat osteoblastic (ROS 17/2.8) cells. Toxicol Appl Pharmacol. 1992;114(1):63–70. doi: 10.1016/0041-008x(92)90097-c. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, et al. Maternal levels of polychlorinated biphenyls in relation to preterm and small-for-gestational-age birth. Epidemiology. 2005;16(5):641–647. doi: 10.1097/01.ede.0000172137.45662.85. [DOI] [PubMed] [Google Scholar]
- Mendez MA, et al. Prenatal organochlorine compound exposure, rapid weight gain, and overweight in infancy. Environ Health Perspect. 2011;119(2):272–278. doi: 10.1289/ehp.1002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckle G, et al. Determinants of polychlorinated biphenyls and methylmercury exposure in inuit women of childbearing age. Environ Health Perspect. 2001;109(9):957–963. doi: 10.1289/ehp.01109957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckle G, et al. Prenatal exposure of the northern Quebec Inuit infants to environmental contaminants. Environ Health Perspect. 2001;109(12):1291–1299. doi: 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LE, et al. Maternal serum preconception polychlorinated biphenyl concentrations and infant birth weight. Environ Health Perspect. 2010;118(2):297–302. doi: 10.1289/ehp.0901150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman K, et al. Toxic and essential elements in placentas of Swedish women. Clin Biochem. 2000;33(2):131–138. doi: 10.1016/s0009-9120(00)00052-7. [DOI] [PubMed] [Google Scholar]
- Patandin S, et al. Effects of environmental exposure to polychlorinated biphenyls and dioxins on birth size and growth in Dutch children. Pediatr Res. 1998;44(4):538–545. doi: 10.1203/00006450-199810000-00012. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, et al. Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science. 1988;241(4863):334–336. doi: 10.1126/science.3133768. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, et al. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects on growth, morbidity, and duration of lactation. Am J Public Health. 1987;77(10):1294–1297. doi: 10.2105/ajph.77.10.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg SJ, et al. Pre- and postnatal lead effect on head circumference: a case for critical periods. Neurotoxicol Teratol. 1999;21(1):1–11. doi: 10.1016/s0892-0362(98)00034-8. [DOI] [PubMed] [Google Scholar]
- Rylander L, et al. Polychlorinated biphenyls in blood plasma among Swedish female fish consumers in relation to low birth weight. Am J Epidemiol. 1998;147(5):493–502. doi: 10.1093/oxfordjournals.aje.a009476. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, et al. Organochlorine exposures during pregnancy and infant size at birth. Epidemiology. 2007;18(1):120–129. doi: 10.1097/01.ede.0000249769.15001.7c. [DOI] [PubMed] [Google Scholar]
- Sauk JJ, Somerman MJ. Physiology of bone: mineral compartment proteins as candidates for environmental perturbation by lead. Environ Health Perspect. 1991;91:9–16. doi: 10.1289/ehp.91919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell LM, et al. Growth of infants’ length, weight, head and arm circumferences in relation to low levels of blood lead measured serially. Am J Hum Biol. 2009;21(2):180–187. doi: 10.1002/ajhb.20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, et al. Relationship between childhood blood lead levels and stature. Pediatrics. 1986;77(3):281–288. [PubMed] [Google Scholar]
- Shukla R, et al. Lead exposure and growth in the early preschool child: a follow-up report from the Cincinnati Lead Study. Pediatrics. 1991;88(5):886–892. [PubMed] [Google Scholar]
- Sonneborn D, et al. Prenatal polychlorinated biphenyl exposures in eastern Slovakia modify effects of social factors on birthweight. Paediatr Perinat Epidemiol. 2008;22(3):202–213. doi: 10.1111/j.1365-3016.2008.00929.x. [DOI] [PubMed] [Google Scholar]
- Tang-Peronard JL, et al. Endocrine-disrupting chemicals and obesity development in humans: a review. Obes Rev. 2011;12(8):622–636. doi: 10.1111/j.1467-789X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- Tang-Peronard JL, et al. Association between prenatal polychlorinated biphenyl exposure and obesity development at ages 5 and 7 y; a prospective cohort study of 656 children from the Faroe Islands. Am J Clin Nutr. 2014;99(1):5–13. doi: 10.3945/ajcn.113.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Rojo MM, et al. Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy. Am J Epidemiol. 2004;160(7):668–678. doi: 10.1093/aje/kwh271. [DOI] [PubMed] [Google Scholar]
- Valvi D, et al. Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study. Environ Health Perspect. 2012;120(3):451–457. doi: 10.1289/ehp.1103862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen T, et al. Birth weight and sex of children and the correlation to the body burden of PCDDs/PCDFs and PCBs of the mother. Environ Health Perspect. 1998;106(2):61–66. doi: 10.1289/ehp.9810661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst SL, et al. Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life. Environ Health Perspect. 2009;117(1):122–126. doi: 10.1289/ehp.0800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtyniak BJ, et al. Association of maternal serum concentrations of 2,2′, 4,4′5,5′-hexachlorobiphenyl (CB-153) and 1,1-dichloro-2,2-bis (p-chlorophenyl)-ethylene (p,p′-DDE) levels with birth weight, gestational age and preterm births in Inuit and European populations. Environ Health. 2010;9:56. doi: 10.1186/1476-069X-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, et al. Prenatal pesticide and PCB exposures and birth outcomes. Pediatr Res. 2007;61(2):243–250. doi: 10.1203/pdr.0b013e31802d77f0. [DOI] [PubMed] [Google Scholar]
- Xie X, et al. The effects of low-level prenatal lead exposure on birth outcomes. Environ Pollut. 2013;175:30–34. doi: 10.1016/j.envpol.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Yamashita F, Hayashi M. Fetal PCB syndrome: clinical features, intrauterine growth retardation and possible alteration in calcium metabolism. Environ Health Perspect. 1985;59:41–45. doi: 10.1289/ehp.59-1568075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, et al. Effects of lead and cadmium exposure from electronic waste on child physical growth. Environ Sci Pollut Res Int. 2013;20(7):4441–4447. doi: 10.1007/s11356-012-1366-2. [DOI] [PubMed] [Google Scholar]
- Zhu M, et al. Maternal low-level lead exposure and fetal growth. Environ Health Perspect. 2010;118(10):1471–1475. doi: 10.1289/ehp.0901561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT. Polychlorinated biphenyls as disruptors of thyroid hormone action. In: Fisher LJ, Hansen L, editors. Recents advances in the environmental toxicology and health effects of PCBs. Lexington, KY: University of Kentucky Press; 2001. pp. 265–272. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.