Abstract
Background
It is often claimed that non-nutritive sweeteners (NNS) are ‘sweeter than sugar’, with the implicit implication high potency sweeteners are super-normal stimuli that encourage exaggerated responses. This study aimed to investigate the perceived sweetness intensity of a variety of nutritive (Sucrose, Maple Syrup, and Agave Nectar) and NNS (Acesulfame-K (AceK), Rebaudioside A (RebA), Aspartame, and Sucralose) in a large cohort of untrained participants using contemporary psychophysical methods.
Methods
Participants (n=401 total) rated the intensity of sweet, bitter, and metallic sensations for nutritive and NNS in water using the general labeled magnitude scale (gLMS).
Results
Sigmoidal Dose-Response functions were observed for all stimuli except AceK. That is, sucrose follows a sigmoidal function if the data are not artifactually linearized via prior training. More critically, there is no evidence that NNS have a maximal sweetness (intensity) greater than sucrose; indeed, the maximal sweetness for AceK, RebA and Sucralose were significantly lower than for concentrated sucrose. For these sweeteners, mixture suppression due to endogenous dose-dependent bitter or metallic sensations appears to limit maximal perceived sweetness.
Conclusions
In terms of perceived sweetness, non-nutritive sweeteners cannot be considered super-normal stimuli. These data do not support the view that non-nutritive sweeteners hijack or over-stimulate sweet receptors to product elevated sweet sensations.
Keywords: Sweetness, perception, psychophysics, potency, activity
Introduction
Evolutionarily, sweet taste has enabled humans to make qualitative judgments about the energy density and nutritional quality of their food. Although sweet taste is no longer fundamentally needed for survival, sweet sensations are innately pleasurable across the lifespan 1. Non-nutritive sweeteners (NNS) have been utilized since the late 1800’s 2 as alternatives to evoke the desired sweetness in food products without the calories associated with mono- and disaccharides. More recently, consumption of NNS by both adults and children has increased greatly, as NNS are now used by 28% to 85% of the American population 3, 4.
Despite increasing use and renewed research interest in non-nutritive sweeteners, there is no standardized nomenclature for these compounds. The terms artificial, alternative, synthetic, low calorie, non-caloric, sugar-substitute, hyper-intense, high-intensity, and high-potency have all been used roughly synonymously (e.g. 5) to describe NNS despite implicit differences in meaning. We choose to use NNS as a blanket term largely through a process of exclusion. For example, Rebaudioside A (RebA), and monkfruit are plant extracts, therefore the terms artificial and synthetic are not appropriate; aspartame is metabolized while sucralose is not, therefore non-caloric and low calorie lack precision.
Critically, the term ‘high-intensity’ has been repeatedly misinterpreted in the scientific literature and popular press to imply that non-nutritive sweeteners are “sweeter than sugar” 6–9. However, there is no evidence to suggest that NNS are sweeter than natural carbohydrate sweeteners like sucrose. NNS are generally high-potency sweeteners, but potency is not synonymous with intensity, and the critical distinction between the two terms has strong implications for public health and health policy. Specifically, referring to NNS as high-intensity sweeteners suggests they are some sort of super-normal stimulus. Originally described by Tinbergen, super-normal stimuli are exaggerated stimuli that evoke behavioral responses more effectively than the stimulus for which the response evolved 10. Describing NNS as high-intensity sweeteners rather than high-potency sweeteners implies NNS evoke a sweetness response greater than natural sugars like sucrose. Although it is often claimed NNS over stimulate sweet taste receptors 11–13, we fail to find evidence that NNS act as supernormal stimuli.
The confusion between high-potency and high-intensity is understandable, given common marketing claims like “sucralose is 600 times sweeter than sugar”14 that are often repeated uncritically in the scientific literature. While strictly correct in one sense, this phrasing is also a gross over simplification that is misleading. It may be helpful to recall the critical distinction between potency and activity in pharmacology. Specifically, because NNS have high pharmacological potency with respect to receptor activation, they have very low psychophysical detection thresholds compared to bulk carbohydrate sweeteners. It takes a very small amount of these compounds to activate a receptor and elicit a sensation; accordingly, a metabolized compound like aspartame is able to provide sweetness without contributing a nutritionally meaningful amount of calories to the diet. With regard to their detection threshold, NNS are ‘sweeter’ than disaccharides like sucrose on a weight-to-weight basis. Nonetheless, this does not imply high potency sweeteners are high-intensity stimuli. That is, the psychophysical intensity (i.e. the quantitative magnitude of a given sensation) is roughly equivalent to the pharmacological concept of activity. The important distinction between potency and activity can be illustrated with the opioids buprenorphine and morphine: buprenorphine has much greater potency than morphine, but the activity of buprenorphine is much less than morphine. Likewise, a sweetener may have a low detection threshold without being intensely sweet. Indeed, it is well known in the food industry that NNS may have low maximal sweetness (e.g. AceK, Saccharin) 15, which limits their utility. DuBois and colleagues 16 demonstrated this in a dose-response study for numerous nutritive and non-nutritive sweeteners using a small trained panel (n=18). They found sweetness functions for NNS were hyperbolic, as perceived sweetness hit a ceiling and did not increase further as concentration increased. Conversely, monosaccharides, disaccharides, and sugar alcohols showed a linear dose-response (D-R) function in their study, although they noted that this linearity was an artifact of the panelist training 16. Given that sweetness is primarily a G protein-coupled receptor (GPCR) mediated phenomenon 17, we would expect a sigmoidal function if other intensity scaling methods were used.
In terms of measuring sweetness perception, magnitude estimation has been commonly used to collect intensity data in relation to sweet taste stimuli 18–20. Because individuals do not have the same sensory experiences (e.g. 21), variation in magnitude estimation data may arise from either true perceptual differences or from differences in how the participant uses numbers. The general Labeled Magnitude Scale (gLMS) reduces this problem because it encourages participants to rate outside of the context of taste stimuli with the top anchor being strongest sensation of any kind 22. Surprisingly, relatively few studies have utilized the gLMS to quantify sweetness intensity perception (e.g. 23–25), despite the ability of the gLMS to generate putative ratio level data and allow more valid across group comparisons 22.
Here, we sought to re-examine and characterize the sweetness intensity dose-response (D–R) functions of NNS (sucralose, AceK, RebA, aspartame) and ‘natural’ caloric sweeteners (sucrose, maple syrup, agave nectar) using modern psychophysical techniques. The present study had two specific goals. First, we revisit the question of whether NNS are able to elicit greater (more intense) sweet sensations than sucrose. Second, we provide new D-R function estimates determined from a large cohort of untrained participants.
2. Methods
2.1 Overview of methods
The purpose of these series of experiments was to investigate the perceived sweetness of NNS (aspartame, AceK, RebA, and sucralose) and nutritive sweeteners (sucrose, agave nectar, and maple syrup). Data were collected in 4 experiments conducted on separate days and pooled, treating observations from each participant as independent measures. For each experiment, the same orientation procedures and testing methods were used. Sucrose concentrations remained constant across experiments to enable comparisons across days. In the fourth experiment, sucrose and aspartame were retested with two additional concentrations to better characterize the D-R functions. Compusense five software, version 5.2 (Guelph, Ontario, Canada) was used for data collection. Presentation order of samples was counterbalanced using a Williams design. All tests were completed at the Sensory Evaluation Center in the Department of Food Science at the Pennsylvania State University. Participants were provided with an explanation of the experiment in a brief orientation prior to testing in isolated testing booths. The orientation consisted of an overview of the gLMS with a warm-up using both imagined sensations (e.g. 26), and presentation of prototypical exemplars of sweet, bitter and metallic stimuli 27. This orientation differs substantially from classical ‘trained panel’ approaches like Quantitative Descriptive Analysis or Spectrum Descriptive Analysis that use small numbers of participants and require tens or hundreds of hours of training to calibrate panelists to attributes and scale usage (28, 29).
2.2 Participants
Reportedly healthy individuals (n=401) were recruited from the Pennsylvania State University campus and surrounding area (State College, PA) via email for their willingness to participate in a taste study. Participants were prescreened for eligibility. Eligibility criteria included: between 18–64 years old; not pregnant or breastfeeding; no known defects of smell or taste; no lip, cheek, or tongue piercings; nonsmoker (had not smoked in last 30 days); no food allergies or sensitivities; no history of choking or difficulty swallowing. Participants were also required to provide 30–35 min of their time for the experiment. A new group of participants were recruited for each experiment described below from a database containing 1200+ individuals; however, due to limitations of our recruitment system, we did not actively exclude those who had participated in a prior experiment, so a small fraction may have participated in more than one study. Retained data are fully anonymized, so we are unable to estimate this proportion. Participants provided informed consent and were paid for their time. All procedures were approved by the Pennsylvania State University Institutional Review Board (protocol number # 33164).
2.3 Psychophysical scaling
A generalized labeled magnitude scale (gLMS) 30, 31 was used to measure the perceived intensities of sweetness, bitterness, and metallic sensation 32 for all stimuli. The gLMS ranges from 0 (no sensation), 1.4 (barely detectable), 6 (weak), 17 (moderate), 35 (strong), 51 (very strong) and 100 (strongest imaginable sensation of any kind). Data were collected using Compusense five software. Prior to rating test stimuli, all participants partook in a brief warm-up to familiarize the participants with the gLMS. The warm-up required participants to make overall intensity ratings for 15 imagined and/or remembered sensations that include oral and non-oral sensations 26, 33. Generalizing the scale outside an oral context allows for more valid comparisons across individuals. More pragmatically, the gLMS also provides two other advantages over magnitude estimation: the gLMS does not require the same degree of numeracy on the part of participants, and gLMS data does not require the same extensive post collection manipulation required by magnitude estimation data.
2.3. Stimuli
2.3.1 Taste Stimuli
All stimuli were presented in 10mL aliquots in 30mL medicine cups at room temperature. Solutions were prepared at least 24 hours prior to testing using reverse osmosis (RO) water and were stored at refrigerated temperature for a maximum of five days. Concentration ranges for sweeteners were determined from results of bench top testing.
2.3.2 Orientation Stimuli
The orientation exemplars were 10mL 292mM sucrose (sweet), 0.032mM quinine monohydrocholoride dehydrate (bitter), a 292mM sucrose/0.032mM quinine mixture (sweet and bitter), and 1.7984 mM ferrus sulfate (metallic) solutions, as used by 27. Participants were told that they ‘may or may not experience all sensations from the orientation samples during the session in the booth’ and that ‘they may receive samples that have more than one taste quality’. Participants were also instructed to avoid rating how much they ‘liked’ or ‘disliked’ samples and separate intensity from hedonic affect (liking).
2.3.3 Dose-Response for Non-Nutritive Sweeteners (Experiments 1,2 & 4)
Participants rinsed with room temperature reverse osmosis (RO) water before and between each sample. Participants were provided with 45s to rinse before the next sample. Five sucrose concentrations were used as constant stimuli across all sessions. In experiment 1, 102 participants rated the sweetness, bitterness, and metallic intensity for 5 sucrose solutions (109.5, 219.1, 303.8, 409.0, and 818.0 mM), 5 aspartame solutions (0.23, 0.70, 1.0, 1.83, and 1.35 mM), and 5 rebaudioside A solutions (0.04, 0.25, 0.52, 1.03, and 1.55 mM). In experiment 2, 91 participants made the same attribute ratings for 5 Sucrose solutions (as above), 5 acesulfame K solutions (1.57, 6.26, 24.90, 99.15, and 394.71 mM), and 5 sucralose solutions (0.20, 0.80, 3.17, 12.6 and 50.18 mM). In experiment 4, 114 participants made sweet, bitter and metallic ratings for 5 sucrose solutions (as above) and 7 aspartame solutions (0.23, 0.70, 1.0, 1.83, 1.35, 6.79, and 9.0 mM).
2.3.4 Dose-Response for Nutritive Sweeteners (Experiment 3)
In experiment 3, participants made ratings for 5 sucrose solutions, 5 maple syrup (Great Value, Bentonville, AR) solutions (37.5, 75, 104, 140, and 280 g/L), and 5 light agave nectar (Madhava, Longmont CA) solutions (37.5, 75, 104, 140, and 280 g/L). In experiment 3 participants wore nose clips to minimize any influence of volatiles on perceived sweetness (e.g. 34–36). Nutritive sweeteners were measured on a weight-to-volume basis (g/L) as they contain a variety of sugars and other components 37, 38.
The sweetener concentrations used in Experiments 1–4 are summarized in Supplemental Table 1.
2.4. Procedure
Participants received instructions, brief instruction on the gLMS, and taste exemplars in a waiting room. After this orientation, the participants entered isolated computerized testing booths. Once in the booths, participants completed a scaling warm-up procedure on the computer, rating imagined or remembered sensations (e.g. 26). Following the scale warm-up, participants received a tray of 15 samples (Test 1, 2 and 3) or 12 samples (Test 4). Participants were instructed to put the entire sample in their mouth, swish for 5s to obtain total mouth coating, and spit the sample out. Participants then waited 10s to allow the sensation to peak before making intensity ratings; 45s breaks between samples were enforced via software to minimize potential carry over and lingering. Ad libitum RO rinse water was also provided.
2.5. Statistical Analysis
Dose-response functions were fit using GraphPad Prism 5.0C for OSX (GraphPad Software, San Diego CA). Descriptive and inferential statistics were calculated using SPSS statistical software. Because sweetness perception is a receptor-mediated process 17, D-R functions for Sucrose, Aspartame, RebA, Sucralose, Maple Syrup and Agave Nectar were fit a priori using the Hill Equation:
where R is the mean response (perceived intensity) across participants, and C is the stimulus concentration. In this model, Rmax is top of the curve, Rmin is the bottom of the curve, the point halfway between min and max is EC50, and the slope of the linear portion of the model is the HillSlope. The min value was constrained to zero, leaving three parameters free. Separate functions were fit for sweet, bitter and metallic ratings obtained on the gLMS. The dose response for AceK could not be fit using this model, so a second-degree polynomial function was used instead:
where R is the mean intensity and C is concentration.
3. Results
3.1 Dose-Response functions for nutritive sweeteners are not linear
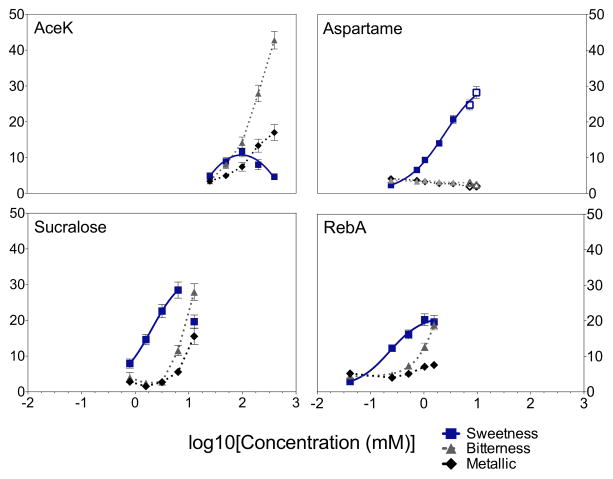
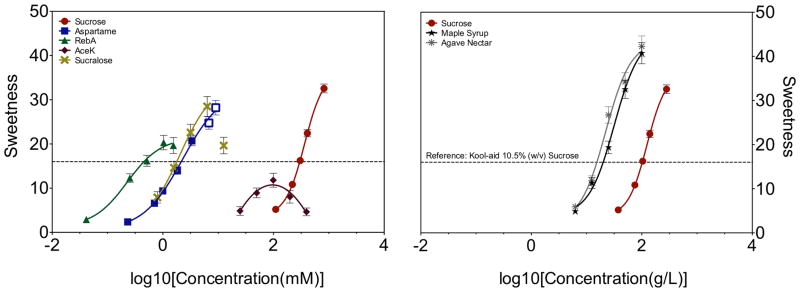
Mean dose-response functions for caloric and non-nutritive sweeteners in a large number of participants are shown in Figure 1. As expected, all four non-nutritive sweeteners are left shifted compared to sucrose, indicating they have higher potency. Notably, both caloric and non-nutritive sweeteners are well described by a four parameter logistic equation, with the exception of AceK. Present data partially conflict with prior reports: in 18 highly trained assessors, sugars and sugar alcohols showed linear dose-response functions while high potency sweeteners were best described by Hill-type models 16.
Figure 1.
3.2 Sugars have higher maximal sweetness than non-nutritive sweeteners
As shown in Figure 1 and Table 1, the caloric sweeteners (sucrose, agave nectar, and maple syrup) all had higher Rmax values (estimated maximal sweetness) than the non-nutritive sweeteners, indicating NNS are not supernormal stimuli as compared to sucrose. To corroborate this, we also used the maximal observed sweetness for each NNS (Supplemental Table 3) rather than the estimated Rmax values in Table 1, testing for differences compared to sucrose. The sweetness of RebA was significantly lower than that of sucrose [t(513)= 5.94; p<0.0001]. Likewise, the maximal observed sweetness for aspartame was lower than that of sucrose [t(501)= 2.03; p<0.043]. Finally, the maximal observed sweetness for sucralose tended to be lower than sucrose [t(490)= 1.73; p = 0.08] in a two tailed test. Collectively, there was no evidence to suggest NNS were sweeter than sucrose.
Table 1.
Hill Equation Dose-Response Parameters for Sucrose, Agave Nectar, Maple Syrup, Aspartame, RebA, and Sucralose.
| Sweetener | Rmax | SE | Hill Slope | SE | LogEC50 | SE | EC50 | R2 |
|---|---|---|---|---|---|---|---|---|
| Maple Syrup | 50.7 | 7.01 | 1.32 | 0.24 | 1.53 | 0.12 | 33.86 | 0.40 |
| Agave Nectar | 45.4 | 3.50 | 1.60 | 0.26 | 1.34 | 0.06 | 21.9 | 0.39 |
| Sucrose | 42.8 | 3.90 | 1.65 | 0.19 | 2.60 | 0.06 | 400.6 | 0.30 |
| Aspartame | 33.4 | 3.22 | 1.15 | 0.14 | 0.37 | 0.09 | 2.33 | 0.34 |
| Sucralose | 34.6 | 7.87 | 1.34 | 0.48 | 0.30 | 0.19 | 1.99 | 0.18 |
| RebA | 22.2 | 2.54 | 1.19 | 0.33 | 0.67 | 0.13 | 0.21 | 0.17 |
All values represent means. EC50 is half of the concentration needed to evoke the maximal perceived sweetness rating. Rmax and SE estimated from the fitted equations were tested via one-way ANOVA using summary statistics with appropriate n’s, assuming n of 102 for aspartame. The Rmax values were significantly different (p<.05) across sweeteners.
The mechanism by which many NNS are unable to show the same efficacy (maximal intensity) as the caloric sweeteners is unknown, but it may be partially due to mixture suppression of sweetness by bitter 39 or metallic sensations. Figure 2 shows the mean D-R functions for sweetness and bitterness. For AceK, sucralose, and RebA, bitterness increases with concentration, eventually equaling or surpassing perceived sweetness. In contrast, Figure 2 shows that Aspartame has a similar function to that of sucrose, maple syrup, and agave nectar (see Supplemental Figure 1) bitterness is minimal and does not increase with concentration.
Figure 2.
4. Discussion
Present data make several important contributions to extant literature. First, these data indicate non-nutritive sweeteners are not super-normal stimuli. That is, they do not evoke sweet sensations that are more intense than sucrose. Second, we find that carbohydrate sweeteners exhibit sigmoid concentration-response functions, as would be expected given this is a receptor-mediated process, and not linear functions as reported previously.
Non-nutritive sweeteners are not super-normal stimuli. Although NNS have low psychophysical detection thresholds compared to sugars, it is not valid to use thresholds or the dose over threshold to estimate the perceived intensity of these sweeteners 36, 40, 41. Indeed, in 1948, Lichtenstein and colleagues noted that comparing thresholds provides invalid information concerning the relative sweetness of sweet stimuli above threshold levels 42. The dose-response functions obtained here indicate non-nutritive sweeteners are more potent but have lower activity than sucrose, maple syrup, and agave nectar, even near maximal concentrations. The lack of activity in the perceived sweetness intensity of AceK, sucralose, and RebA is likely a function of increasing bitterness with concentration. Bitterness is a side taste that is associated with many NNS including RebA and AceK 43, 44. In contrast to prior reports which indicate sucralose has minimal bitterness 44, we find clear evidence that sucralose is bitter, consistent with unpublished data showing sucralose can activate bitter receptors (hT2Rs) in vitro. Notably, the bitterness of sucralose and AceK are sufficiently intense to depress sweetness ratings. That is, endogenous bitterness not only provides a ceiling on maximal sweetness, but can actually reverse the slope of the function at the highest concentrations. Similar effects have been shown recently for the stevia glycoside Rubusoside 45. Here, the D-R function for RebA is suggestive of this pattern: accordingly, we would expect a similar reversal if higher concentrations were used. In contrast, aspartame lacks any bitterness, showing a pattern like sucrose, maple syrup, and agave nectar. The absence of bitterness in aspartame is well documented in the literature as well as its similar taste qualities to sucrose 46–48.
Furthermore, the nutritive sweeteners sucrose, maple syrup, and agave nectar follow sigmoidal, not linear, functions. The literature disagrees whether nutritive sweeteners like saccharides and sugar alcohols follow linear functions in which intensity increases as a function of concentration 16, 49–52 or whether sugars diverge from linearity 20. Present data support the existence of sigmoidal dose-response functions, consistent with the underlying biology 17.
The previously reported linearity of the sucrose D-R function is likely a result of extensive panel training with reference samples 16 or the choice of regression model. To prevent artifactual linearization in our data, we avoided extensive panel training (e.g. Sensory Spectrum® universal scaling) by using the gLMS. It is typically claimed that the gLMS generates ratio level comparable to magnitude estimation, although it should be noted this assumption is based on a limited number of studies. Nonetheless, present data show that using the gLMS enables successful sweetener differentiation. Also, these data suggest the gLMS can be used to efficiently generate dose-response functions in a large number of naïve participants, precluding the need for labor intensive forced choice methods (e.g. 53) or use of highly trained assessors (e.g. 16).
5. Conclusion
Our data indicate that NNS are not super-normal stimuli with regard to perceived sweetness intensity. That is, although NNS may have greater binding affinity to sweet receptors, this does not imply NNS over stimulate sweet receptors as has been implied previously 11, 13. We also show nutritive sweeteners (sucrose, maple syrup, and agave nectar) do not follow linear dose-response functions as previously described in the literature; instead they follow the sigmoidal dose response function one would expect from receptor dependent phenomenon. Present data also clarify the bitter and metallic functions of the NNS as a function of concentration, although we must point out that the use of such high concentrations of NNS in commercial applications would be unlikely. Also, we should note present stimuli were presented in a simple aqueous model system; whether they might behave differently in mixtures with each other or in real foods remains to be tested. Nonetheless, AceK, sucralose, and RebA are not ‘sweeter than sugar’ in that they do not surpass the perceived sweetness intensities of natural sweeteners like sucrose, maple syrup, and agave nectar. Finally, we do not take a broader position on the safety of NNS (cf 54 and 55), the nutritional consequences of non-nutritive sweetener intake (e.g. the energy signaling/decoupling hypothesis 5), or the role of extraoral taste receptors (e.g. 56). Additional randomized trials, as opposed to observational studies (cf. 57 and 58), are needed to determine the utility of NSS in caloric reduction for weigh loss or weight maintenance. Nonetheless, present data suggest that NNS do not result in deleterious health effects by over-stimulating sweet taste receptors to produce hyper-intense sweet sensations.
Supplementary Material
Acknowledgments
The authors thank Alissa L. Allen, MS and Emma L. Feeney, PhD for assistance with protocol development and testing, and Rachel Primrose, BS for participant scheduling, and assistance in the Sensory Evaluation Center. We also thank our study participants for their time and participation. Funding was provided by the Pennsylvania State University and a National Institutes of Health grant from the National Institute National of Deafness and Communication Disorders [DC010904] to JEH.
Footnotes
Conflict of Interest Disclosure
RGA declares no potential conflict of interest. JEH has previously accepted speaking and consulting fees from Tate & Lyle PLC, Symrise AG, PepisCo, and General Mills, Inc. for unrelated projects. He also serves on the Scientific Advisory Board of Medifast, Inc. His laboratory also conducts routine taste tests for the food industry to facilitate practical student training. None of these organizations have had any influence over study conception, design or interpretation, or the decision to publish these data.
Supplementary information is available at International Journal of Obesity’s website.
References
- 1.Beauchamp GK, Mennella JA. Flavor perception in human infants: development and functional significance. Digestion. 2011;83 (Suppl 1):1–6. doi: 10.1159/000323397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Research Council (U.S.). Food Protection Committee. . The safety of artificial sweeteners for use in foods, a report. Washington: 1955. [Google Scholar]
- 3.Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89(1):1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sylvetsky AC, Welsh JA, Brown RJ, Vos MB. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96(3):640–6. doi: 10.3945/ajcn.112.034751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swithers SE. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends in Endocrinology & Metabolism. 2013;24(9):431–441. doi: 10.1016/j.tem.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao L, Tepper BJ. Perception and acceptance of selected high-intensity sweeteners and blends in model soft drinks by propylthiouracil (PROP) non-tasters and super-tasters. Food Quality and Preference. 2007;18(3):531–540. [Google Scholar]
- 7.Hutchinson SA, Ho GS, Ho CT. Stability and degradation of the high-intensity sweeteners: Aspartame, alitame, and sucralose. Food Reviews International. 1999;15(2):249–261. [Google Scholar]
- 8.Shankar P, Ahuja S, Sriram K. Non-nutritive sweeteners: Review and update. Nutrition. 2013;29(11–12):1293–9. doi: 10.1016/j.nut.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Pereira MA, Odegaard AO. Artificially Sweetened Beverages-Do They Influence Cardiometabolic Risk? Current atherosclerosis reports. 2013;15(12):1–6. doi: 10.1007/s11883-013-0375-z. [DOI] [PubMed] [Google Scholar]
- 10.Tinbergen N, Perdeck AC. On the stimulus situation releasing the begging response in the newly hatched herring gull chick (Larus argentatus argentatus Pont.) Behaviour. 1950:1–39. [Google Scholar]
- 11.Ludwig DS. Artificially sweetened beverages: cause for concern. JAMA. 2009;302(22):2477–8. doi: 10.1001/jama.2009.1822. [DOI] [PubMed] [Google Scholar]
- 12.Bloomgarden ZT. Nonnutritive sweeteners, fructose, and other aspects of diet. Diabetes Care. 2011;34(5):e46–51. doi: 10.2337/dc11-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alpert B, Brooke Alpert M, Farris P. The Sugar Detox: Lose Weight, Feel Great, and Look Years Younger. Da Capo Press; 2013. [Google Scholar]
- 14.McNeil Nutritionals L. Splenda Products FAQs. Fort Washington, PA: 2013. [Google Scholar]
- 15.Paulus K, Braun M. Sweetening Capacity and Taste Profile of Sweeteners. Ernahrungs-Umschau. 1988;35(11):384. [Google Scholar]
- 16.DuBois Grant E, Walters DE, Schiffman Susan S, Warwick Zoe S, Booth Barbara J, Pecore Suzanne D, et al. Sweeteners. Vol. 450. American Chemical Society; 1991. Concentration-Response Relationships of Sweeteners; pp. 261–276. [Google Scholar]
- 17.Vigues S, Dotson C, Munger S. Chemosensory Systems in Mammals, Fishes, and Insects. Springer; 2009. The receptor basis of sweet taste in mammals; pp. 20–23. [DOI] [PubMed] [Google Scholar]
- 18.Cadena RS, Bolini HMA. Ideal and relative sweetness of high intensity sweeteners in mango nectar. International Journal of Food Science & Technology. 2012;47(5):991–996. [Google Scholar]
- 19.Souza VR, Pereira PA, Pinheiro ACM, Bolini H, Borges SV, Queiroz F. Analysis of various sweeteners in low-sugar mixed fruit jam: equivalent sweetness, time-intensity analysis and acceptance test. International Journal of Food Science & Technology. 2013 [Google Scholar]
- 20.Moskowitz HR. The sweetness and pleasantness of sugars. The American journal of psychology. 1971:387–405. [PubMed] [Google Scholar]
- 21.Snyder DJ, Prescott J, Bartoshuk LM. Modern psychophysics and the assessment of human oral sensation. Adv Otorhinolaryngol. 2006;63:221–41. doi: 10.1159/000093762. [DOI] [PubMed] [Google Scholar]
- 22.Snyder D, Fast K. Valid comparisons of suprathreshold sensations. Journal of Consciousness Studies. 2004;11(7–8):7–8. [Google Scholar]
- 23.Sartor F, Donaldson LF, Markland DA, Loveday H, Jackson MJ, Kubis HP. Taste perception and implicit attitude toward sweet index and soft drink supplementation. Appetite. 2011;57(1):237–246. doi: 10.1016/j.appet.2011.05.107. [DOI] [PubMed] [Google Scholar]
- 24.Thai PK, Tan EC, Tan WL, Tey TH, Kaur H, Say YH. Sweetness intensity perception and pleasantness ratings of sucrose, aspartame solutions and cola among multi-ethnic Malaysian subjects. Food Quality and Preference. 2011;22(3):281–289. [Google Scholar]
- 25.Green BG, Lim J, Osterhoff F, Blacher K, Nachtigal D. Taste mixture interactions: suppression, additivity, and the predominance of sweetness. Physiol Behav. 2010;101(5):731–7. doi: 10.1016/j.physbeh.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes JE, Allen AL, Bennett SM. Direct comparison of the generalized Visual Analog Scale (gVAS) and general Labeled Magnitude Scale (gLMS) Food Qual Prefer. 2013;28(1):36–44. doi: 10.1016/j.foodqual.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamerud JK, Delwiche JF. Individual differences in perceived bitterness predict liking of sweeteners. Chem Senses. 2007;32(9):803–10. doi: 10.1093/chemse/bjm050. [DOI] [PubMed] [Google Scholar]
- 28.Murray JM, Delahunty CM, Baxter IA. Descriptive sensory analysis: past, present and future. Food Res Int. 2001;34(6):461–471. [Google Scholar]
- 29.Hootman RC, editor. Manual on descriptive analysis testing for sensory evaluation. ASTM; Philadelphia, PA: 1992. [Google Scholar]
- 30.Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993;18(6):683–702. [Google Scholar]
- 31.Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ. Labeled scales (eg, category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Quality and Preference. 2003;14(2):125–138. [Google Scholar]
- 32.Riera CE, Vogel H, Simon SA, le Coutre J. Artificial sweeteners and salts producing a metallic taste sensation activate TRPV1 receptors. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2007;293(2):R626–R634. doi: 10.1152/ajpregu.00286.2007. [DOI] [PubMed] [Google Scholar]
- 33.Allen AL, McGeary JE, Knopik VS, Hayes JE. Bitterness of the non-nutritive sweetener acesulfame potassium varies with polymorphisms in TAS2R9 and TAS2R31. Chem Senses. 2013;38(5):379–89. doi: 10.1093/chemse/bjt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank RA, Ducheny K, Mize SJS. Strawberry Odor, but Not Red Color, Enhances the Sweetness of Sucrose Solutions. Chem Senses. 1989;14(3):371–377. [Google Scholar]
- 35.Schifferstein HN, Verlegh PW. The role of congruency and pleasantness in odor-induced taste enhancement. Acta Psychol (Amst) 1996;94(1):87–105. doi: 10.1016/0001-6918(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 36.Bartoshuk LM, Klee HJ. Better Fruits and Vegetables through Sensory Analysis. Current Biology. 2013;23(9):R374–R378. doi: 10.1016/j.cub.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 37.Taga A, Kodama S. Analysis of Reducing Carbohydrates and Fructosyl Saccharides in Maple Syrup and Maple Sugar by CE. Chromatographia. 2012;75(17–18):1009–1016. [Google Scholar]
- 38.Willems JL, Low NH. Major carbohydrate, polyol, and oligosaccharide profiles of agave syrup. Application of this data to authenticity analysis. J Agric Food Chem. 2012;60(35):8745–54. doi: 10.1021/jf3027342. [DOI] [PubMed] [Google Scholar]
- 39.Lawless HT. Evidence for neural inhibition in bittersweet taste mixtures. J Comp Physiol Psychol. 1979;93(3):538. doi: 10.1037/h0077582. [DOI] [PubMed] [Google Scholar]
- 40.Frijters JER. Critical Analysis of Odor Unit Number and Its Use. Chem Sens Flav. 1978;3(2):227–233. [Google Scholar]
- 41.Hayes JE. Transdisciplinary Perspectives on Sweetness. Chemosensory Perception. 2008;1(1):48–57. [Google Scholar]
- 42.Lichtenstein P. The relative sweetness of sugars: sucrose and dextrose. Journal of Experimental Psychology. 1948;38(5):578. doi: 10.1037/h0058760. [DOI] [PubMed] [Google Scholar]
- 43.Allen AL, McGeary JE, Hayes JE. Rebaudioside A and Rebaudioside D Bitterness do not Covary with Acesulfame-K Bitterness or Polymorphisms in TAS2R9 and TAS2R31. Chemosensory Perception. 2013;6(3):109–117. doi: 10.1007/s12078-013-9149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiffman SS, Booth BJ, Losee ML, Pecore SD, Warwick ZS. Bitterness of sweeteners as a function of concentration. Brain Res Bull. 1995;36(5):505–13. doi: 10.1016/0361-9230(94)00225-p. [DOI] [PubMed] [Google Scholar]
- 45.Hellfritsch C, Brockhoff A, Stähler F, Meyerhof W, Hofmann T. Human psychometric and taste receptor responses to steviol glycosides. J Agric Food Chem. 2012;60(27):6782–6793. doi: 10.1021/jf301297n. [DOI] [PubMed] [Google Scholar]
- 46.Schiffman SS, Booth BJ, Losee ML, Pecore SD, Warwick ZS. Bitterness of sweeteners as a function of concentration. Brain Res Bull. 1995;36(5):505–513. doi: 10.1016/0361-9230(94)00225-p. [DOI] [PubMed] [Google Scholar]
- 47.Larsonpowers N, Pangborn RM. Paired Comparison and Time-Intensity Measurements of Sensory Properties of Beverages and Gelatins Containing Sucrose or Synthetic Sweeteners. Journal of Food Science. 1978;43(1):41–46. [Google Scholar]
- 48.Mazur RH. Discovery of aspartame. Aspartame: Physiology and biochemistry. 1984:3–9. [Google Scholar]
- 49.Fujimaru T, Park JH, Lim J. Sensory Characteristics and Relative Sweetness of Tagatose and Other Sweeteners. Journal of Food Science. 2012;77(9):S323–S328. doi: 10.1111/j.1750-3841.2012.02844.x. [DOI] [PubMed] [Google Scholar]
- 50.Stone H, Oliver SM. Measurement of the relative sweetness of selected sweeteners and sweetener mixtures. Journal of Food Science. 1969;34(2):215–222. [Google Scholar]
- 51.McBride RL. Category scales of sweetness are consistent with sweetness-matching data. Percept Psychophys. 1983;34(2):175–179. doi: 10.3758/bf03211345. [DOI] [PubMed] [Google Scholar]
- 52.MacLeod S. A construction and attempted validation of sensory sweetness scales. Journal of Experimental Psychology. 1952;44(5):316. doi: 10.1037/h0057860. [DOI] [PubMed] [Google Scholar]
- 53.Fry JC, Yurttas N, Biermann KL, Lindley MG, Goulson MJ. The Sweetness Concentration-Response of R,R-Monatin, a Naturally Occurring High-Potency Sweetener. J Food Sci. 2012;77(10):S362–S364. doi: 10.1111/j.1750-3841.2012.02885.x. [DOI] [PubMed] [Google Scholar]
- 54.Fitch C, Keim KS. Position of the academy of nutrition and dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112(5):739–758. doi: 10.1016/j.jand.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Schiffman SS. Rationale for Further Medical and Health Research on High-Potency Sweeteners. Chem Senses. 2012;37(8):671–679. doi: 10.1093/chemse/bjs053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dotson CD, Vigues S, Steinle NI, Munger SD. T1R and T2R receptors: the modulation of incretin hormones and potential targets for the treatment of type 2 diabetes mellitus. Current opinion in investigational drugs (London, England: 2000) 2010;11(4):447. [PMC free article] [PubMed] [Google Scholar]
- 57.Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16(8):1894–900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- 58.Peters JC, Wyatt HR, Foster GD, Pan Z, Wojtanowski AC, Vander Veur SS, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring) 2014;22(6):1415–21. doi: 10.1002/oby.20737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.