Abnormalities of regional brain functional connectivity have been suggested for the syndromes of schizophrenia since the origin of the name using the Greek roots: skhizein (to split) and phren (mind) by Eugen Bleuler in 1908 (Kuhn, 2004). The neuropsychologist Norman Geschwind generalized this pathophysiological concept in his classic papers about what he called the disconnexion syndromes (Geschwind, 1965). He suggested that deficits in higher functions resulted from the disruption of pathways involving the signal relay functions of the association cortices. Discontinuities in white matter observed in diffusion tensor imaging and inferences from fMRI have been interpreted as evidence for cortical network disconnections in patients with schizophrenia (Bullmore et al, 1997).
More dynamical approaches to functional pathophysiological connections between brain regions have assessed their nearly simultaneous mutual similarities in frequency, wavelength, and phase (Uhlhaas and Singer, 2006). In addition, analysis of the envelope of oscillatory activity has yielded additional measures of connectivity. However, the brain does not operate by copying information from one region to another. The more general approach is to observe how broadband electrophysiological activity in one region shares information with other regions. To accomplish this we have expanded on our previous work using nonlinear dynamical measures of complexity for cortical activity (Robinson et al, 2012; Mandell, 2013).
A unique non-parametric measure of dynamical complexity, the symbolic rank vector approach (Robinson et al, 2012), was invoked to characterize the activity of the beamformer localized, time-dependent sources of the resting 275-channel whole-head magnetoencephalogram (MEG). MEG is the magnetic counterpart of EEG and has the advantage of yielding measures of cortical activity that are nearly independent of intervening conductivities. The source time series for an array of voxel locations is estimated from the multi-channel measurements using a linearly constrained minimum variance beamformer (Robinson and Rose, 1993). Each voxel time series is then converted to a symbolic time series by ranking their source magnitudes within a short n-place moving window. A measure of shared information, symbolic mutual information (SMI), is then computed for all voxel pairs from the probability of occurrence of their symbolic states (see Kraskov et al, 2004).
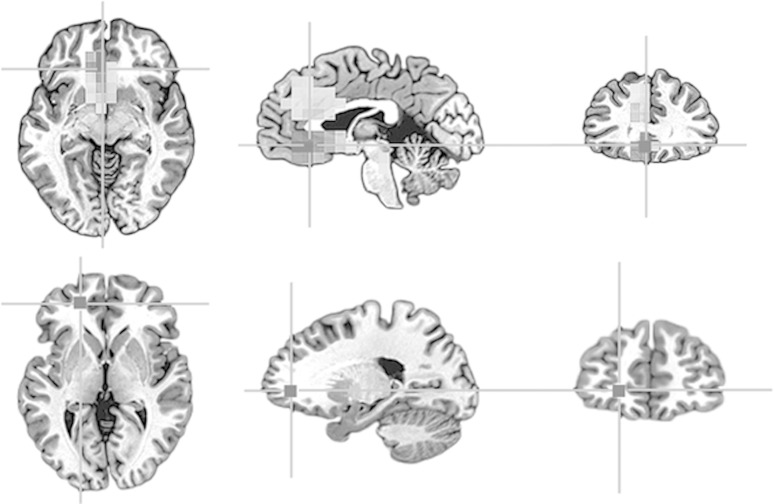
Subjects were recruited nationwide as part of an ongoing family study of schizophrenia at the Clinical Brain Disorders Branch Sibling Study (Protocol 95-M-0150) at the NIH and supported by the NIMH Intramural Research Program. MEG was collected under Protocol 99-M-0172. SMI analysis was applied to resting MEG data from 15 proband schizophrenics (10 M/5 F, 20–45 years) and 14 normal control subjects (5 M/9 F, 19–36 years). The schizophrenic subjects all met the DSM-IV-TR criteria and were medicated to ∼500 CPZ equivalents. Figure 1 illustrates a typical example of the network differences, showing characteristically higher values for SMI in schizophrenic than in normal controls, suggesting hyper-connectivity in rostral prefrontal cortex for short-range connections. Hypo-connectivity was also observed for long-range connections to lateral prefrontal cortical regions. The left prefrontal lobe location of this hyperconnectivity example is consistent with the literature implicating this area in the primary thought disorder, ambivalence, and thought-blocking characteristics of this patient group (Kuhn, 2004).
Figure 1.
Group difference (top) between schizophrenics (n=15) and normal control subjects (n=14) for symbolic mutual information during rest with FDR threshold q<0.01. The seed location is shown at the bottom. This is one example of hyperconnectivity in rostral prefrontal cortex.
These preliminary findings are subject to refinement by incorporation of cofactors such as diagnostic scale, medication, and gender.The SMI measure of shared information is not dependent upon task-related activity and may be applicable to assessing psychiatric disorders and their response to psychopharmaceuticals.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the National Institute of Mental Health, NIMH Intramural Research Program (Robinson), and the Fetzer-Franklin Trust of the Fetzer Foundation (Mandell).
References
- Bullmore E, Frangou S, Murray R (1997). The dysplastic net hypothesis: integration of developmental and dysconnectivity theories of schizophrenia. Schiz Res 28: 143–156. [DOI] [PubMed] [Google Scholar]
- Geschwind N (1965). Disconnexion syndromes in animals and man. I. Brain 88: 237–294. [DOI] [PubMed] [Google Scholar]
- Kraskov A, Stögbauer H, Grassberger P (2004). Estimating mutual information. Phys Rev E 69: 066138. [DOI] [PubMed] [Google Scholar]
- Kuhn R (2004). Eugen Bleuler's concepts of psychopathology. History Psychiatry 15: 361–366. [DOI] [PubMed] [Google Scholar]
- Mandell AJ (2013). Can a metaphor of physics contribute to neuroscience research; intermittent turbulent eddies in brain magnetic fields. Chaos Solitons Fractals 55: 95–101. [Google Scholar]
- Robinson SE, Mandell AJ, R. Coppola R (2012). Spatiotemporal imaging of complexity. Front Comput Neurosci 6: 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SE, Rose DF (1993). Current source image estimation by spatially filtered MEG. In: Hoke M, Erné SN, Okada YC, Romani GL, (eds). Biomagnetism: Clinical Aspects. Elsevier Science Publishers B. V.: Amsterdam. pp 761–765. [Google Scholar]
- Uhlhaas P, Singer W (2006). Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 52: 155–168. [DOI] [PubMed] [Google Scholar]