Abstract
The related neuropeptides oxytocin and vasopressin are involved in species-typical behavior, including social recognition behavior, maternal behavior, social bonding, communication, and aggression. A wealth of evidence from animal models demonstrates significant modulation of adult social behavior by both of these neuropeptides and their receptors. Over the last decade, there has been a flood of studies in humans also implicating a role for these neuropeptides in human social behavior. Despite popular assumptions that oxytocin is a molecule of social bonding in the infant brain, less mechanistic research emphasis has been placed on the potential role of these neuropeptides in the developmental emergence of the neural substrates of behavior. This review summarizes what is known and assumed about the developmental influence of these neuropeptides and outlines the important unanswered questions and testable hypotheses. There is tremendous translational need to understand the functions of these neuropeptides in mammalian experience-dependent development of the social brain. The activity of oxytocin and vasopressin during development should inform our understanding of individual, sex, and species differences in social behavior later in life.
INTRODUCTION
After a century of study, two tiny nine-amino-acid peptide hormones continue to capture the attention and fuel, the scientific curiosity of diverse fields from biology to sociology. Numerous disciplines have contributed to the copious studies indicating a role for oxytocin and vasopressin systems in adult social behavior (for detailed reviews, see Carter et al, 2008; Donaldson and Young, 2008; Insel, 2010; Gordon et al, 2011; Kavaliers and Choleris, 2011; Meyer-Lindenberg et al, 2011; Young et al, 2011; Albers, 2012; Bosch and Neumann, 2012; Ebstein et al, 2012; Wacker and Ludwig, 2012; Zink and Meyer-Lindenberg, 2012; Goodson, 2013). We know that manipulating these systems in adulthood alters brain and behavior, so why should we consider oxytocin and vasopressin in the developmental emergence of brain and behavior? Given the prevalence of developmental disorders and the contribution of early life experience to lasting outcomes, it is important to uncover the underlying mechanisms of experience-dependent development. What are the effector molecules of the early life social environment that lead to species-typical developmental trajectories for social behavior, such as preference for social contact with a conspecific, which requires developmental entrainment (Denenberg et al, 1964)? As a result of their prominent roles in adult social behavior, brain oxytocin and vasopressin systems appear as low hanging fruit in the garden of potential effector molecules underlying the developmental mechanisms of social behavior. Their experience-dependent activity during developmental sensitive periods likely contributes to individual, sex, and species differences in social behavior later in life.
There is a long history of studying oxytocin and vasopressin in social behavior in adult animals, which set the stage for exploring oxytocin and vasopressin in brain and social behavior development. Peter Klopfer (Klopfer and Klopfer, 1968; Klopfer, 1971) first proposed a role for oxytocin in the brain to modulate adult social behavior, specifically the transition to maternal behavior in goats. Testing that hypothesis, Pedersen and Prange (1979) showed in rats that, in an estrogen-dependent manner, oxytocin acting in the brain facilitated the transition to maternal behavior (pup grouping, licking, crouching, nest building, and pup retrieval). This was followed by a hypothesis that oxytocin acting in the brain in adults may facilitate the development of sociosexual bonds between adult partners (Witt et al, 1990). This was tested in prairie voles—a rodent species with the neural capacity to form a selective social bond in adulthood with another adult (eg, monogamy). Central injection of oxytocin facilitated the formation of a selective partner preference in the laboratory partner preference test. Vasopressin and oxytocin are evolutionary paralogs, so it is not surprising that vasopressin was subsequently tested in the prairie vole and also observed to facilitate selective partner preference behavior (Winslow et al, 1993), especially as vasopressin was already appreciated for its central role in flank marking in hamsters, which is a species-typical olfactory social communication behavior (Ferris et al, 1984). There is now an abundance of evidence that both oxytocin and vasopressin contribute to adult species-typical social behavior, including effects on human social behavior. As such examples of species-typical social behavior are influenced by both genetics (Lim et al, 2004; Hammock and Young, 2005) and early life experience (Denenberg et al, 1964; Pedersen and Boccia, 2002), a full understanding of the mechanisms of social behavior must include uncovering the genetic and neural circuit mechanisms of experience-dependent development.
The main tools used to investigate oxytocin and vasopressin systems in social behavior are pharmacological, genetic (viral vectors, transgenic and knock-out mice), biomarker, and gene association studies. Although pharmacological manipulation of neuropeptide systems in adult and developing animals can yield clues about how receptor activation changes behavior, knockout and gene association studies can tell us only if there is an association between gene and behavior: congenital knockout and gene association studies fail to distinguish between genetic contributions to developmental processes (‘organizational’ effects) and genetic contribution to adult function of the gene product itself (‘activational’ effects). Thus, the role of these systems in development must be directly probed and addressed separately from activational effects in adulthood. Pharmacological activation of receptors during development as well as temporally regulated genetic manipulation during development can both be used to answer developmental questions. So far, the neurobiological research on oxytocin, vasopressin, and development falls into two non-mutually exclusive categories: one asks if developmental events affect adult levels of oxytocin and vasopressin system function (eg, reviewed in Bales and Perkeybile, 2012; Veenema, 2012) and the other asks if oxytocin and vasopressin activity during sensitive periods shapes brain and behavior development (reviewed here and Bales and Perkeybile, 2012). Both kinds of questions are informative and lead to a greater understanding of the roles of oxytocin and vasopressin in the experience-dependent development of social behavior.
In the remainder of this review, I discuss brain oxytocin and vasopressin systems as mediators of experience-dependent development. First, I discuss the neuroanatomy of these systems during development. Then, I describe the effects of oxytocin and vasopressin on infant behavior and on adult behavior after neonatal manipulation of these systems. Next, I discuss data demonstrating short- and long-term effects of oxytocin and vasopressin on brain development. Then, I describe data that support a gene by environment development model of oxytocin and vasopressin systems. The main ideas and key points in this review are summarized in Table 1. I conclude the review with a synthesis of current findings and future directions. This review should arm the reader with a fresh perspective on the roles of oxytocin and vasopressin in species-typical experience-dependent development and provide a theoretical framework for the interpretation of surprising clinical and gene association studies in humans.
Table 1. Review Highlights.
| Oxytocin and vasopressin have significant roles in adult species-typical social behavior. |
| More direct research on oxytocin and vasopressin function in experience-dependent development is needed. |
| Vasopressin and oxytocin are available to the developing brain: vasopressin emerges before oxytocin and reaches higher levels in males than females. |
| Vasopressin receptors (V1aR) and oxytocin receptors (OXTR) show dynamic developmental profiles. Their distribution patterns in the brain depend on age and species, with quantitative levels modulated by gonadal hormones and experience. |
| Vasopressin and oxytocin both influence a primordial social behavior (neonatal familiar orienting bias), but in opposite directions. Oxytocin promotes, while vasopressin inhibits neonatal orienting bias. |
| Parental engagement activates the oxytocin system in the infant. |
| Vasopressin and oxytocin activity in the neonate impact behavior into adulthood, indicating a role for activity-dependent development. |
| Both oxytocin and vasopressin activity during development have long-term impacts on the brain. |
| If oxytocin and vasopressin mediate developmental experience, then genetic variation in system components should interact with the developmental environment to contribute to individual, sex, and species differences in social behavior. |
| Oxytocin (and perhaps other neuroendocrine hormones) may have a key role in experience-dependent programming of sensory systems during development for species-typical social expertise in adulthood. |
| The pre-clinical data suggest that clinical effectiveness of oxytocin and vasopressin during development will be strongly influenced by environmental factors. |
Dynamic Developmental Profiles: When and Where are the Factors Present in Development?
Neuropeptide distribution in the brain
Oxytocin and vasopressin are produced in and available to the developing brain, including the developing human brain (reviewed in Swaab, 1995). In all species examined, vasopressin mRNA and protein emerge before oxytocin mRNA and protein, and in greater quantities (Choy and Watkins, 1979; Buijs et al, 1980; Burford and Robinson, 1982; Wolf et al, 1984; Van Der Sluis et al, 1986; Altstein and Gainer, 1988; Iqbal and Jacobson, 1995a, 1995b). In humans, vasopressin has been detected as early as 11 weeks gestation and oxytocin as early as 14 weeks, and both peptides appear to have adult-like levels of immunoreactive cell number in the paraventricular nucleus (PVN) by 26 weeks gestation (Goudsmit et al, 1992), although the volume of the PVN is still immature (Rinne et al, 1962). In rats and mice, based on in situ hybridization studies, immunohistochemistry, and quantitative immunoassay, vasopressin has consistently been detected prenatally, while oxytocin is sometimes detected prenatally, and sometimes not detected until the first or second postnatal day (Sinding et al, 1980a; Wolf et al, 1984; Whitnall et al, 1985; Reppert and Uhl, 1987; Altstein and Gainer, 1988; Yamashita et al, 1988a, 1988b; Laurent et al, 1989; Bloch et al, 1990; Hyodo et al, 1992; Jing et al, 1998; Lipari et al, 2001). Both vasopressin- and oxytocin-producing cells continue to develop postnatally (Krisch, 1980; Sinding et al, 1980a; Wolf et al, 1984; Van Tol et al, 1986; Almazan et al, 1989). Oxytocin and vasopressin are both produced in the PVN and the supraoptic nucleus (SON) of the hypothalamus, while only vasopressin is produced in the suprachiasmatic nucleus, which emerges slightly later with a day–night rhythm of vasopressin content by E21 in rats (Reppert and Uhl, 1987). The rat extrahypothalamic vasopressin system (vasopressin-producing cells in the bed nucleus of the stria terminalis and the medial amygdala) is established early postnatally and is sexually dimorphic with much greater vasopressin production in males (De Vries et al, 1981; Szot and Dorsa, 1993; Rood et al, 2013). Sex differences in extrahypothalamic vasopressin production, which emerge by postnatal day 12 in rats, are testosterone dependent, leading to more immunoreactive fibers in the lateral septum and habenula (De Vries et al, 1981). Compared with fibers in adult rats, fibers in the adult mouse appear to be even more widespread (Rood and De Vries, 2011), although a complete developmental profile in mice has not yet been performed for vasopressin immunoreactivity. Sex differences are apparent in adult mice too (Rood et al, 2013). Vasopressin development has been studied in golden hamsters (Delville et al, 1994b), and while male hamsters produce more vasopressin than female hamsters, the sex differences in hamsters are evident in the hypothalamus, and not the extrahypothalamic areas (Delville et al, 1994a). A transient vasopressin immunoreactivity in the cortex in the first postnatal week of golden hamsters has also been reported (Delville et al, 1994b). Vasopressin neurons are also detected in the locus coeruleus of rats (Caffe and Van Leeuwen, 1983) and have also been detected in the adult rat olfactory bulb (Tobin et al, 2010), but the development of this population is unknown. The genes encoding oxytocin and vasopressin each include a neurophysin, which serves as a carrier protein. The detection of the neurophysin component precedes the fetal detection of either vasopressin or oxytocin (Silverman, 1975; Choy and Watkins, 1979; Sinding et al, 1980a, 1980b). The developmental significance of this earlier onset of neurophysin expression is unknown, although one early study indicated that neurophysin II (vasopressin’s carrier protein) enhances proliferation of non-neuronal hypothalamic cells in vitro (Worley and Pickering, 1984).
In rat, primary fetal brain cultures from the hypothalamus, vasopressin mRNA and peptide can be detected, but oxytocin cannot (Di Scala-Guenot et al, 1990a, 1990b) without stimulation by potassium or thyroid hormone (Madarasz et al, 1992). This difference is consistent with in vivo results that vasopressin emerges earlier than oxytocin and is also consistent with the hypothesis that developmental oxytocin is activity dependent (Zheng et al, 2014). However, oxytocin mRNA levels in primary cultures derived from postnatal rat brains did not appear to be affected by lack of serum in the culture media or the presence of tetrodotoxin (Wray et al, 1991). In summary, vasopressin and oxytocin are produced in and available to the developing brain, vasopressin production precedes oxytocin production, and oxytocin production may be activity dependent.
Vasopressin and oxytocin receptors in the brain
Although the peptides are available early in development, their receptors have a more dynamic developmental time course of availability. Receptors for oxytocin and vasopressin are the oxytocin receptor (OXTR), vasopressin 1a (V1aR), 1b (V1bR) and V2 (V2R) receptors. OXTR and V1aR are abundant in the developing and adult brain and are typically assumed to be the main brain receptors in the adult, with V1bR expression limited to the pituitary and V2R receptor in the kidney. The presence of V1bR (Hernando et al, 2001) and V2R (Kato et al, 1995;Vargas et al, 2009) in the brain during development has not been ruled out, and this could be an important research avenue for V1bR in particular (Wersinger et al, 2002, 2004, 2008; Caldwell et al, 2008; Stevenson and Caldwell, 2012; Zai et al, 2012). OXTR and V1aR mRNA and ligand-binding capacity have both been detected prenatally in rats, but their interesting transient developmental patterns appear postnatally, with an adult-like pattern around the time of typical weaning, and further maturing in quantity into adulthood (Lukas et al, 2010) Some, but not all adult sex differences in OXTR expression are influenced by developmental exposure to testosterone (Tribollet et al, 1990; Uhl-Bronner et al, 2005; Dumais et al, 2013) and are actively regulated in adulthood (Bale and Dorsa, 1995a, 1995b; Bale et al, 1995a, 1995b). There do not appear to be gonadal steroid-dependent sex differences in brain V1aR expression, as determined by tritiated vasopressin ligand binding (Tribollet et al, 1990).
In voles, rats, and mice, the binding profiles for OXTR (Shapiro and Insel, 1989; Snijdewint et al, 1989; Tribollet et al, 1989; 1991a; 1992; Wang and Young, 1997; Hammock and Levitt, 2013) and V1aR (Snijdewint et al, 1989; Tribollet et al, 1991a, 1991b; Wang et al, 1997; Wang and Young, 1997; Hammock and Levitt, 2012) have been mapped during postnatal development. Receptor mRNA has also been measured in development (Yoshimura et al, 1996; Chen et al, 2000) in rats. Each species studied to date has a specific transient map of receptor ligand binding. In rodents, there is a transient peak of ligand binding in some brain areas in the first week for V1aR and in the second week in some brain areas for OXTR. The brain regions that display these transient appearances of receptors are not conserved across rodent species. For example, in separate experiments using the same radioactive ligand, pre-weanling rats have peak OXTR specifically in the cingulate neocortex (Shapiro and Insel, 1989; Tribollet et al, 1989), while mice have a peak in OXTR throughout the entire neocortex (Hammock and Levitt, 2013). Readers are referred to the primary literature for more details of their species of interest. This suggests that experience-dependent oxytocin and vasopressin activity during these time points may help to shape activity-dependent development in a species-specific manner, in which species differences in genetics influence the brain distribution of receptors.
Species differences and human relevance
Species specificity of receptor distribution patterns in the adult brain is a common theme in the oxytocin/vasopressin family of receptors. There are numerous examples of species-specific distribution of binding sites for these receptors—in primates, rodents and even non-mammals such as birds and fishes. This diversity of receptor locations affects behavior. For example, the monogamous prairie vole has an abundance of V1aR in the ventral pallidum, while the closely related non-monogamous meadow vole does not (Young, 1999; Lim and Young, 2006). Adding V1aR by viral vector gene transfer to the ventral pallidum of the meadow vole confers the males with the ability to form a partner preference (Lim et al, 2004), which is a laboratory test of the pair bonding component of monogamous social behavior. Receptor locations in adulthood and throughout development have significant implications for the underlying mechanisms of the influence of oxytocin and vasopressin on social behavior. Importantly, these receptor densities vary by species, sex, age, and to a lesser extent, by individual, providing a putative neurobiological mechanism for individual, sex, and species differences in experience-dependent developmental trajectories.
Are there transient profiles of OXTR and V1aR in human brain development? Yes. Data from the Allen Brain Atlas indicate that V1aR mRNA expression is high in the human neocortex during the second trimester (Kang et al, 2011). In mice, the V1aR transient peak occurs around P7, and includes intense transient ligand binding in the hippocampus and neocortex that is not present in the adult mouse (Hammock and Levitt, 2012). The translation of neurodevelopmental events occurring at this time suggests that this time is similar to the second trimester in human brain development (Clancy et al, 2007). Human OXTR expression seems to be high at birth and for the first 3 years in the neocortex, with prolonged expression in the medial prefrontal cortex (Kang et al, 2011). The peak of OXTR ligand binding in the neocortex (see Figure 4b) in mice around P14 (Hammock and Levitt, 2013) translates to perinatal human neurodevelopmental events (Clancy et al, 2007). This consistent and direct neurodevelopmental translation of peak expression for both AVPR1A and OXTR suggests that mice will be very valuable models of the activity-dependent effects of oxytocin and vasopressin on neocortical development. The functional significance of the robust transient peak of OXTR in the neocortex of mice has recently been demonstrated by Zheng et al (2014), and will be discussed in much further detail below.
Behavioral Influence During Development
Oxytocin and vasopressin both have a role in adult behavior. Their role in neonatal behavior is less well established, but deserves careful consideration, as this may significantly contribute to species-typical trajectories of experience-dependent development. Social expertise in adulthood is initiated by experience during development (eg, Denenberg et al, 1964), so alterations in infant activity that bring the infant in contact with social stimuli will positively contribute to the development of social expertise, while behavior that removes them from social contexts may negatively impact the development of species-typical social behavior.
Vasopressin influences early social behavior and blocks early familiarity preference
Several lines of evidence indicate both long-term and short-term behavioral effects of neonatal vasopressin exposure. A single study of enhanced vasopressin exposure during the neonatal period in prairie voles resulted in heightened aggression in adult voles, while exposure to an antagonist reduced aggression, with no effects on exploration in the elevated plus maze and partner preference behavior (Stribley and Carter, 1999). A few studies in the neonatal period in rats also indicate a role for vasopressin in social behavior. Neonatal mice and rats emit ultrasonic vocalizations when separated from their mother, perhaps in a reflex pattern that may elicit her attention. Large doses (100 ng) of vasopressin given intracerebroventricularly to neonatal rats (Winslow and Insel, 1993) reduced ultrasonic vocalizations but also core body temperature, and while vasopressin reduced the latency for righting in an inclined surface assay indicating potential regulation of sensory-motor integration, it also reduced motor activity in general. Consistent with a motor inhibiting effect of central injections of large doses of vasopressin, the vasopressin-deficient Brattleboro rat neonate is hyperactive and social contact fails to elicit a typical quiescence response in this mutant line (Schank, 2009). Further, Brattleboro neonates show disturbances in early odor orienting (Nelson and Panksepp, 1998) and dysregulation of the HPA axis in response to maternal separation as neonates (Fodor and Zelena, 2014). Flank marking (a social communication behavior) in hamsters emerges around P19–P22 and is tied to a robust increase in vasopressin in the hypothalamus (Ferris et al, 1996). Together, these data strongly suggest an early role for vasopressin in the modulation of species-typical social behavior.
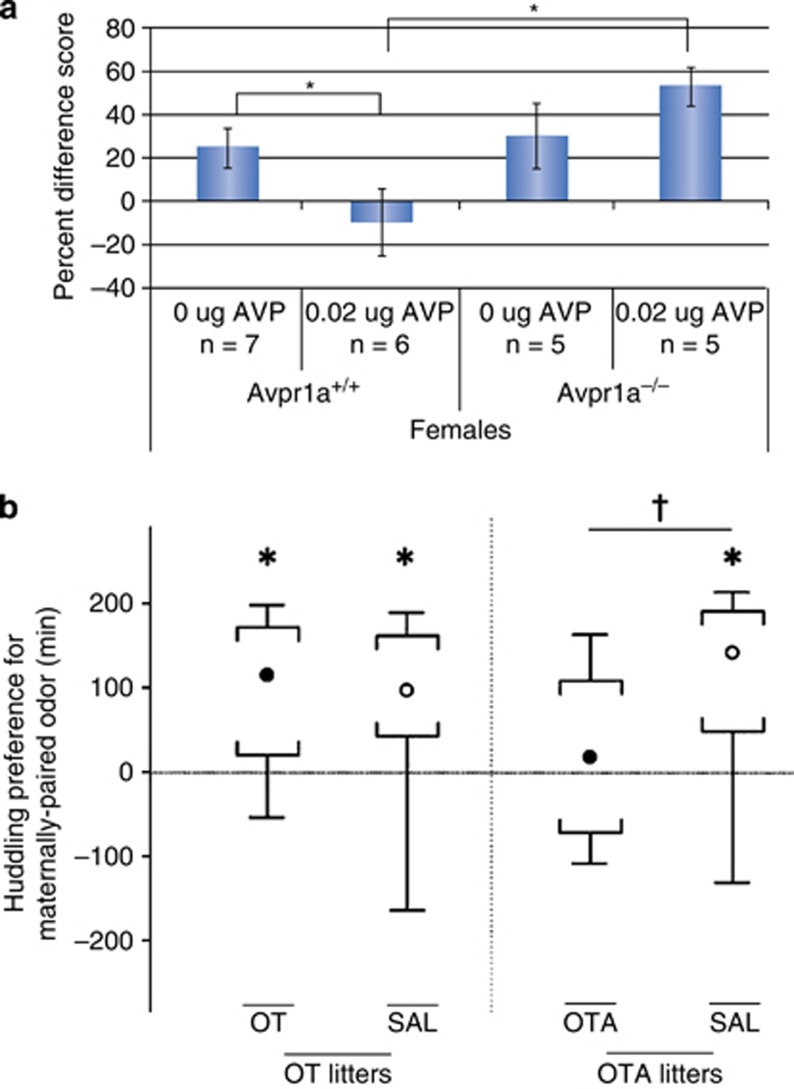
Early orienting to specific odor cues is a significant contributor to mammalian species-typical social behavior developmental trajectories (Denenberg et al, 1964; Brunjes and Alberts, 1979; Rosenblatt, 1983; Balogh and Porter, 1986). Not unlike visual or auditory imprinting in many birds (Hess, 1959), olfactory imprinting in rodents helps to orient to the initial food source (the mother) and allows the infant to begin to build a neural representation of the social world through classical conditioning of innately social odor sources (eg, the mom, the littermates, species-typical food odors, and species-typical nest materials) with other stimuli (Rosenblatt, 1983; Hammock and Levitt, 2006; Hammock et al, 2013). Therefore, it is important to understand the neural mechanisms that underlie these important early orienting processes as they are at the foundation of building a social brain. We have explored the role of vasopressin in early olfactory learning in neonatal mice. Vasopressin, acting through the brain V1a receptor, acts as a barrier to the display of olfactory preferences in neonatal female mice (Figure 1a; Hammock et al, 2013). In these experiments, a novel odor was presented for 1 h to C57BL/6J mouse litters on postnatal day 8, in the home cage with the mother. After odor exposure, the mom and odor source were removed from the cage. Two hours later, the neonates were individually tested for an odor preference for the trained odor vs a novel odor. Wild-type females showed a preference for the odor when that odor had been presented with the mom in the home cage, but failed to show a preference if the odor was presented without the mom in the cage. V1aR knock-out females showed a strong preference for the odor after training with the mom and also showed a strong preference for the odor when trained without the mom. This suggested that the absence of V1aR signaling was permissive for exposure alone (or the odor plus littermates) to generate strong olfactory preferences and indicated that activation of V1aR may inhibit the expression of early olfactory preferences. To test for a blocking effect for vasopressin on the expression of maternally paired odors, we next trained neonates as before, and just before testing gave them a central (intracerebroventricular) injection of vasopressin and demonstrated that vasopressin blocked the expression of a trained odor preference, at a low dose that did not affect motor activity. Further, vasopressin failed to block olfactory preferences in V1aR knock-out females, indicating that this receptor was required for the preference-blocking effects of vasopressin. Surprisingly, the wild-type and V1aR knock-out male mouse neonates were poor performers in this task and showed no evidence of olfactory preferences in any condition. A similar role for vasopressin activation of V1aR to reduce familiarity preference was also observed for rats at postnatal day 17 (Sigling et al, 2009) and postnatal day 33 (Veenema et al, 2012). Although the most straightforward interpretation of these data indicate a blocking effect by vasopressin on familiarity bias, it may also be possible that vasopressin acting at the V1aR imposes more stringent conditioning expectations, such that a neonate will not condition to a novel odor without an appropriate social context. Regardless of the interpretation, these data demonstrate a role for vasopressin at V1aR in very early sensory-dependent development of the social brain.
Figure 1.
Vasopressin blocks and oxytocin promotes primordial social orienting behaviors in neonatal rodents. Early orienting to social cues increases exposure to social stimuli required for the development of species-specific social behavior expertise. (a) Vasopressin acting at the V1aR eliminates preferences for maternally associated odors in neonatal female mice on postnatal day 8. Copied with permission from Hammock et al (2013). Copyright Elsevier. *Significant difference. (b) Oxytocin antagonism eliminates preferences for maternally associated odors in rats on postnatal day 15. Copied with permission from Kojima and Alberts (2011a). Copyright Elsevier. *Significant huddling preference, †Significant difference in huddling preference.
Oxytocin influences early separation distress vocalizations and social contact behavior in neonates
Neonates of some species vocalize, which may elicit parental care when needed. Both oxytocin knock-out (Winslow et al, 2000) and OXTR knock-out mice (Takayanagi et al, 2005) show low rates of isolation-induced vocalizations, but instead of increasing ultrasonic vocalizations, oxytocin delivered to the brains of neonatal rats actually inhibits isolation-induced vocalizations (Insel and Winslow, 1991). Oxytocin application in decerebrate rat neonates reduced vocalizations in response to electrical stimulation of the whiskers, a pain-inducing stimulus (Mazzuca et al, 2011). CD38 has been identified as a potent releaser of oxytocin in the hypothalamus (Jin et al, 2007), and these mice have altered social behavior in the neonatal period (Higashida et al, 2010), including a reduction in isolation-induced ultrasonic vocalizations (Liu et al, 2008). Therefore, as presence of oxytocin, total lack of oxytocin, or poor oxytocin secretion, all result in reduced separation or stress-induced vocalizations, the role of oxytocin in ultrasonic vocalizations in neonatal rodents is unclear. In addition to vocalizing, and learning social odors (described above), neonatal rodents in a litter also modulate their huddle size based on ambient temperature and activity levels. Tighter more compact huddling by a litter of rats was observed after brain injections of oxytocin at postnatal day 10, but not earlier at postnatal day 7 (Alberts, 2007), indicating a role for oxytocin in a very early social behavior. A similar increase in tighter rat pup huddling after oxytocin treatment compared with antagonist treatment was also observed on postnatal day 14 (Kojima and Alberts, 2011a). Oxytocin also induces oral/suckling behaviors and grooming, especially during somatosensory vibrissae stimulation (Nelson and Alberts, 1997). As described earlier, rodent neonates learn to prefer a novel odor if that odor is paired with their mother. An oxytocin antagonist injected into the brain of 14-day-old rats just before pairing a novel odor with the mother resulted in the elimination of preference for that paired odor when tested the next day (Figure 1b). In contrast, rats injected with saline or oxytocin both demonstrated strong odor preferences for the odor that had been paired with the mother (Nelson and Panksepp, 1996; Kojima and Alberts, 2011a). The oxytocin antagonist may block the formation of these olfactory preferences by interfering with huddling and pup–mother contact during the odor exposure period, which is positively correlated with the strength of the olfactory preference (Kojima and Alberts, 2009, 2011b). In short, oxytocin activity in the neonate may enhance the required proximity to social cues that permit social learning.
Oxytocin in the infant is associated with parental engagement
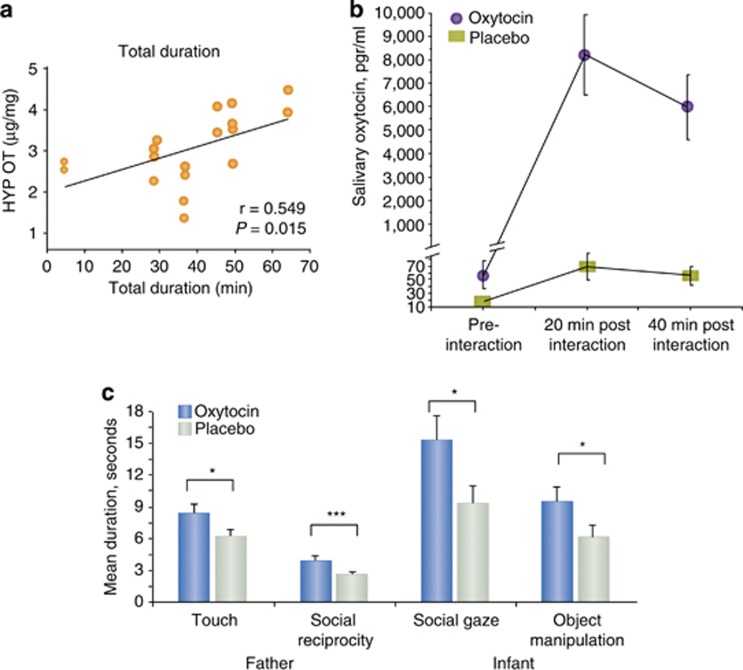
Although there are several studies in rodents that demonstrate long-term effects of the quality of maternal care on the adult levels of oxytocin and vasopressin function (eg, Francis et al, 2000, 2002; Champagne et al, 2001; Pedersen and Boccia, 2002), there are fewer studies that measure an effect of parental care on oxytocin or vasopressin systems during development. In non-human primates, mother-reared monkeys show increased CSF oxytocin levels measured at 18, 24, and 36 months compared with nursery-reared infants, which displayed maladapted social and self-regulatory behaviors (Winslow et al, 2003). In neonatal rats, OXTR levels were lower in the dorsal hippocampus during postnatal development after repeated maternal separations (Noonan et al, 1994). Mogi et al (2011) report that oxytocin content is lower in the cingulate cortex of rats at postnatal day 14 when separated from their mother. Oxytocin responds to maternal separation and skin-to-skin contact in pre-weanling rats (Kojima et al, 2012). Oxytocin concentrations were sampled from pre-weanling rat hypothalamus and blood at several time points during a separation and reunion paradigm with a foster mother, which in previous studies elicits a huddling preference in the pups. In the hypothalamus, 1 h of separation from a maternal figure resulted in increased oxytocin concentrations in the hypothalamus. Prolonged isolation for another 2 h induced further increases in oxytocin concentration in the hypothalamus, while 2-h treatment with a foster mother reduced oxytocin concentrations in the hypothalamus. In contrast, in serum a 1-h separation increased oxytocin concentrations as it did in the hypothalamus, but serum concentrations were decreased after a further 2-h period of isolation from a maternal figure. Perhaps the initial loss of contact with the mother results in an increased production of oxytocin in the hypothalamus and release into the periphery to organize the infant behavior to solicit social contact, but that further isolation results in conservation of activity of the oxytocin system. During the interaction with the foster mother, skin-to-skin contact was recorded, and there was a significant positive correlation between duration of contact and hypothalamic oxytocin concentrations (Figure 2a), but no relationship between skin-to-skin contact and peripheral oxytocin concentrations. At baseline, central hypothalamic and peripheral serum oxytocin concentrations were not correlated. However, after 1 h of separation, the infant’s central and peripheral oxytocin were positively correlated. The group that received contact with a foster mother maintained a correlation between peripheral and central oxytocin concentrations, but the group that remained isolated from a maternal figure for 3 h lost their initial isolation-induced central and peripheral correlation, and the oxytocin concentrations in the hypothalamus and the blood were no longer correlated. These results indicate a relationship between nurturing contact and oxytocin in the neonate, but the relationship is dynamic, and will require very careful further study to understand fully the relationship between nurturing social contact and the activity of the oxytocin system within the brain and periphery. Nelson and Panksepp (1996) argued that ‘…gradual elevation and inhibition of oxytocinergic activity may be a critical aspect of the natural dynamics of social interaction in young rats, and it is possible that a patterned release of oxytocin in the presence of a social stimulus may be necessary to form conditioned odor preferences’. This important consideration still must be carefully addressed. Oxytocin may be released at social transitions rather than being continuously released during social contact.
Figure 2.
Social contact is associated with oxytocin system activity in rodent and human infants. (a) In neonatal rats, the duration of skin-to-skin contact with a surrogate mother after a period of isolation was positively correlated with the concentration of oxytocin in the hypothalamus. Copied with permission from Kojima et al (2012). Copyright Wiley. (b, c) In 4- to 8-month-old human infants, intranasal oxytocin delivery to their fathers resulted in a very robust increase in infant salivary oxytocin (b) and a significant increase in social gaze and object manipulation in the infants (c), perhaps mediated by enhanced social contact induced by oxytocin in the father (c). Copied with permission from Weisman et al (2012). Copyright Elsevier. *P<0.05, ***P<0.001.
Single injections of oxytocin just after birth has long-term sex-specific effects on behavior in prairie voles
As oxytocin is used frequently to induce labor, Bales and Carter (2003) attempted to determine the long-term behavioral effects, if any, of a single perinatal application of oxytocin. Early postnatal manipulations of prairie voles with oxytocin or an oxytocin antagonist have sexually dimorphic effects on later behavior, with males seemingly more affected by such perinatal treatments (reviewed in Carter et al, 2009). A single postnatal injection of oxytocin in male prairie voles increased the probability of partner preference and reduced anxiety-like behavior in adulthood (Bales and Carter, 2003), and a neonatal dose of OXTR antagonist reduced alloparental behavior in adult male prairie voles, without affecting partner preference behavior (Bales et al, 2004). The same perinatal dose in females did not affect partner preference behavior in adulthood, although higher doses did affect partner preference behavior, but in a non-linear manner (Bales et al, 2007b; Carter et al, 2009).
Chronic oxytocin during postnatal development alters later social behavior
As oxytocin has been considered as a potential therapeutic agent for autism and schizophrenia, Bales et al (2013) tested the effects of chronic exposure to exogenous oxytocin in the postnatal brain. Daily intranasal delivery of oxytocin to prairie voles during the juvenile period interferes with species-typical partner preference behavior in adulthood in males, but not females (Bales et al, 2013). Four groups of male and female prairie vole weanlings underwent chronic exposure to either one of three doses of oxytocin or a saline control. The doses were chosen such that the medium dose approximates doses being used in clinical trials on children. Importantly, social behavior was measured in the acute phase of drug delivery as well as in the long term. Immediately after drug delivery, male juveniles receiving intranasal oxytocin showed increases in social behavior (contact time) with a familiar cage mate and decreased time spent autogrooming at low, medium, and high doses of oxytocin. Juvenile females failed to show a change in social contact time immediately after intranasal oxytocin delivery. As adults, males that received low and medium doses of oxytocin as juveniles were, without a change in total social contact time, less likely to form a partner preference that is an important species-typical social behavior for prairie voles. On the surface, this appears as if chronic exogenous oxytocin exposure has a negative outcome (ie, reduction in the probability of an important species-typical behavior), however, prairie voles can adapt their mating strategy from monogamy to non-monogamy to adjust to various selection pressures, thus non-monogamy is not necessarily a pathological trait, but potentially an adaptive trait (argued in Young, 2013). Pedersen and Boccia (2002) delivered oxytocin subcutaneously to female rat pups on postnatal days 2–10 and observed that females treated with oxytocin grew up to show higher levels of maternal attention to pups (pup licking) than saline-treated females. Further, adults who as pups were given daily injections of an OXTR antagonist showed lower levels of pup licking compared with saline-treated females. These data highlight important timing (acute vs chronic and developing vs mature), dose, and sex effects on social behavior outcomes after exogenous manipulation of oxytocin. A recent study in adult male mice also revealed a counterintuitive reduction in social investigation after chronic intranasal oxytocin (Huang et al, 2014). Combined, these data indicate caution and careful study design for future chronic use of oxytocin in clinical populations. If oxytocin facilitates environmental encoding or social learning, then the context in which oxytocin is applied deserves special attention. To be successful clinically, it will probably be important for oxytocin to be given along with behavioral and/or environmental intervention. Chronic oxytocin treatment in the absence of an ethologically appropriate social setting may actually degrade the value of oxytocin as a social signal.
In human infants, there is evidence that the infant’s oxytocin system responds to social engagement with the parent (Figures 2b and c; Feldman et al, 2010; Weisman et al, 2012). Thirty-five healthy fathers were given either placebo or oxytocin intranasally, and 45 min later interacted with their 4- to 8-month-old infants. The fathers that were given oxytocin showed enhanced social engagement with their infant, and those infants showed markedly increased levels of oxytocin in their saliva. This very interesting result needs replication and is consistent with the hypothesis that nurturing parental engagement activates the human infant oxytocin system.
Oxytocin and Vasopressin Impact Brain Development
Given that vasopressin and oxytocin appear to influence neonatal behavior and that there are brain areas with a developmental transience of oxytocin and vasopressin receptor expression, what is the experimental evidence that either receptor has a role in brain development? The Brattleboro rat exhibits a natural mutation rendering them vasopressin knock-outs. Boer et al (1982a, 1982b) published some very early studies demonstrating the non-subtle changes in brain development observed in these rats, which included changes in the cerebellum (Boer, 1985) and noradrenergic content (Boer et al, 1995). Those early studies gave the first clues that neuropeptide signaling may regulate brain development.
Hormonal imprinting confers long-term changes
Perinatal manipulations of the levels of oxytocin and vasopressin or steroid hormones can alter the long-term programming of oxytocin and vasopressin systems and impact other neurotransmitter systems. This programming phenomenon was first described in rats (Swaab and Boer, 1983; Boer, 1993; Boer et al, 1994) and named ‘hormonal imprinting’ by Csaba (1986). Reviewed in Bales and Perkeybile (2012), single doses of oxytocin given to newborn prairie vole pups have long-term sex-specific consequences on PVN oxytocin and on other systems such as V1aR and estrogen receptor alpha (Yamamoto et al, 2004, 2006; Kramer et al, 2006; Bales et al, 2007a; Pournajafi-Nazarloo et al, 2007). For example, as adults, prairie voles treated with a single dose of oxytocin or an OXTR antagonist given intraperitoneally on postnatal day 1 had altered levels of V1aR in various brain regions in a sex-dependent manner (Bales et al, 2007a), although surprisingly, no treatment effects on OXTR or the dopamine D2 receptor were observed. Chronic intraperitoneal postnatal treatments in rats also altered estrogen receptor alpha (Perry et al, 2009). Although intraperitoneal delivery of oxytocin or oxytocin antagonists may not be an ideal way to investigate central effects, it does result in differential c-fos activation of neonatal prairie vole brains (Cushing et al, 2003), indicating that peripheral injection of oxytocin does affect the brain. Subcutaneous injections of oxytocin within 24 h of birth also has long-term brain and behavioral consequences in mandarin voles (Jia et al, 2008a, 2008b). A single perinatal injection of oxytocin impacts serotonin and dopamine metabolism in the hypothalamus and striatum in adult rats (Hashemi et al, 2013). This potent capacity for a single exposure of oxytocin in the peripartum period has raised concerns about the potential consequences of routine use of oxytocin in labor and delivery (Swaab and Boer, 1983; Carter, 2003; Hashemi et al, 2013).
Oxytocin is neuroprotective and analgesic during labor and delivery
Despite concerns about exogenous application of oxytocin in the peripartum period, it appears as though oxytocin released during birth acts within the neonatal brain to dampen excitatory neurotransmission, thus serving as a putative neuroprotective factor. In adult tissues, GABA is hyperpolarizing and inhibitory because of the low concentration of chloride ions inside the cell relative to higher concentrations outside the cell. During development, however, GABA is excitatory and depolarizing because of high intracellular chloride concentration. Acting in a manner that may alleviate the stress of the birth process and its potential excitatory neurotoxicity, at the time of birth, oxytocin temporarily shifts the chloride concentrations in neurons such that GABA becomes hyperpolarizing and inhibitory (Tyzio et al, 2006; Khazipov et al, 2008). Most recently, Tyzio et al (2014) observed that the transient hyperpolarization induced by oxytocin at birth is absent in two rodent models of autism: the sodium valproate model and a fragile X genetic model. The authors were able to rescue this deficit by pretreating the mothers before delivery with bumetanide that targets the chloride pump required to alter the gradient of chloride ions. In addition to modulating GABA signals in the forebrain and protecting against potential excitotoxicity, oxytocin also reduces the depolarizing actions of GABA on nociceptive neurons during birth giving it a powerful analgesic property (Mazzuca et al, 2011). These potential neuroprotective and analgesic effects of endogenous oxytocin at birth must be reconciled with the data described above demonstrating long-term ‘hormonal imprinting’ by exogenous oxytocin. Is the dose or exact timing of exposure to large quantities of oxytocin key to the distinction of neuroprotective vs potentially harmful perinatal exposure to oxytocin? Do the effects vary by brain region or system?
Developmental vasopressin and oxytocin have long-term effects on cortical plasticity
As we observed transient V1aR in the mouse neocortex, we questioned whether V1aR knock-out mice would have a measurably atypical neocortex as adults. We focused on parvalbumin interneurons because of their role in experience-dependent plasticity (Hensch, 2005) and their relationship with schizophrenia (Lewis et al, 2005) and mouse models of autism (Gogolla et al, 2009). We observed that adult V1aR knock-out mice had greater numbers of parvalbumin immunoreactive cells than their wild-type littermates (Hammock and Levitt, 2012). This indicates that transient V1aR signaling during postnatal development affects neocortical developmental outcomes. The mechanisms and the behavioral contributions of these excess interneurons remains to be elucidated.
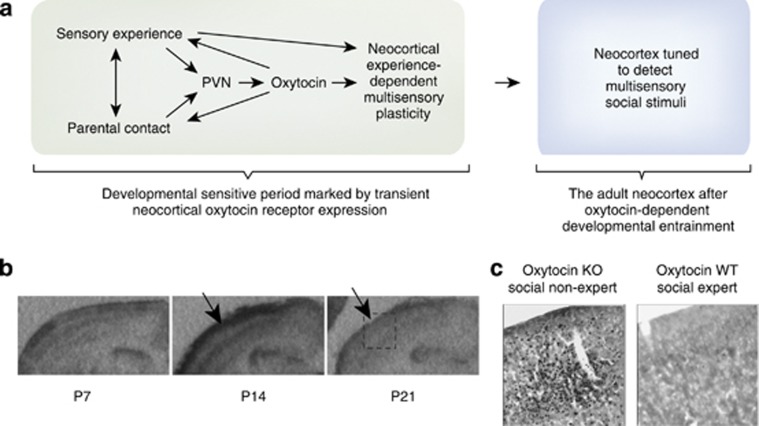
There is also indirect evidence that developmental exposure to oxytocin leaves a long-term mark on the function of the neocortex. An intriguing and orphan result from the earliest data from the oxytocin knock-out mouse (Ferguson et al, 2001) suggests an important organizational role for oxytocin in neocortical neurocircuitry: after introduction to a social stimulus, the primary somatosensory neocortex of adult oxytocin knock-out mice shows exceptionally robust c-Fos immunoreactivity compared with similarly exposed wild-type mice (see panel c in Figure 4). This has remained unexplained because in the adult, when the c-Fos study was performed, there are relatively few oxytocin (or vasopressin) receptors. This suggests that oxytocin may promote the experience-dependent development of the neocortex (see below): the absence of developmental oxytocin signaling through transient neocortical OXTR may lead to atypical or labored processing of multisensory information in the adult neocortex (Figure 4).
Oxytocin modulates signal:noise processing
Neocortical OXTRs appear poised to facilitate signal to noise processing for multi-sensory signals. Oxytocin enhances signal:noise processing in the neocortex and the hippocampus, although through different mechanisms. Oxytocin applied to acute slices made from 3- to 4-week-old mouse medial prefrontal cortex (Ninan, 2011) caused a reduction in post-synaptic excitatory currents (EPSCs) in layer V pyramidal cells at baseline (a reduction in noise), but an increase in glutamate transmission when a 5 Hz stimulus was applied to inputs from layer II/III neurons. The stimulus-evoked long-term potentiation induced by oxytocin required an NMDA receptor-dependent mechanism. More recently, Owen et al (2013) showed the same phenomenon of oxytocin enhancing signal:noise processing in the adult mouse hippocampus, although they determined that the effect was through oxytocin modulation of fast spiking GABAergic interneurons, rather than modulation of NMDA currents.
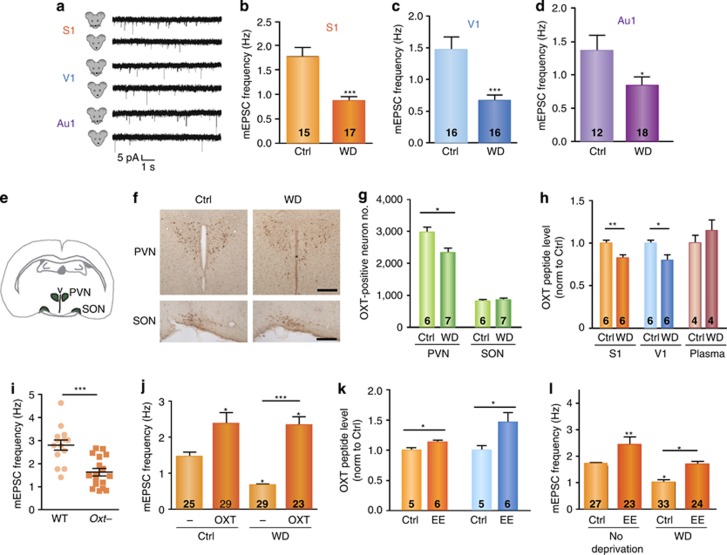
Oxytocin has recently been shown to enhance neocortical experience-dependent plasticity during postnatal mouse development (Figure 3; Zheng et al, 2014). After unisensory deprivation by either whisker trimming from birth, or rearing in total darkness, at postnatal day 14, layer II/III pyramidal neurons in auditory, somatosensory, and visual primary sensory neocortex showed reduced spontaneous firing rates and reduced excitatory synaptic transmission. The prefrontal cortex was not affected under these conditions. To identify potential mediators of this cross-modal plasticity in primary sensory neocortex, Zheng et al screened for potential transcript changes between control and sensory deprived mice. Although transcriptomic analysis of the neocortical areas failed to reveal a consistent pattern, transcriptomic analysis of combined hypothalamus and thalamus mRNAs revealed that oxytocin mRNA was consistently downregulated after both kinds of sensory deprivation (whisker trimming and dark rearing). These results were confirmed with qPCR and immunohistochemical analysis clearly demonstrating reduced oxytocin levels in the PVN after developmental sensory deprivation. Sensory deprivation reduced the firing of oxytocinergic cells in the PVN, and resulted in lower detectable oxytocin peptide in S1 and V1 neocortex. This oxytocin detected in the neocortex comes from the hypothalamus and most likely reaches the neocortex through the CSF, and less likely through blood or direct projections: sensory deprivation did not change plasma levels, no direct projections from PVN to S1 neocortex were detected, and injecting an oxytocin sequestering antibody into the ventricles reduced the frequency of miniature excitatory post-synaptic currents (mEPSCs) in the neocortex in non-deprived animals. Oxytocin knock-out mice showed reduced mEPSC frequency like the sensory deprived mice, and exogenous oxytocin in the neocortex increased mEPSC frequency of layer II/III pyramidal cells and rescued the effects of sensory deprivation on cross-modal plasticity. This effect was mediated through OXTR as the OXTR antagonists OTA and atosiban, and siRNA against OXTR, each prevented the ability of exogenous oxytocin to increase mEPSC frequency in layer II/III neurons. In a final experiment, environmental enrichment during postnatal development increased oxytocin mRNA in the PVN and oxytocin protein in S1 and V1 cortex, increased mEPSC frequency in S1 and V1 cortex, and finally, rescued the mEPSC frequency of whisker deprived and dark reared pups to control levels reared in standard housing. Together, these data indicate that sensory experience drives oxytocin activity in the PVN of the hypothalamus, leading to enhanced oxytocin availability at neocortical receptor sites to modulate cross-modal neocortical developmental plasticity. The identification of this novel action of oxytocin in experience-dependent neocortical developmental plasticity is a profound and potentially transformative result.
Figure 3.
Oxytocin in the neocortex modulates experience-dependent multisensory plasticity. (a) Representative miniature post-synaptic current (mEPSC) recordings for conditions as indicated from the primary somatosensory cortex (S1), primary visual cortex (V1), and primary auditory cortex (Au1). (b) Whisker-deprivation (WD) significantly reduced mEPSC frequencies in S1. (c) WD significantly reduced mEPSC frequencies in V1. (d) WD significantly reduced mEPSC frequencies in Au1. (e) Illustration of the location of the paraventricular (PVN) and supraoptic (SON) nuclei, both in the mouse hypothalamus, in a coronal brain slice. (f) Representative images of oxytocin-positive neurons in the PVN and SON of P14 mice, conditions as indicated; scale bar=200 μm. (g) WD reduced the number of oxytocin-positive neurons in the PVN, but not SON. (h) WD significantly reduced oxytocin peptide level in S1 and V1, but not blood plasma. (i) Oxytocin knockout mice showed reduced mEPSC frequencies in S1. (j) Oxytocin injection increased mEPSC frequencies and rescued the effects of whisker-deprivation in S1. (k) Environmental enrichment (EE) significantly increased oxytocin peptide level in S1 and V1. (l) EE increased mEPSC frequencies and rescued the effects of whisker deprivation in S1. Figure and Figure legend courtesy of Dr Xiang Yu (Zheng et al, 2014), copyright Nature Publishing Group. *P<0.05, **P<0.01, ***P<0.001.
Gene by Environment Interactions
The data described above point to an organizational role for oxytocin and vasopressin where their early activity during developmental sensitive periods may developmentally program later social behavior. Emerging evidence suggests a critical interaction of early environment and oxytocin function in humans. For example, adult women retrospectively reporting childhood abuse have decreased oxytocin in CSF (Heim et al, 2009). Both men (Meinlschmidt and Heim, 2007) and women (Riem et al, 2013) show altered responses to intranasal oxytocin after a childhood with significant disruptions in the parent-child relationship. Further, peripheral (urine) oxytocin levels were lower after a social encounter in children with early life histories in institutional settings compared with children who were reared in typical home settings (Wismer Fries et al, 2005). These data in humans suggest that careful attention to experiences in childhood may help disentangle the true nature of the influence of oxytocin and vasopressin system genetics in developing and adult behavior. Two main research areas support this assumption: oxytocin system differences during development may alter the methylation of the OXTR gene leading to developmentally programmed levels of adult OXTR function, and recent gene association studies indicate significant gene × environment interactions. The emerging picture points to experience-dependent organizational effects of oxytocin and vasopressin systems during mammalian development of brain and behavior.
Methylation of OXTR with experience
Methylation of DNA contributes to tissue-specific and dynamic or stimulus-evoked regulation of gene expression. Methylation of the mouse Oxtr gene is associated with brain region-specific expression levels of Oxtr mRNA (Mamrut et al, 2013; Harony-Nicolas et al, 2014) and is dynamically regulated in the uterine myometrium and mammary tissue at parturition and lactation (Mamrut et al, 2013) and in the brain after drug exposure in adulthood (Aguilar-Valles et al, 2013). There is significant associative evidence that methylation of the human OXTR gene may also contribute to developmental and dynamic regulation of OXTR gene expression (reviewed in Kumsta et al, 2013). Methylation of the third intron of the human OXTR was associated with repressive binding proteins from non-expressing peripheral blood leukocytes, while relative hypomethylation of this region was associated with myometrium from non-pregnant and term pregnancy (Mizumoto et al, 1997). Kim et al (2013) assessed some methyl sites in the 5’ UTR of the OXTR in term, pre-term labored and term unlabored amnion. Methylation of the human OXTR gene predominantly at a site called MT2 contributes to tissue-specific repression of OXTR mRNA expression, in vitro (Kusui et al, 2001) and is associated with OXTR mRNA repression in human brain (Gregory et al, 2009).
Gregory et al (2009) showed increased methylation in the MT2 region of OXTR in blood cells in 20 individuals with autism compared with 20 matched controls. Further, a similar pattern of methylation was observed in DNA form temporal cortex from 10 individuals with autism compared with matched controls, and the increased methylation was associated with reduced OXTR expression in temporal cortex. In addition to an association between methylation and OXTR expression levels, OXTR methylation has also been associated with brain activity during an fMRI task. OXTR methylation at site −934 in whole blood has been associated with increased fMRI BOLD signal in the left superior temporal gyrus/supramarginal gyrus and right dorsal anterior cingulate cortex during a task that involved perception of ‘animacy’ and the ability to attribute mental states to geometric shapes (Jack et al, 2012).
The methylation status of the OXTR gene has also been explored in conduct disorder (Dadds et al, 2014), a developmental antecedent to psychopathic traits in adulthood. Significantly higher methylation status of OXTR was observed in older children aged 9–16 years with comorbid callous-unemotional traits compared with the same age individuals with low callous-unemotional traits. No methylation differences related to callous-unemotional traits were observed in younger children aged 3–8 years. Further, the older children with high levels of callous-unemotional traits also had lower peripheral levels of oxytocin in plasma.
In addition to methylation, Oxtr is also regulated by estrogen receptors, and methylation of that gene. Licking and grooming of rodent pups is a strong signal of high-quality care with long-term positive developmental effects (Francis et al, 1999). Adult rodents who received low levels of licking and grooming in the neonatal period have reduced OXTR levels in the medial preoptic area (MPOA) and show low levels of licking and grooming to their own offspring (Champagne et al, 2001). Estrogen receptor alpha regulates the levels of OXTR in the MPOA (Young et al, 1998), and is itself methylated and repressed after an early history of low levels of licking and grooming (Champagne et al, 2003, 2006). The low levels of ER alpha in the MPOA of these offspring make them less responsive to the effects of estrogen and less able to increase the necessary complement of OXTR in the MPOA to facilitate high levels of maternal licking grooming. This represents a neural mechanism of the transgenerational effects of low levels of maternal–infant interaction, with a sensitive period between postnatal days 6 and 10 in rats (Pena et al, 2013). This developmental time window in rodents translates to the perinatal period in human neonates (Clancy et al, 2007). These effects are not immutable, however, a socially enriched peri-adolescent environment can rescue the low levels of OXTR in the MPOA in individual rats who did not have much licking and grooming as neonates as well as promote the transmission of high levels of licking and grooming to the next generation (Champagne and Meaney, 2007).
Interaction between OXTR genotype and early social experience
OXTR genotype interacts with early adversity to increase risk for depression and social anxiety in female adolescents (Thompson et al, 2011), and depression in a population of college students (Mcquaid et al, 2013). Intranasal oxytocin has ‘prosocial’ effects in women who had more nurturing childhoods, suggesting important individual differences in the activity of the OXT system during experience-dependent refinement of neural circuits.
Recently, Feldman et al (2013) tracked first time parents and their infants from 1 month to 3 years of age and measured the relationship between parental oxytocin-related genes, maternal care, and infant salivary oxytocin. They report that infant salivary oxytocin at 3 years is related to maternal care and maternal oxytocin genotypes. In a separate study (Apter-Levy et al, 2013), maternal depression was linked with overall lower oxytocin measures in saliva among mothers, fathers, and their 6-year-old children. Further, variation in the OXTR rs2254298 (GG genotype) was more prevalent in the depressed mothers and significantly predicted the low oxytocin concentrations in the saliva. Children of depressed mothers were more likely to have a behavioral disorder such as anxiety or conduct disorder and had lower empathy and social engagement scores. These findings underscore the importance of gene by environment interaction models in gene association studies for genes that may be involved in experience-dependent developmental plasticity.
Future Research Directions
What are the unanswered questions?
The available evidence is consistent with the hypothesis that oxytocin and vasopressin contribute to experience-dependent development. There are numerous unanswered questions about the specific mechanisms of this special developmental plasticity. At this stage, we could potentially distill our working models of oxytocin and vasopressin in to very simple approach/avoid models: does oxytocin increase the ability to orient/make social contact in the neonatal period, and increase the encoding of the environment? Does vasopressin decrease the ability to orient/make social contact in the neonatal period and decrease encoding of the environment? Through this lens, oxytocin may facilitate the internalization of stimuli in the environment (both nurturing/appropriate and toxic/inappropriate environments), while vasopressin may block the internalization of the external social environment. These predictions must be vetted with direct experiments in which environment is precisely controlled.
Oxytocin and vasopressin can act at synapses as classical neurotransmitters through synaptic release, and also as hormones, by bolus release and diffusion through the extracellular space after dendritic release or by perfusion through direct release into the CSF (reviewed in Landgraf and Neumann, 2004; Neumann, 2007; Stoop, 2012). We do not yet know if in infancy oxytocin and vasopressin have more of a hormonal role (bathing the tissue through CSF and extracellular space) or by modulation of synapses from direct projections of oxytocin and vasopressin cells. Also, we do not know if constant contact or perhaps transitions between social contexts are more potent releasers of oxytocin and vasopressin. In infants, we do not know if the amplitude, frequency, or both of oxytocin and vasopressin release convey environmental signals. The rhythm of secretion of hypothalamic hormones is physiologically significant in adults (reviewed in Gan and Quinton, 2010). Oxytocin is released with parturition, lactation, and chronic dehydration in the adult. This oxytocin results in a reduction in glia around oxytocin cells in the SON and PVN, which places oxytocin neurons in close juxtaposition with each other (Theodosis et al, 1986). This contributes to the pulsatility of release, which is a critical aspect of the efficacy of oxytocin in milk ejection, for example. Oxytocin measured in blood in 1-day-old human infants points to pulsatile release (Marchini and Stock, 1996). The pattern of release and the mechanisms that contribute to pattern generation are important future research targets.
Oxytocin and vasopressin systems interact in adult animals (reviewed in Neumann and Landgraf, 2012). As described above, the available data suggest that in very young animals, oxytocin may facilitate social orienting, while vasopressin may inhibit social orienting. These opposing actions are reminiscent of the opposing aspects of oxytocin and vasopressin function on emotional behavior in adult animals. However, in contrast to opposing actions on social orienting in neonates, oxytocin and vasopressin often, but not always, regulate social behavior in the same direction in adults. The significance of potential developmental interaction of oxytocin and vasopressin tone remains to be fully explored.
What are the behavioral or perceptual implications of oxytocin modulating early postnatal excitatory activity in the neocortex? Is this a potential mechanism of neocortical hypoactivity or delayed maturation observed by EEG in Romanian orphans after extreme social and sensory neglect (Marshall et al, 2004) or children at psychosocial risk for cognitive disability (Otero et al, 2003)? Does more oxytocin activation of the neocortex in the context of a rich social and sensory environment improve early perceptual skills? Newer gene association studies have identified sensory processing relationships with OXTR genotype in adults, including decoding speech in a noisy environment (Tops et al, 2011), sensitivity to infant cues (Bakermans-Kranenburg and Van Ijzendoorn, 2008), and facial emotion processing (O’connell et al, 2012; Melchers et al, 2013). As described above, perception of ‘animacy’ was also recently associated with OXTR methylation status (Jack et al, 2012). These gene association data cannot differentiate between an organizing role for OXTR genotype in the developing sensory neocortex (Figures 3 and 4), or an activational role in adult sensory cortex. In light of the model of oxytocin-dependent neocortical development, we would predict an organizational role for the individual differences in OXTR genetics. For example, we would predict that infant T carriers of the rs2268498 SNP and GG carriers of the rs53576 single-nucleotide polymorphism would have more robust sensory encoding in layers II/III of neocortex during a postnatal developmental sensitive period. In combination with an enriched social-sensory developmental environment, this enhanced developmental expression of neocortical OXTR should lead to enhanced social expertise in adulthood because of more facile neural computation of sensory input as adults (Figure 4). Atypical neocortical multisensory processing is a proposed etiological factor in autism, indicating that this mechanism may help explain some of the gene association studies implicating OXTR in autism (Wu et al, 2005; Jacob et al, 2007; Israel et al, 2008; Lerer et al, 2008; Liu et al, 2010; Campbell et al, 2011), and also that it may be difficult to detect the effect of OXTR alleles on autism risk (Tansey et al, 2010) without controlling for social and sensory environment in early life. As sensory systems are exceptionally tractable experimental targets in human neuroscience research, exploring the interaction between oxytocin and neocortical function should be a highly informative research area in the near term. For example, some individuals with autism show atypical multisensory integration in psychophysical tasks like the flash-beep illusion (Foss-Feig et al, 2010; Stevenson et al, 2014), which involves combining visual and auditory cues into a single percept. Will intranasal delivery of oxytocin narrow the window of sensory integration in these individuals, but perhaps only at younger ages when neocortical OXTR levels are highest? Perhaps oxytocin permits developmental ‘binding’ of sensory information into a single unified perceptual whole.
Figure 4.
Hypothesis in which development shines light on the ‘Dark Matter of Social Neuroscience’, or the unclarified neural mechanisms between social sensory inputs and social behavior outputs (Insel, 2010). (a) During developmental sensitive periods, parental nurturing (Kojima et al, 2012; Weisman et al, 2012) and/or sensory inputs (Zheng et al, 2014) drive the activity of the PVN resulting in increased release of oxytocin. This oxytocin can regulate multi-sensory plasticity in the neocortex. (b) OXTRs are transiently available during experience-dependent developmental sensitive periods (Hammock and Levitt, 2013). Arrows point to layer II/III of neocortex, which has a high density at postnatal day 14, but not postnatal day 21 or later ages. Perhaps this entrains the developing brain to attend to relevant stimuli, which acquire social definitions because of their proximity in time and space to the nurturing caregiver. After such a developmental trajectory, an adult brain is tuned to ascribe social, affect-modulating properties to multisensory information. (c) For example, wild-type mice are tuned to social stimuli and can rapidly resolve sensory input to determine if a mouse is familiar or novel. In contrast, oxytocin knock-out mice are unable to resolve this social sensory information, despite very active neural processing in primary sensory cortex as measured by c-Fos immunoreactivity after a social encounter (Ferguson et al, 2001), c-Fos images courtesy of Dr Larry Young, copyright The Society for Neuroscience; hatched box at P21 in ‘b’ indicates cortical barrel fields measured in young adult (>P50) mice in panel (c). Thus, developmentally transient experience-dependent neocortical oxytocin signals may allow social support (ie, maternal contact) to fine tune the function of the entire neocortex during a developmental sensitive period for social contact. This layer II/III positioning makes OXTR a promising modulator of multi-sensory integration across modalities by changing the window of opportunity in which separate neural signals could be bound into one unified percept. Developmental modulation in this way by other classic neuroendocrine modulators (eg, CRF, vasopressin) during various sensitive periods may have a significant role in the species-typical development of the brain.
What general direction is the field heading?
In the next 10 years, we should have a clearer picture of how oxytocin and vasopressin relate to social support in development, and the risks and/or benefits of early exogenous exposure to these neuropeptides. The work of Zheng et al, although serendipitous in their discovery of a novel role for oxytocin in the neocortex, will open some exciting doors in helping to unravel mysteries in oxytocin and vasopressin contributions to development. We do not yet know how sensory experience, like whisker deprivation or total darkness, could directly reduce the production and release of oxytocin in the hypothalamus. An alternative explanation is that maternal factors rather than sensory deprivation per se mediate the enhanced oxytocin seen very early: it is possible that sensory deprived pups elicit lower maternal contact than intact pups. Further, an enriched environment may induce more maternal contact and thus more oxytocin activity, as a deprived environment disturbs maternal care in mice (Rice et al, 2008). Maternal care was not directly measured and although the effects of whisker deprivation were obtained in litters with whisker-deprived and intact control littermates, suggesting that maternal care toward the litter may not have a role, it is possible that dams show differential provisioning of care (eg, Cavigelli et al, 2010) to injured vs intact neonates. A lack of change in maternal care toward deprived pups will be needed to confirm this very exciting hypothesis that sensory inputs directly modulate oxytocin release from the PVN during experience-dependent development.
Social neuroscience has grown markedly in the past decade, and foundational research in behavioral neuroendocrinology, neuroethology, and sensory neuroscience have led the way to uncover brain mechanisms of social stimulus perception and social behavior action (Insel, 2010). The ‘dark matter of social neuroscience’ (Insel, 2010) is the gray area between perception and action, and is represented in a question like ‘how does the adult brain identify a stimulus as ‘social’ so that it may act upon it?’ This is both a mechanistic and a developmental question (Denenberg et al, 1964; Rosenblatt, 1983): early unconditioned stimuli in the environment such as maternal odor become paired with multisensory stimuli, which then acquire reinforcing properties. To many, it may come as a shock to see an effect of whisker trimming or dark rearing on a ‘social neuropeptide’ system like oxytocin—why does a non-social intervention like whisker trimming or total darkness reduce oxytocin content? Given the rather robust effects on oxytocin in these studies, why aren’t there obvious effects of intranasal oxytocin on vision or somatosensation in the human studies? I would argue that the effects of oxytocin on the neocortex are limited to early development and that they are part of the neural mechanism of mammalian social imprinting. The ability of experience to yield high levels of oxytocin in the neocortex appears time delimited: neocortical oxytocin levels were high at P7 and P14, but reached low adult levels by typical weaning age of P21, and further, robust receptor availability in the neocortex is limited to the pre-weaning period. These results are from developing animals—animals that are still acquiring their experience-dependent definitions of what is and what is not social. Ethologically, for altricial and helpless infants, everything in infancy is social: smells, touches, temperatures—there is nowhere the infant can go or exist on their own outside of a social context. Developmental comparisons of neuroendocrine systems in precocial vs altricial species should be particularly informative. In mice, this developmental period is when they are learning what it means to be a mouse (Denenberg et al, 1964). If any kind of sensory input at this age can drive oxytocin function, then it is going to be refined and entrained to be a very easy neural computation and perhaps a releaser of oxytocin later in life—the smell of the mother, the feel of her fur, her body temperature, her posture, the way she grooms herself and others, etc—these are the multisensory subtleties that mice should process quickly after such developmental experience is encoded in the brain. The recent exciting findings by Zheng et al provide a potential neurodevelopmental substrate to marry neuroendocrinological and sensory neuroscience approaches through an integration of hypothalamic and neocortical function around sensory experience. These findings shed light on ‘the dark matter of social neuroscience’ (Insel, 2010), by demonstrating oxytocin effects on sensory processing capacity in the developing neocortex (Figure 4).
What will the next big trends or discoveries be and what techniques will need to be developed or implemented to address these questions?
Rapid progress continues in all areas of social neuroscience at many levels of analysis. Well designed and precisely controlled behavioral, pharmacological, and optogenetic studies in developing animals will help to further our mechanistic understanding of oxytocin and vasopressin in experience-dependent developmental plasticity. Intranasal drug delivery and gene association studies in humans that take special care to include early life history variables (eg, childhood maltreatment, maternal depression) will be an interesting and likely productive research effort (Tabak, 2013). Ongoing clinical trials will do well to include life history variables, environmental context, and consider the potential contribution of individual differences in the genetics of these signaling pathways. As a result of the limitations of human research, we could use better biomarkers and a clear picture of the distribution of OXTR and V1aR in human tissue via the development of selective PET ligands (Smith et al, 2012), and endophenotype approaches to gene–brain–behavior relationships (Haas et al, 2013).
Clinical Implications
The clinical implications of the developmental roles for oxytocin and vasopressin are weighty. As oxytocin and vasopressin systems contribute to different aspects of brain physiology in different species thus having a role in species-typical social behaviors (Goodson, 2013), it is important to sample from a wide variety of animal systems to get a broad picture of both the potential and limitations of these systems in humans. The ability to translate appropriately across species and developmental time points becomes imperative as the enthusiasm grows for intranasal oxytocin and vasopressin therapies in various developmental disabilities and mental illness (Guastella et al, 2009, 2010; Andari et al, 2010; Feifel et al, 2010; Goldman et al, 2011; Pedersen et al, 2011; Simeon et al, 2011; Hall et al, 2012). In addition to gathering data from a wide variety of species, it will be important to understand the behavioral context in which oxytocin and vasopressin function. For example, if, in addition to enhancing social orienting, oxytocin facilitates developmental encoding of the environment, then oxytocin given to a child while in a toxic environment could potentially exacerbate the negative effects of that toxic environment.
Summary
Oxytocin and vasopressin contribute to the brain basis of social behavior. They influence brain and behavior development, yet more mechanistic research is needed in the area of the developmental contributions of these two neuropeptides.
CONCLUSIONS
The past decade has seen a robust and enthusiastic translation of neuropeptide research in animal models into human social behavior. As we move into the next era, we should see rapid gains in our understanding of the mechanisms of the roles of oxytocin and vasopressin in the experience-dependent development of the social brain. This is an exciting time.
FUNDING AND DISCLOSURE
Dr. Hammock has received funding from Vanderbilt University Department of Pediatrics, the Vanderbilt Kennedy Center for Research on Human Development, and a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation.
Acknowledgments
Dr. EADH gratefully acknowledges the anonymous reviewers, and Drs Larry Young and Xiang Yu for providing images and helpful comments on earlier drafts of the manuscript.
References
- Aguilar-Valles A, Vaissiere T, Griggs EM, Mikaelsson MA, Takacs IF, Young EJ et al (2013). Methamphetamine-associated memory is regulated by a, writer and an eraser of permissive histone methylation. Biol Psychiatry pii: S0006-3223(13)00855-X. doi:10.1016/j.biopsych.2013.09.014 (e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Albers HE (2012). The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav 61: 283–292. [DOI] [PubMed] [Google Scholar]
- Alberts JR (2007). Huddling by rat pups: ontogeny of individual and group behavior. Dev Psychobiol 49: 22–32 Group huddling behavior responds to central administration of oxytocin on day 10 in neonatal rats. [DOI] [PubMed] [Google Scholar]
- Almazan G, Lefebvre DL, Zingg HH (1989). Ontogeny of hypothalamic vasopressin, oxytocin and somatostatin gene expression. Brain Res Dev Brain Res 45: 69–75. [DOI] [PubMed] [Google Scholar]
- Altstein M, Gainer H (1988). Differential biosynthesis and posttranslational processing of vasopressin and oxytocin in rat brain during embryonic and postnatal development. J Neurosci 8: 3967–3977 This classic paper (among others) demonstrates the earlier emergence of vasopressin relative to oxytocin in rat brain development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A (2010). Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA 107: 4389–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter-Levy Y, Feldman M, Vakart A, Ebstein RP, Feldman R (2013). Impact of maternal depression across the first 6 years of life on the child's mental health, social engagement, and empathy: the moderating role of oxytocin. Am J Psychiatry 170: 1161–1168. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van Ijzendoorn MH (2008). Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci 3: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM (1995a). Regulation of oxytocin receptor messenger ribonucleic acid in the ventromedial hypothalamus by testosterone and its metabolites. Endocrinology 136: 5135–5138. [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM (1995b). Sex differences in and effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the ventromedial hypothalamus. Endocrinology 136: 27–32. [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM, Johnston CA (1995a). Oxytocin receptor mRNA expression in the ventromedial hypothalamus during the estrous cycle. J Neurosci 15: 5058–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Pedersen CA, Dorsa DM (1995b). CNS oxytocin receptor mRNA expression and regulation by gonadal steroids. Adv Exp Med Biol 395: 269–280. [PubMed] [Google Scholar]
- Bales KL, Carter CS (2003). Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster). Behav Neurosci 117: 854–859 A single dose of oxytocin given within 24 hours of birth results in increasesd probability of partner prefrence behavior in male prairie voles as adults. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM (2012). Developmental experiences and the oxytocin receptor system. Horm Behav 61: 313–319. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM et al (2013). Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry 74: 180–188 Chronic oxytocin delivered intranasally to juvenile prairie voles resulted in a surprising and counterintuitive reduction in selective partner preference behavior, a hallmark social behavior of prairie voles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Pfeifer LA, Carter CS (2004). Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Dev Psychobiol 44: 123–131. [DOI] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E et al (2007a). Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience 144: 38–45 This is an example of hormonal imprinting in which oxytocin in the neonate affects the adult expression of vasopressin 1a receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS (2007b). Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm Behav 52: 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh R, Porter RH (1986). Olfactory preferences resulting from mere exposure in human neonates. Infant Behavior and Development 9: 395–401 This interesting paper highlights the translational value of olfaction in understanding the ontogeny of mammalian social behavior. [Google Scholar]
- Bloch B, Guitteny AF, Chouham S, Mougin C, Roget A, Teoule R (1990). Topography and ontogeny of the neurons expressing vasopressin, oxytocin, and somatostatin genes in the rat brain: an analysis using radioactive and biotinylated oligonucleotides. Cell Mol Neurobiol 10: 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer GJ (1985). Vasopressin and brain development: studies using the Brattleboro rat. Peptides 6(Suppl 1): 49–62. [DOI] [PubMed] [Google Scholar]
- Boer GJ (1993). Chronic oxytocin treatment during late gestation and lactation impairs development of rat offspring. Neurotoxicol Teratol 15: 383–389. [DOI] [PubMed] [Google Scholar]
- Boer GJ, Feenstra MG, Botterblom MJ, Korse V, Te Riele P (1995). Early postnatal appearance of enhanced noradrenaline content in the brain of vasopressin-deficient Brattleboro rat; normal adrenoceptor densities and aberrant influences of vasopressin treatment. Int J Dev Neurosci 13: 63–74. [DOI] [PubMed] [Google Scholar]
- Boer GJ, Quak J, De Vries MC, Heinsbroek RP (1994). Mild sustained effects of neonatal vasopressin and oxytocin treatment on brain growth and behavior of the rat. Peptides 15: 229–236. [DOI] [PubMed] [Google Scholar]
- Boer GJ, Uylings HB, Patel AJ, Boer K, Kragten R (1982a). The regional impairment of brain development in the Brattleboro diabetes insipidus rat; some vasopressin supplementation studies. Ann N Y Acad Sci 394: 703–717. [DOI] [PubMed] [Google Scholar]
- Boer GJ, Van Rheenen-Verberg CM, Uylings HB (1982b). Impaired brain development of the diabetes insipidus Brattleboro rat. Brain Res 255: 557–575. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID (2012). Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav 61: 293–303. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Alberts JR (1979). Olfactory stimulation induces filial preferences for huddling in rat pups. J Comp Physiol Psychol 93: 548–555 This classic paper describes early familiar odor bias behavior in infant rats which could be considered analogous to imprinting behavior in geese. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Velis DN, Swaab DF (1980). Ontogeny of vasopressin and oxytocin in the fetal rat: early vasopressinergic innervation of the fetal brain. Peptides 1: 315–324. [DOI] [PubMed] [Google Scholar]
- Burford GD, Robinson IC (1982). Oxytocin, vasopressin and neurophysins in the hypothalamo-neurohypophysial system of the human fetus. J Endocrinol 95: 403–408. [DOI] [PubMed] [Google Scholar]
- Caffe AR, Van Leeuwen FW (1983). Vasopressin-immunoreactive cells in the dorsomedial hypothalamic region, medial amygdaloid nucleus and locus coeruleus of the rat. Cell Tissue Res 233: 23–33. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Wersinger SR, Young WS 3rd (2008). The role of the vasopressin 1b receptor in aggression and other social behaviours. Prog Brain Res 170: 65–72. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Datta D, Jones ST, Batey Lee E, Sutcliffe JS, Hammock EA et al (2011). Association of oxytocin receptor (OXTR) gene variants with multiple phenotype domains of autism spectrum disorder. J Neurodev Disord 3: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS (2003). Developmental consequences of oxytocin. Physiol Behav 79: 383–397. [DOI] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL (2009). Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci 31: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW (2008). Oxytocin, vasopressin and sociality. Prog Brain Res 170: 331–336. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, Ragan CM, Barrett CE, Michael KC (2010). Within-litter variance in rat maternal behaviour. Behav Processes 84: 696–704. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ (2001). Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA 98: 12736–12741 This paper reveals potential mechanisms of intergenerational transmission of maternal care through regulation of oxytocin receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ (2007). Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci 121: 1353–1363. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ (2006). Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 147: 2909–2915. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ (2003). Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology 144: 4720–4724. [DOI] [PubMed] [Google Scholar]
- Chen Q, Schreiber SS, Brinton RD (2000). Vasopressin and oxytocin receptor mRNA expression during rat telencephalon development. Neuropeptides 34: 173–180. [DOI] [PubMed] [Google Scholar]
- Choy VJ, Watkins WB (1979). Maturation of the hypothalamo-neurohypophysial system. I. Localization of neurophysin, oxytocin and vasopressin in the hypothalamus and neural lobe of the developing rat brain. Cell Tissue Res 197: 325–336. [DOI] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL (2007). Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics 5: 79–94 This reference and its associated web tool are an important resource for identifying similar neurodevelopmental events across mammalian model systems and humans. [DOI] [PubMed] [Google Scholar]
- Csaba G (1986). Receptor ontogeny and hormonal imprinting. Experientia 42: 750–759. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Yamamoto Y, Hoffman GE, Carter CS (2003). Central expression of c-Fos in neonatal male and female prairie voles in response to treatment with oxytocin. Brain Res Dev Brain Res 143: 129–136. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Moul C, Cauchi A, Dobson-Stone C, Hawes DJ, Brennan J et al (2014). Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Dev Psychopathol 26: 33–40. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Swaab DF (1981). Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain--presence of a sex difference in the lateral septum. Brain Res 218: 67–78 These authors demonstrate robust sex differences in the vasopressin system of the rat and although some of the details are variable, this observation is found across many species. [DOI] [PubMed] [Google Scholar]
- Delville Y, Koh ET, Ferris CF (1994a). Sexual differences in the magnocellular vasopressinergic system in golden hamsters. Brain Res Bull 33: 535–540. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Quan EW, Yules BM, Ferris CF (1994b). Postnatal development of the vasopressinergic system in golden hamsters. Brain Res Dev Brain Res 81: 230–239. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Hudgens GA, Zarrow MX (1964). Mice reared with rats: modification of behavior by early experience with another species. Science 143: 380–381 This is a classic paper for any reader with interests in the potency of early experience on later social preferences. [DOI] [PubMed] [Google Scholar]
- Di Scala-Guenot D, Strosser MT, Felix JM, Richard P (1990a). Expression of vasopressin and opiates but not of oxytocin genes studied by in situ hybridization in embryonic rat brain primary cultures. Brain Res Dev Brain Res 56: 35–39. [DOI] [PubMed] [Google Scholar]
- Di Scala-Guenot D, Strosser MT, Sarlieve LL, Legros JJ, Richard P (1990b). Development of neurophysin-containing neurons in primary cultures of rat hypothalami is related to the age of the embryo: morphological study and comparison of in vivo and in vitro neurophysins, oxytocin, and vasopressin content. J Neurosci Res 25: 94–102. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322: 900–904. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH (2013). Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav 64: 693–701. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS (2012). The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm Behav 61: 359–379. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B et al (2010). Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry 68: 678–680. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Influs M, Gutbir T, Ebstein RP (2013). Parental oxytocin and early caregiving jointly shape children's oxytocin response and social reciprocity. Neuropsychopharmacology 38: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O (2010). The cross-generation transmission of oxytocin in humans. Horm Behav 58: 669–676. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ (2001). Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21: 8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE (1984). Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science 224: 521–523. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Brewer JA, Mansour K, Yules B, Melloni RH Jr (1996). Vasopressin and developmental onset of flank marking behavior in golden hamsters. J Neurobiol 30: 192–204. [DOI] [PubMed] [Google Scholar]
- Fodor A, Zelena D (2014). The effect of maternal stress activation on the offspring during lactation in light of vasopressin. Scientific World J 2014: 265394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Kwakye LD, Cascio CJ, Burnette CP, Kadivar H, Stone WL et al (2010). An extended multisensory temporal binding window in autism spectrum disorders. Exp Brain Res 203: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ (1999). Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286: 1155–1158. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ (2000). Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol 12: 1145–1148. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR (2002). Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol 14: 349–353. [DOI] [PubMed] [Google Scholar]
- Gan EH, Quinton R (2010). Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones. Prog Brain Res 181: 111–126. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Sudhof TC, Fagiolini M, Hensch TK (2009). Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord 1: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Gomes AM, Carter CS, Lee R (2011). Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology (Berl) 216: 101–110. [DOI] [PubMed] [Google Scholar]
- Goodson JL (2013). Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology 38: 465–478 This review highlights important considerations to understand oxytocin and vasopressin in a species-specific context. [DOI] [PubMed] [Google Scholar]
- Gordon I, Martin C, Feldman R, Leckman JF (2011). Oxytocin and social motivation. Dev Cogn Neurosci 1: 471–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit E, Neijmeijer-Leloux A, Swaab DF (1992). The human hypothalamo-neurohypophyseal system in relation to development, aging and Alzheimer's disease. Prog Brain Res 93: 237–247 discussion 247-238. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA et al (2009). Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med 7: 62 The authors demonstrate that OXTR methylation can lead to expression changes in the temporal cortex in the context of autism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ et al (2010). Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry 67: 692–694. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS (2009). A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology 34: 917–923. [DOI] [PubMed] [Google Scholar]
- Haas BW, Anderson IW, Smith JM (2013). Navigating the complex path between the oxytocin receptor gene () and cooperation: an endophenotype approach. Front Hum Neurosci 7: 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Mccarthy BE, Parker KJ, Reiss AL (2012). Effects of intranasal oxytocin on social anxiety in males with fragile X syndrome. Psychoneuroendocrinology 37: 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock E, Levitt P (2013). Oxytocin receptor ligand binding in embryonic tissue and postnatal brain development of the C57BL/6J mouse. Front Behav Neurosci 7. [DOI] [PMC free article] [PubMed]
- Hammock EA, Law CS, Levitt P (2013). Vasopressin eliminates the expression of familiar odor bias in neonatal female mice through V1aR. Horm Behav 63: 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Levitt P (2012). Modulation of parvalbumin interneuron number by developmentally transient neocortical vasopressin receptor 1a (V1aR). Neuroscience 222C: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Young LJ (2005). Microsatellite instability generates diversity in brain and sociobehavioral traits. Science 308: 1630–1634. [DOI] [PubMed] [Google Scholar]
- Hammock EaD, Levitt P (2006). The discipline of neurobehavioral development: the emerging interface of processes that build circuits and skills. Human Dev 49: 294–309. [Google Scholar]
- Harony-Nicolas H, Mamrut S, Brodsky L, Shahar-Gold H, Barki-Harrington L, Wagner S (2014). Brain region-specific methylation in the promoter of the murine oxytocin receptor gene is involved in its expression regulation. Psychoneuroendocrinology 39: 121–131. [DOI] [PubMed] [Google Scholar]
- Hashemi F, Tekes K, Laufer R, Szegi P, Tothfalusi L, Csaba G (2013). Effect of a single neonatal oxytocin treatment (hormonal imprinting) on the biogenic amine level of the adult rat brain: could oxytocin-induced labor cause pervasive developmental diseases? Reprod Sci 20: 1255–1263. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB (2009). Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry 14: 954–958. [DOI] [PubMed] [Google Scholar]
- Hensch TK (2005). Critical period mechanisms in developing visual cortex. Curr Top Dev Biol 69: 215–237. [DOI] [PubMed] [Google Scholar]
- Hernando F, Schoots O, Lolait SJ, Burbach JP (2001). Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology 142: 1659–1668. [DOI] [PubMed] [Google Scholar]
- Hess EH (1959). Imprinting, an effect of early experience, imprinting determines later social behavior in animals. Science 130: 133–141. [DOI] [PubMed] [Google Scholar]
- Higashida H, Lopatina O, Yoshihara T, Pichugina YA, Soumarokov AA, Munesue T et al (2010). Oxytocin signal and social behaviour: comparison among adult and infant oxytocin, oxytocin receptor and CD38 gene knockout mice. J Neuroendocrinol 22: 373–379. [DOI] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Manago F, Sannino S, Scheggia D et al (2014). Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology 39: 1102–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo S, Yamada C, Takezawa T, Urano A (1992). Expression of provasopressin gene during ontogeny in the hypothalamus of developing mice. Neuroscience 46: 241–250. [DOI] [PubMed] [Google Scholar]
- Insel TR (2010). The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65: 768–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Winslow JT (1991). Central administration of oxytocin modulates the infant rat's response to social isolation. Eur J Pharmacol 203: 149–152. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Jacobson CD (1995a). Ontogeny of arginine vasopressin-like immunoreactivity in the Brazilian opossum brain. Brain Res Dev Brain Res 89: 11–32. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Jacobson CD (1995b). Ontogeny of oxytocin-like immunoreactivity in the Brazilian opossum brain. Brain Res Dev Brain Res 90: 1–16. [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, Uzefovsky F, Reibold M, Bachner-Melman R et al (2008). Molecular genetic studies of the arginine vasopressin 1a receptor (AVPR1a) and the oxytocin receptor (OXTR) in human behaviour: from autism to altruism with some notes in between. Prog Brain Res 170: 435–449. [DOI] [PubMed] [Google Scholar]
- Jack A, Connelly JJ, Morris JP (2012). DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Front Hum Neurosci 6: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH Jr. (2007). Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett 417: 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia R, Tai F, An S, Broders H, Sun R (2008a). Neonatal manipulation of oxytocin influences the partner preference in mandarin voles (Microtus mandarinus). Neuropeptides 42: 525–533. [DOI] [PubMed] [Google Scholar]
- Jia R, Tai FD, An SC, Broders H, Ding XL, Kong Q et al (2008b). Effects of neonatal oxytocin treatment on aggression and neural activities in mandarin voles. Physiol Behav 95: 56–62. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O et al (2007). CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446: 41–45. [DOI] [PubMed] [Google Scholar]
- Jing X, Ratty AK, Murphy D (1998). Ontogeny of the vasopressin and oxytocin RNAs in the mouse hypothalamus. Neurosci Res 30: 343–349. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M et al (2011). Spatio-temporal transcriptome of the human brain. Nature 478: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Igarashi N, Hirasawa A, Tsujimoto G, Kobayashi M (1995). Distribution and developmental changes in vasopressin V2 receptor mRNA in rat brain. Differentiation 59: 163–169. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E (2011). Sociality, pathogen avoidance, and the neuropeptides oxytocin and arginine vasopressin. Psychol Sci 22: 1367–1374. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Tyzio R, Ben-Ari Y (2008). Effects of oxytocin on GABA signalling in the foetal brain during delivery. Prog Brain Res 170: 243–257. [DOI] [PubMed] [Google Scholar]
- Kim J, Pitlick MM, Christine PJ, Schaefer AR, Saleme C, Comas B et al (2013). Genome-wide analysis of DNA methylation in human amnion. Scientific World J 2013: 678156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfer PH (1971). Mother love: what turns it on? Am Sci 59: 404–407. [PubMed] [Google Scholar]
- Klopfer PH, Klopfer MS (1968). Maternal ‘imprinting’ in goats: fostering of alien young. Z Tierpsychol 25: 862–866. [Google Scholar]
- Kojima S, Alberts JR (2009). Maternal care can rapidly induce an odor-guided huddling preference in rat pups. Dev Psychobiol 51: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Alberts JR (2011a). Oxytocin mediates the acquisition of filial, odor-guided huddling for maternally-associated odor in preweanling rats. Horm Behav 60: 549–558 In early post-natal life, oxytocin injected into the brain enhances familiarity preferences in neonatal rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Alberts JR (2011b). Warmth from skin-to-skin contact with mother is essential for the acquisition of filial huddling preference in preweanling rats. Dev Psychobiol 53: 813–827. [DOI] [PubMed] [Google Scholar]
- Kojima S, Stewart RA, Demas GE, Alberts JR (2012). Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother-pup interaction that induces a filial huddling preference. J Neuroendocrinol 24: 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KM, Choe C, Carter CS, Cushing BS (2006). Developmental effects of oxytocin on neural activation and neuropeptide release in response to social stimuli. Horm Behav 49: 206–214. [DOI] [PubMed] [Google Scholar]
- Krisch B (1980). Electron microscopic immunocytochemical investigation on the postnatal development of the vasopressin system in the rat. Cell Tissue Res 205: 453–471. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Hummel E, Chen FS, Heinrichs M (2013). Epigenetic regulation of the oxytocin receptor gene: implications for behavioral neuroscience. Front Neurosci 7: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusui C, Kimura T, Ogita K, Nakamura H, Matsumura Y, Koyama M et al (2001). DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochem Biophys Res Commun 289: 681–686. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID (2004). Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol 25: 150–176. [DOI] [PubMed] [Google Scholar]
- Laurent FM, Hindelang C, Klein MJ, Stoeckel ME, Felix JM (1989). Expression of the oxytocin and vasopressin genes in the rat hypothalamus during development: an in situ hybridization study. Brain Res Dev Brain Res 46: 145–154. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP (2008). Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry 13: 980–988. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW (2005). Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6: 312–324. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ (2004). Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429: 754–757 This paper demonstrated that the location of the receptor in the adult brain alters the probability of the display of species-typical behavior. This finding has implications for the ability of transient receptor profiles during development, along with experience, to help shape species-typical behavioral patterns. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ (2006). Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav 50: 506–517. [DOI] [PubMed] [Google Scholar]
- Lipari EF, Lipari D, Gerbino A, Di Liberto D, Bellafiore M, Catalano M et al (2001). The hypothalamic magnocellular neurosecretory system in developing rats. Eur J Histochem 45: 163–168. [DOI] [PubMed] [Google Scholar]
- Liu HX, Lopatina O, Higashida C, Tsuji T, Kato I, Takasawa S et al (2008). Locomotor activity, ultrasonic vocalization and oxytocin levels in infant CD38 knockout mice. Neurosci Lett 448: 67–70. [DOI] [PubMed] [Google Scholar]
- Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T et al (2010). Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet 55: 137–141. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Neumann ID, Veenema AH (2010). Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology 58: 78–87. [DOI] [PubMed] [Google Scholar]
- Madarasz E, Kornyei Z, Poulain DA, Theodosis DT (1992). Development of oxytocinergic neurons in monolayer cultures derived from embryonic, fetal and postnatal rat hypothalami. J Neuroendocrinol 4: 433–439. [DOI] [PubMed] [Google Scholar]
- Mamrut S, Harony H, Sood R, Shahar-Gold H, Gainer H, Shi YJ et al (2013). DNA methylation of specific CpG sites in the promoter region regulates the transcription of the mouse oxytocin receptor. PLoS One 8: e56869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini G, Stock S (1996). Pulsatile release of oxytocin in newborn infants. Reprod Fertil Dev 8: 163–165. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Fox NA, Bucharest Early Intervention Project Core, G. (2004). A comparison of the electroencephalogram between institutionalized and community children in Romania. J Cogn Neurosci 16: 1327–1338. [DOI] [PubMed] [Google Scholar]
- Mazzuca M, Minlebaev M, Shakirzyanova A, Tyzio R, Taccola G, Janackova S et al (2011). Newborn analgesia mediated by oxytocin during delivery. Front Cell Neurosci 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcquaid RJ, Mcinnis OA, Stead JD, Matheson K, Anisman H (2013). A paradoxical association of an oxytocin receptor gene polymorphism: early-life adversity and vulnerability to depression. Front Neurosci 7: 128 These authors demonstrate that a previously described 'prosocial' OXTR allele (G rs53576) may actually exacerbate the risk for depression after childhood maltreatment. This allele may favor social sensitivity leading to opposing outcomes depending on the rearing environment: vulnerability during adverse early experience or prosociality during emotionally healthy childhood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C (2007). Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol Psychiatry 61: 1109–1111. [DOI] [PubMed] [Google Scholar]
- Melchers M, Montag C, Markett S, Reuter M (2013). Relationship between oxytocin receptor genotype and recognition of facial emotion. Behav Neurosci 127: 780–787. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12: 524–538. [DOI] [PubMed] [Google Scholar]
- Mizumoto Y, Kimura T, Ivell R (1997). A genomic element within the third intron of the human oxytocin receptor gene may be involved in transcriptional suppression. Mol Cell Endocrinol 135: 129–138. [DOI] [PubMed] [Google Scholar]
- Mogi K, Nagasawa M, Kikusui T (2011). Developmental consequences and biological significance of mother-infant bonding. Prog Neuropsychopharmacol Biol Psychiatry 35: 1232–1241. [DOI] [PubMed] [Google Scholar]
- Nelson E, Alberts JR (1997). Oxytocin-induced paw sucking in infant rats. Ann N Y Acad Sci 807: 543–545. [DOI] [PubMed] [Google Scholar]
- Nelson E, Panksepp J (1996). Oxytocin mediates acquisition of maternally associated odor preferences in preweanling rat pups. Behav Neurosci 110: 583–592. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J (1998). Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev 22: 437–452. [DOI] [PubMed] [Google Scholar]
- Neumann ID (2007). Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans 35: 1252–1257. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R (2012). Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 35: 649–659. [DOI] [PubMed] [Google Scholar]
- Ninan I (2011). Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. J Neurochem 119: 324–331. [DOI] [PubMed] [Google Scholar]
- Noonan LR, Caldwell JD, Li L, Walker CH, Pedersen CA, Mason GA (1994). Neonatal stress transiently alters the development of hippocampal oxytocin receptors. Brain Res Dev Brain Res 80: 115–120 This is one of the few early studies where the effects of environmental variables on the oxytocin system was measured during development. [DOI] [PubMed] [Google Scholar]
- O’connell G, Whalley HC, Mukherjee P, Stanfield AC, Montag C, Hall J et al (2012). Association of genetic variation in the promoter region of OXTR with differences in social affective neural processing. J Behav Brain Sci 2: 60–66. [Google Scholar]
- Otero GA, Pliego-Rivero FB, Fernandez T, Ricardo J (2003). EEG development in children with sociocultural disadvantages: a follow-up study. Clin Neurophysiol 114: 1918–1925. [DOI] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW (2013). Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 500: 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML (2002). Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress 5: 259–267 This important review links oxytocin to the infant side of the maternal-infant bond and suggests this as a mechanism of intergenerational transmission of maternal care. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL et al (2011). Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res 132: 50–53. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ Jr. (1979). Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci USA 76: 6661–6665 This is the classic foundation paper identifying the role of brain oxytocin in social behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena CJ, Neugut YD, Champagne FA (2013). Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats. Endocrinology 154: 4340–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Paramadilok A, Cushing BS (2009). Neonatal oxytocin alters subsequent estrogen receptor alpha protein expression and estrogen sensitivity in the female rat. Behav Brain Res 205: 154–161. [DOI] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Carr MS, Papademeteriou E, Schmidt JV, Cushing BS (2007). Oxytocin selectively increases ERalpha mRNA in the neonatal hypothalamus and hippocampus of female prairie voles. Neuropeptides 41: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Uhl GR (1987). Vasopressin messenger ribonucleic acid in supraoptic and suprachiasmatic nuclei: appearance and circadian regulation during development. Endocrinology 120: 2483–2487. [DOI] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ (2008). A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 149: 4892–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Huffmeijer R, Van Ijzendoorn MH (2013). Does intranasal oxytocin promote prosocial behavior to an excluded fellow player? A randomized-controlled trial with Cyberball. Psychoneuroendocrinology 38: 1418–1425. [DOI] [PubMed] [Google Scholar]
- Rinne UK, Kivalo E, Talanti S (1962). Maturation of human hypothalamic neurosecretion. Biol Neonat 4: 351–364. [DOI] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ (2011). Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol 519: 2434–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Stott RT, You S, Smith CJ, Woodbury ME, De Vries GJ (2013). Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J Comp Neurol 521: 2321–2358. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS (1983). Olfaction mediates developmental transition in the altricial newborn of selected species of mammals. Devel Psychobiol 16: 347–375. [DOI] [PubMed] [Google Scholar]
- Schank JC (2009). Early locomotor and social effects in vasopressin deficient neonatal rats. Behav Brain Res 197: 166–177. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Insel TR (1989). Ontogeny of oxytocin receptors in rat forebrain: a quantitative study. Synapse 4: 259–266. [DOI] [PubMed] [Google Scholar]
- Sigling HO, Wolterink-Donselaar IG, Spruijt BM (2009). Home seeking behavior in rat pups: attachment vs kin selection, oxytocin vs vasopressin. Eur J Pharmacol 612: 48–53. [DOI] [PubMed] [Google Scholar]
- Silverman AJ (1975). The hypothalamic magnocellular neurosecretory system of the guinea pig. II. Immunohistochemical localization of neurophysin and vasopressin in the fetus. Am J Anat 144: 445–459. [DOI] [PubMed] [Google Scholar]
- Simeon D, Bartz J, Hamilton H, Crystal S, Braun A, Ketay S et al (2011). Oxytocin administration attenuates stress reactivity in borderline personality disorder: a pilot study. Psychoneuroendocrinology 36: 1418–1421. [DOI] [PubMed] [Google Scholar]
- Sinding C, Czernichow P, Seif SM, Robinson AG (1980a). Quantitative changes in neurohypophyseal peptides in the developing brain. Peptides 1: 45–50. [DOI] [PubMed] [Google Scholar]
- Sinding C, Robinson AG, Seif SM, Schmid PG (1980b). Neurohypophyseal peptides in the developing rat fetus. Brain Res 195: 177–186. [DOI] [PubMed] [Google Scholar]
- Smith AL, Freeman SM, Stehouwer JS, Inoue K, Voll RJ, Young LJ et al (2012). Synthesis and evaluation of C-11, F-18 and I-125 small molecule radioligands for detecting oxytocin receptors. Bioorg Med Chem 20: 2721–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijdewint FG, Van Leeuwen FW, Boer GJ (1989). Ontogeny of vasopressin and oxytocin binding sites in the brain of Wistar and Brattleboro rats as demonstrated by lightmicroscopical autoradiography. J Chem Neuroanat 2: 3–17. [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK (2012). The vasopressin 1b receptor and the neural regulation of social behavior. Horm Behav 61: 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Schneider BC, Eberly HE, Woynaroski TG, Camarata SM et al (2014). Multisensory temporal integration in autism spectrum disorders. J Neurosci 34: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R (2012). Neuromodulation by oxytocin and vasopressin. Neuron 76: 142–159. [DOI] [PubMed] [Google Scholar]
- Stribley JM, Carter CS (1999). Developmental exposure to vasopressin increases aggression in adult prairie voles. Proc Natl Acad Sci USA 96: 12601–12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF (1995). Development of the human hypothalamus. Neurochem Res 20: 509–519. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Boer GJ (1983). Neuropeptides and brain development. Current perils and future potential. J Dev Physiol 5: 67–75. [PubMed] [Google Scholar]
- Szot P, Dorsa DM (1993). Differential timing and sexual dimorphism in the expression of the vasopressin gene in the developing rat brain. Brain Res Dev Brain Res 73: 177–183. [DOI] [PubMed] [Google Scholar]
- Tabak BA (2013). Oxytocin and social salience: a call for gene-environment interaction research. Front Neurosci 7: 199 This review and references therein provide a compelling argument for studying gene by environment interactions in OXTR and social-emotional behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T et al (2005). Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA 102: 16096–16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey KE, Brookes KJ, Hill MJ, Cochrane LE, Gill M, Skuse D et al (2010). Oxytocin receptor (OXTR) does not play a major role in the aetiology of autism: genetic and molecular studies. Neurosci Lett 474: 163–167. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Montagnese C, Rodriguez F, Vincent JD, Poulain DA (1986). Oxytocin induces morphological plasticity in the adult hypothalamo-neurohypophysial system. Nature 322: 738–740 Using electron microscopy, these authors demonstrate that oxytocin given to the third ventricle results in increased cellular contact among oxytocinergic cells in the supraoptic nucleus due to retreating glia. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH (2011). Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology 36: 144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C et al (2010). An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature 464: 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, Van Ijzendoorn MH, Riem MM, Boksem MA, Bakermans-Kranenburg MJ (2011). Oxytocin receptor gene associated with the efficiency of social auditory processing. Front Psychiatry 2: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Audigier S, Dubois-Dauphin M, Dreifuss JJ (1990). Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats. An autoradiographical study. Brain Res 511: 129–140. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Charpak S, Schmidt A, Dubois-Dauphin M, Dreifuss JJ (1989). Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied by autoradiography and electrophysiology. J Neurosci 9: 1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Barberis C, Jard S (1992). Oxytocin receptors in the central nervous system. Distribution, development, and species differences. Ann N Y Acad Sci 652: 29–38. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Goumaz M, Raggenbass M, Dreifuss JJ (1991a). Appearance and transient expression of vasopressin and oxytocin receptors in the rat brain. J Recept Res 11: 333–346. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Goumaz M, Raggenbass M, Dubois-Dauphin M, Dreifuss JJ (1991b). Early appearance and transient expression of vasopressin receptors in the brain of rat fetus and infant. An autoradiographical and electrophysiological study. Brain Res Dev Brain Res 58: 13–24. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hubner CA, Represa A et al (2006). Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science 314: 1788–1792 This important paper describes potential neuroprotective effects of oxytocin during labor and delivery. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S et al (2014). Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science 343: 675–679. [DOI] [PubMed] [Google Scholar]
- Uhl-Bronner S, Waltisperger E, Martinez-Lorenzana G, Condes Lara M, Freund-Mercier MJ (2005). Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat. Neuroscience 135: 147–154. [DOI] [PubMed] [Google Scholar]
- Van Der Sluis PJ, Boer GJ, Swaab DF (1986). Vasopressin and oxytocin in the developing rat brain as shown by isoelectric focusing of radioimmunoassayable peptides. Brain Res 391: 85–90. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Snijdewint FG, Boer GJ, Burbach JP (1986). Postnatal development of vasopressin mRNA content in supraoptic and paraventricular nucleus of the Wistar rat. Neurosci Lett 65: 1–6. [DOI] [PubMed] [Google Scholar]
- Vargas KJ, Sarmiento JM, Ehrenfeld P, Anazco CC, Villanueva CI, Carmona PL et al (2009). Postnatal expression of V2 vasopressin receptor splice variants in the rat cerebellum. Differentiation 77: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH (2012). Toward understanding how early-life social experiences alter oxytocin- and vasopressin-regulated social behaviors. Horm Behav 61: 304–312. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ (2012). Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav 61: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker DW, Ludwig M (2012). Vasopressin, oxytocin, and social odor recognition. Horm Behav 61: 259–265. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu Y, Young LJ, Insel TR (1997). Developmental changes in forebrain vasopressin receptor binding in prairie voles (Microtus ochrogaster) and montane voles (Microtus montanus). Ann N Y Acad Sci 807: 510–513. [DOI] [PubMed] [Google Scholar]
- Wang Z, Young LJ (1997). Ontogeny of oxytocin and vasopressin receptor binding in the lateral septum in prairie and montane voles. Brain Res Dev Brain Res 104: 191–195. [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R (2012). Oxytocin administration to parent enhances infant physiological and behavioral readiness for social engagement. Biol Psychiatry 72: 982–989. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O'carroll AM, Lolait SJ, Young WS 3rd (2002). Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry 7: 975–984. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, K RK, Zufall F, Lolait SJ, O'carroll AM, Young WS 3rd (2004). Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm Behav 46: 638–645. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Temple JL, Caldwell HK, Young WS 3rd (2008). Inactivation of the oxytocin and the vasopressin (Avp) 1b receptor genes, but not the Avp 1a receptor gene, differentially impairs the Bruce effect in laboratory mice (Mus musculus). Endocrinology 149: 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnall MH, Key S, Ben-Barak Y, Ozato K, Gainer H (1985). Neurophysin in the hypothalamo-neurohypophysial system. II. Immunocytochemical studies of the ontogeny of oxytocinergic and vasopressinergic neurons. J Neurosci 5: 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR (1993). A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365: 545–548 This foundation paper identifies vasopressin as a significant contributor to adult social bonds. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR (2000). Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav 37: 145–155. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR (1993). Effects of central vasopressin administration to infant rats. Eur J Pharmacol 233: 101–107. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR (2003). Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology 28: 910–918. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD (2005). Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci USA 102: 17237–17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt DM, Carter CS, Walton DM (1990). Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster). Pharmacol Biochem Behav 37: 63–69. [DOI] [PubMed] [Google Scholar]
- Wolf G, Kiessig R, Landgraf R (1984). Levels of vasopressin and oxytocin in neurohypophysis and plasma of the postnatally developing rat and the influence of hypothyroidism on rat fetuses. Exp Clin Endocrinol 83: 251–255. [DOI] [PubMed] [Google Scholar]
- Worley RT, Pickering BT (1984). Non-neuronal cells of rat hypothalamus in dissociated cell culture. Evidence that neurophysin modulates growth and DNA synthesis of non-neuronal cells. Cell Tissue Res 237: 161–168. [DOI] [PubMed] [Google Scholar]
- Wray S, Kusano K, Gainer H (1991). Maintenance of LHRH and oxytocin neurons in slice explants cultured in serum-free media: effects of tetrodotoxin on gene expression. Neuroendocrinology 54: 327–339. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M et al (2005). Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry 58: 74–77. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Carter CS, Cushing BS (2006). Neonatal manipulation of oxytocin affects expression of estrogen receptor alpha. Neuroscience 137: 157–164. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS (2004). Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience 125: 947–955. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kawamoto K, Kawashima S (1988a). Arginine vasopressin contents of the hypothalamus and pituitary during fetal and postnatal-development in the mouse. Dev Growth Differ 30: 563–571. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kawamoto K, Kawashima S (1988b). Fetal and postnatal-development of arginine vasopressin-immunoreactive neurons in the mouse. Zoological Science 5: 1019–1032. [Google Scholar]
- Yoshimura R, Kimura T, Watanabe D, Kiyama H (1996). Differential expression of oxytocin receptor mRNA in the developing rat brain. Neurosci Res 24: 291–304. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z (2011). The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol 32: 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ (1999). Frank A. Beach Award. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm Behav 36: 212–221. [DOI] [PubMed] [Google Scholar]
- Young LJ (2013). When too much of a good thing is bad: chronic oxytocin, development, and social impairments. Biol Psychiatry 74: 160–161. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Donaldson R, Rissman EF (1998). Estrogen receptor alpha is essential for induction of oxytocin receptor by estrogen. Neuroreport 9: 933–936. [DOI] [PubMed] [Google Scholar]
- Zai CC, Muir KE, Nowrouzi B, Shaikh SA, Choi E, Berall L et al (2012). Possible genetic association between vasopressin receptor 1B and child aggression. Psychiatry Res.. [DOI] [PubMed]
- Zheng JJ, Li SJ, Zhang XD, Miao WY, Zhang D, Yao H et al (2014). Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat Neurosci 17: 391–399 This truly exceptional paper is one of the few which measures the direct impact of oxytocin on brain function during development leading to novel and transformative results. [DOI] [PubMed] [Google Scholar]
- Zink CF, Meyer-Lindenberg A (2012). Human neuroimaging of oxytocin and vasopressin in social cognition. Horm Behav 61: 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]