Abstract
Background:
Sleep fragmentation (SF) is highly prevalent and may constitute an important contributing factor to excessive weight gain and the metabolic syndrome. Increased endoplasmic reticulum (ER) stress and activation of the unfolded protein response (UPR) leading to the attenuation of leptin receptor signaling in the hypothalamus leads to obesity and metabolic dysfunction.
Methods:
Mice were exposed to SF and sleep control (SC) for varying periods of time during which ingestive behaviors were monitored. UPR pathways and leptin receptor signaling were assessed in hypothalami. To further examine the mechanistic role of ER stress, changes in leptin receptor (ObR) signaling were also examined in wild-type mice treated with the ER chaperone tauroursodeoxycholic acid (TUDCA), as well as in CHOP −/+ transgenic mice.
Results:
Fragmented sleep in male mice induced increased food intake starting day 3 and thereafter, which was preceded by increases in ER stress and activation of all three UPR pathways in the hypothalamus. Although ObR expression was unchanged, signal transducer and activator of transcription 3 (STAT3) phosphorylation was decreased, suggesting reduced ObR signaling. Unchanged suppressor of cytokine signaling-3 (SOCS3) expression and increases in protein-tyrosine phosphatase 1B (PTP1B) expression and activity emerged with SF, along with reduced p-STAT3 responses to exogenous leptin. SF-induced effects were reversed following TUDCA treatment and were absent in CHOP −/+ mice.
Conclusions:
Sleep fragmentation (SF) induces hyperphagic behaviors and reduced leptin signaling in hypothalamus that are mediated by activation of endoplasmic reticulum (ER) stress, and ultimately lead to increased PTP1B activity. ER stress pathways are therefore potentially implicated in SF-induced weight gain and metabolic dysfunction, and may represent a viable therapeutic target.
Citation:
Hakim F, Wang Y, Carreras A, Hirotsu C, Zhang J, Peris E, Gozal D. Chronic sleep fragmentation during the sleep period induces hypothalamic endoplasmic reticulum stress and ptp1b-mediated leptin resistance in male Mice. SLEEP 2015;38(1):31–40.
Keywords: endoplasmic reticulum stress, leptin resistance, sleep fragmentation
INTRODUCTION
The prevalence of obesity has been steadily increasing around the globe over the past 100 years, and has led to marked increases in the incidence of obesity-associated morbidities, along with the attendant increases in health care costs.1 In parallel with these trends, progressive reductions in sleep duration and sleep integrity have occurred, leading to the hypothesis that these two phenomena may be causally related. Indeed, chronic sleep restriction and consequent sleepiness have been associated with increased propensity for development of obesity and diabetes.2–5 Although fragmented sleep is not necessarily accompanied by curtailed sleep duration, it is also associated with daytime sleepiness, and preliminary studies in humans have further suggested that similar to sleep restriction, sleep fragmentation (SF) also adversely affects metabolic homeostasis, leading to increased appetite and food consumption, and also promotes insulin resistance.6
The adipose tissue secretes a variety of signaling molecules, including lipids and numerous polypeptides that regulate systemic glucose and lipid metabolism. Leptin, one of the most important adipose tissue-derived hormones, plays a major role in the regulation of energy intake and expenditure in terms of appetite and metabolic control, with leptin circulating levels being directly proportional to the total amount of body fat. Leptin binds to its specific hypothalamic receptors, and inhibits appetite by counteracting the effects of the orexigenic neuro-peptide Y (NPY), while enhancing the synthesis of alpha melanocyte stimulating hormone (α-MSH), an appetite suppressant. The absence of leptin, or its receptor, or alternatively decreased leptin receptor signaling in the hypothalamus leads to uncontrolled food intake, resulting in weight gain and obesity.7 Multiple isoforms of the leptin receptor (ObR) are produced as a result of the alternate splicing of the gene. Among these, the long isoform (Ob-Rb) is highly expressed in the hypothalamus, where it plays a critical role in the regulation of metabolic rate.8 ObR signaling involves Janus kinase 2 (JAK2) auto-phosphor-ylation and activation of JAK2, as well as the subsequent phosphorylation of Tyr residues on the cytoplasmic region of ObR.9 pTyr residues on ObR act as docking sites for downstream signaling proteins, such as STAT3, STAT5, and SHP-2, which then translocate to the nucleus to induce target gene transcription.10 Dephosphorylation of JAK2 is an important mechanism for terminating leptin signal transduction, and can be induced by either increased hypothalamic expression of suppressor of cytokine signaling-3 (SOCS3)11 or by the activity of protein-tyrosine phosphatase 1B (PTB1b).12–14 Indeed, PTPb1 has been implicated as a negative regulator of the leptin signaling pathway in the hypothalamus,15 as evidenced by previous reports that PTP1B is elevated in high-fat–fed rats,16 deficiency of hypothalamic PTP1B results in resistance to diet-induced obesity caused by leptin hypersensitivity in mice. Nevertheless, the use of tissue-specific PTP1B knockout further implicates the role of neuronal PTP1B in the control of whole-body leptin sensitivity and the development of leptin resistance.15
Over the past decade, it has become clear that obesity is associated with the activation of cellular stress signaling and inflammatory pathways in multiple organs including the hypothalamus.17–19 A key player in the cellular stress response is the endoplasmic reticulum (ER); pathological stress conditions will disrupt ER homeostasis and lead to accumulation of unfolded or misfolded proteins in the ER lumen. To cope with this stress, cells will activate a signal transduction system linking the ER lumen with the cytoplasm and nucleus, i.e., the unfolded protein response (UPR). During ER stress, the glucose-regulated protein of 78 kDa (GRP78), a molecular chaperone also known as binding immunoglobulin protein (BiP), initiates the UPR signaling cascade. After initiation by GRP78, the main UPR signaling is propagated by three ER-localized protein sensors: inositol-requiring transmembrane kinase-endoribonuclease-1α (IRE1α), double-stranded RNA-dependent protein kinase-like ER kinase (PERK), and activating transcription factor 6 (ATF6). In the resting state, GRP78 binds the N-termini of IRE1α, PERK, and ATF6, preventing their activation. Upon activation, GRP78 binds to unfolded or misfolded proteins, and releases IRE1α, PERK, and ATF6, triggering UPR signaling. The intrinsic ribonuclease activity of IRE1α results in the production of X-box binding protein-1 (XBP-1), a transcription factor that induces the expression of genes involved in restoring protein folding or in degrading unfolded proteins. However, PERK activates initiation factor 2α phosphorylation, preventing general protein synthesis through translation repression. However, under sustained ER stress, the cells may fail to resolve the protein-folding defect and to restore homeostasis in the ER; the resultant UPR then initiates the programmed cell death machinery consisting of apoptosis and autophagy.20
Recent lines of evidence initially described by Ozcan and colleagues17 have revealed that obesogenic behaviors and metabolic dysfunction are promoted by the attenuation of leptin receptor signaling in the hypothalamus, via induction of ER stress and activation of the UPR.17,18,21,22 ER stress and UPR activation are also induced by sleep deprivation.23,24 Thus, we hypothesized that SF will induce hypothalamic ER stress, activate the UPR, and down-regulate leptin receptor signaling, thereby favoring increased orexigenic behaviors and increased weight accrual, ultimately leading to obesity.
METHODS
Animals
Male C57BL/6J mice weighing 22–25 g were purchased from Jackson Laboratories (Bar Harbor, ME, USA), were housed in a 12 h light/dark cycle (light on 07:00 to 19:00) at a constant temperature (24 ± 1°C) and were allowed access to food and water ad libitum. All the experimental procedures took place after at least 1 w of habituation to the facility and started at 8–9 w of age. The experimental protocols were approved by the Institutional Animal Use and Care Committee and are in close agreement with the (Animal Research: Reporting In Vivo Experiments) ARRIVE guidelines and National Institutes of Health Guide in the Care and Use of Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Sleep Fragmentation
The device used to induce SF in rodents has been previously described20,25 (catalog #Model 80390, Lafayette Instruments, Lafayette, IN, USA), and uses intermittent tactile stimulation of freely behaving mice in a standard laboratory mouse cage, using a near-silent motorized mechanical sweeper. This method prevents the need for human contact and intervention, introduction of foreign objects or touching of the animals during sleep, and is therefore superior to other existing methods of sleep fragmentation. To induce moderate to severe sleep fragmentation that is present in multiple sleep disorders, we chose a 2-min interval between each sweep, implemented during the light period (07:00 to 19:00). The sweeper required 9 sec to travel the distance of the cage. Of note, four to five mice were housed in each sleep fragmentation cage to prevent isolation stress, and all mice had ad libitum access to food and water. Sleep control mice (SC) were housed in the same conditions with the sweeper turned off.
Acclimatization and Sleep Recordings
After complete recovery from surgery (supplemental material), mice were transferred to the SF devices for acclimatization and initial habituation to the sweeper movement. During habituation, the device was switched on for 15 min (twice per day) at random intervals during the light period. The recording cages were mounted on a DSI telemetry receiver (RPC-1) (DSI, St. Paul, MN, USA), which were in turn connected to an acquisition computer through a data exchange matrix. Although four mice were housed at each cage, only one of the mice in each cage was instrumented with the telemetric transponder and recorded. After 1 w of acclimatization in the cages, the magnetic switch of the transmitter was activated, and polygraphic recordings were begun at 07:00. Physiological data were continuously acquired for 24 h using Dataquest ART acquisition software, version 3.1 (DSI), at a sampling rate of 500 Hz.
Sleep-Wake Parameters
The behavior data were first scored automatically using Sleepsign software (Kissei Comtec, Japan), and records were visually confirmed or corrected as needed. Many researchers have adopted and successfully applied this software for sleep-wake analyses.25 Behavior was classified into three different states: wake (W), slow wave sleep (SWS) and rapid eye movement (REM) sleep. Electroencephalography (EEG) during W had low-amplitude, high-frequency (desynchronized) waves. During wake, electromyography (EMG) records showed gross body movement artifacts and behaviorally, animals had grooming, scratching and orienting activity. The SWS was characterized by low-frequency, high-amplitude (synchronized) EEG with a considerable reduction in EMG amplitude. The mice assumed a curled recumbent posture during this period. REM sleep was characterized by desynchronized EEG, and a drastic reduction in EMG (muscle atonia). Sleep related low-frequency (delta) activity was also derived from the records using bandpass filtering of 1–4.0 Hz. Delta power, although not changed by SF,25 was computed using the SleepSign software by fast Fourier transform (FFT), which was based on 512 points corresponding to 2-sec epochs, at a sampling rate of 250 Hz with Hanning as the window filter of FFT. The SWS epoch, which showed movement artifacts, were excluded when computing delta power, because EEG signals are especially sensitive to movement, with the resulting artifact specifically enhancing signals in the delta band. The mean wake episodes were computed throughout 24 h in 2 h bins. The SWS latency was determined as the time elapsed after each wake episode to the initiation of the subsequent SWS episode, and was calculated for each arousal throughout the 24-h period.
Body Weight
Body weight was measured twice weekly for a period of up to 8 w (12 mice/experimental group), and always at the same time of the day (middle of the light cycle period). Body weight gain was determined by subtracting the body weight on first day of SF exposure from the body weight on subsequent days.
Food Intake
All animals had free access to regular chow diet. Food in-take was monitored on daily basis, by measuring the amount of food consumed in each cage and dividing it by the number of animals for each cage (usually three to four animals per cage). Food intake is presented as g/mouse/day.
Tissue Harvesting
At the end of each exposure, mice were euthanized using CO2 exposures followed by cervical dislocation, the skull was rapidly opened, and the brain was extracted, immediately placed on dry ice, and the hypothalami were dissected under surgical microscopy and immediately snap frozen in liquid nitrogen (n = 6 for each group at every time point).
Western Blotting
Hypothalamic protein samples were loaded onto 6–10% polyacrylamide gel electrophoresis gels, blots were normalized using β-actin as a housekeeper. Antibodies included ATF6 from Imgenex Corporation (San Diego, CA, USA), HSP70, HSP90, GRP78, and FAT10 were obtained from Enzo Life Sciences (Ann Arbor, MI, USA), phosphorylated-STAT3, STAT3, and eIF2α from Cell Signaling Technology (Beverley, MA, USA), phosphorylatedeIF2α from Invitrogen, Life Technologies (Grand Island, NY, USA), and PTP1B, SOCS3, ObR, and β-Actin from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). For more details please see Table S1, supplemental material.
Quantification of XBP-1 Splicing
qRT-PCR analysis was used to assess the splicing of the X-Box Protein 1(XBP-1)26 and was performed using ABI PRISM 7500 System (Applied Biosystems, Foster City, CA, USA). Taqman primer and probes for XBP-1 were purchased from Applied Biosystems (assay #: 4351372- Mm00457360_ g1) (n = 6/condition) (supplemental material).
Plasma Leptin Levels
Leptin plasma levels were assessed in plasma obtained from blood drawn at the end of each SF exposure (19:00) directly from the hepatic vein after a fast surgical exposure following pentobarbital anesthesia (n = 10–12 experimental group). Plasma samples were kept at -80°C until assay. The assay was carried out using a commercially available enzyme-linked immunosorbent assay kit (Millipore; St. Charles, MO, USA) according to the manufacturer's instructions. The linear range was 0.2 ng/mL up to 30 ng/mL with the sensitivity threshold at 0.05 ng/mL (∼3.13 pM). The intra-assay variation coefficient was up to 1.76%, and the interindividual coefficient of variation was 4.59%.
Leptin Sensitivity and Signaling
For all studies involving leptin signaling we implemented previously published protocols that were slightly modified to accommodate the current SF exposure design.15,27 In brief, both SF and SC-exposed mice fasted for 8 h (n = 6/experimental group), always starting at 07:00, and at 15:00 received a single intraperitoneal injection (3 μg/g of body weight) of recombinant mouse leptin (Sigma-Aldrich, St Louis, MO, USA), with normal saline being used as vehicle. All mice were sacrificed 60 min later, and the hypothalamus was harvested and processed for protein extraction as previously described. To quantify leptin signaling, immunoblotting for pSTAT3, STAT3 (Cell Signaling) and β-actin (Santa Cruz Biotechnologies), were performed.
Tauroursodeoxycholic Acid Treatment
Tauroursodeoxycholic acid (TUDCA) is a chemical chaperone that is used to stabilize protein conformation, improve ER folding capacity, and facilitate the trafficking of mutant proteins. Therefore, we used TUDCA to abrogate SF induction of ER stress and its effects on hypothalamic function. TUDCA was administered intraperitoneally (previously shown to cross the blood-brain barrier28) always at the same time of the day (500 mg/kg at 08:00) starting 48 h prior to initiation of SF and throughout the duration of SF (n = 6/group). Controls for TUDCA-treated group received the same volume of vehicle by intraperitoneal injection (n = 6/group).
Protein Extraction, Immunoprecipitation, and PTP1b Activity Assay
Hypothalamic samples from both SF and SC conditions (n = 6/group) were subjected to PTP1B activity assay using commercial PTP1B assay kit (Calbiochem, EMD Millipore, Billerica,MA, USA) according to manufacturer's protocol. The protein concentration of supernatants was determined by (Detergent Compatible) DC protein assay (Biorad, Hercules, CA, USA) (supplemental material).
CCAAT/Enhancer-Binding Protein Homologous Protein Mice
CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) was identified as an ER stress-induced transcription factor that is a significant mediator of apoptosis in response to ER stress and activation of the UPR. We used CHOP heterozygous null mice (n = 12), which lack the CHOP-mediated ER signaling, to further explore the role of SF on UPR activation and induction of ER stress. Male hemizygous CHOP −/+ mice weighing 22–25 g were purchased from Jackson Laboratories, were housed in a 12 h light/dark cycle (light on 07:00 to 19:00) at a constant temperature (24 ± 1°C) and were allowed access to food and water ad libitum. All the experimental procedures took place after at least a week of habituation to the facility and started at 8–9 w of age.
Statistical Analysis
All data are reported as mean ± standard error. Repeated-measures analysis of variance (ANOVA) was used to compare food intake and weight gain, one-way ANOVA followed by unpaired Student t-test with Bonferroni correction was used to compare other time course experiments. Comparison of all other quantitative data between SF and SC conditions was performed using unpaired Student t-tests. For all comparisons, P < 0.05 was considered as statistically significant.
RESULTS
Sleep Fragmentation Induces Hyperphagic Behaviors and Increases Daily Food Intake
Increased food intake that began within the first 2–4 days from the initiation of the SF exposures became manifest in SF-exposed mice. The hyperphagic behavior was maintained throughout the duration of fragmented sleep (P < 0.01; Figure 1A). Body weight gain differences between the two treatment groups, with increased body weight accrual in SF-exposed mice emerged around 4 weeks of SF, and were sustained thereafter (Figure 1B). Therefore, all experiments described in the following paragraphs were performed up to 2 w of SF exposures, i.e., well before the divergence in body weight trajectories became apparent.
Figure 1.
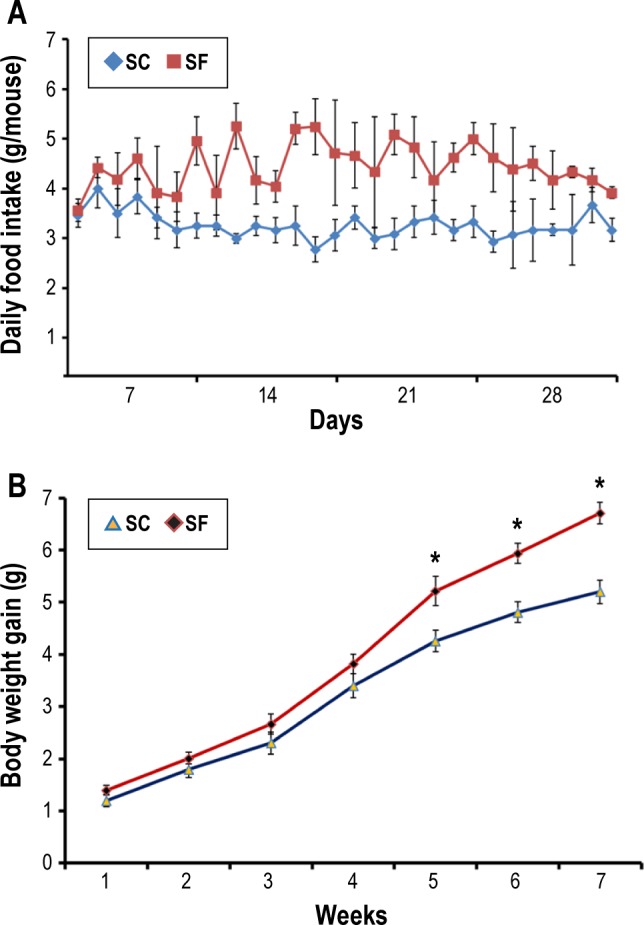
Sleep fragmentation (SF) exposed mice exhibit increased food intake behaviors and accelerated weight gain. (A) Mice exposed to sleep fragmentation (n = 24) developed increased food intake starting within the first 2–4 days from initiation of SF. The hyperphagic behaviors were sustained throughout the duration of disrupted sleep (P < 0.01). (B) Body weight gain differences between the two groups emerged after 4 w of SF (n = 24) and sustained thereafter (P < 0.02). SC, sleep control.
Sleep Fragmentation Induces ER Stress in the Hypothalamus and Activates the UPR
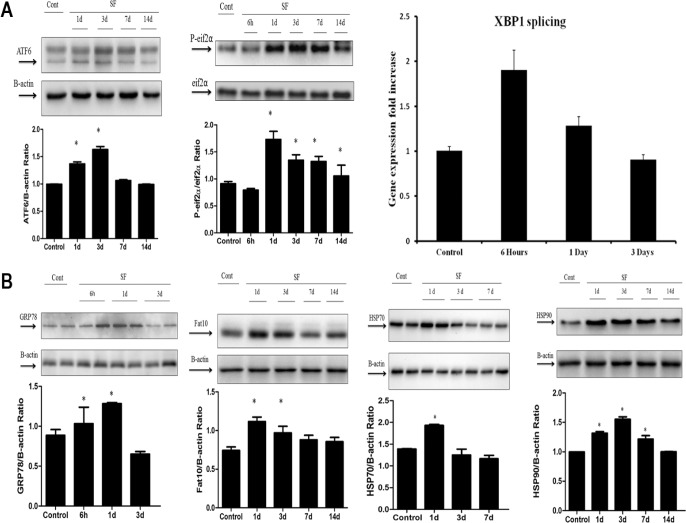
In time-course SF experiments, ER stress in the hypothalamus was apparent in the first 7 days of SF (n = 6/time point), and involved all three major UPR pathways (Figure 2A). Indeed, increased in cleaved ATF6 expression (P < 0.001), increased p-eIF2α/total eIF2α expression (P < 0.001), and increased splicing of XBP1 messenger RNA (P < 0.005) emerged. In addition, the expression of downstream proteins including the molecular chaperones GRP78 (P < 0.002), HSP70 (P < 0.001), HSP90 (P < 0.001), and the downstream target of sustained UPR activation, FAT10 (P < 0.005) were all enhanced (Figure 2B).
Figure 2.
Sleep fragmentation (SF) induces endoplasmic reticulum stress in the hypothalamus and activates the unfolded protein response. (A) Sleep fragmentation induced temporal changes in endoplasmic reticulum (ER) stress in the hypothalamus, across the three major UPR pathways (n = 6) as shown in a representative time course Western blot with an increase in cleaved ATF6 expression (P < 0.001), p-eIF2α expression (P < 0.001), and splicing of XBP1 messenger RNA levels (P < 0.005) when compared with sleep control conditions (SC). (B) A time course Western blot showing the expression of downstream proteins such as GRP78 (P < 0.002), HSP70 (P < 0.001), HSP90 (P < 0.001), and FAT10 (P < 0.005), that also exhibited increased expression levels in hypothalamic extracts after SF compared with SC conditions, in all blots β-actin expression was used as the house keeping control. ATF6, activating transcription factor 6; eIF2α, eukaryotic initiation factor 2; FAT10, HLA-F adjacent transcript 10; GRP78, glucose-regulated protein of 78 kDa; HSP70- 70, kilodalton heat shock protein; HSP90, 90 kilodalton heat shock protein; UPR, unfolded protein response.
Sleep Fragmentation Attenuates Leptin Receptor Signaling in the Hypothalamus, which is Restored by TUDCA Treatment
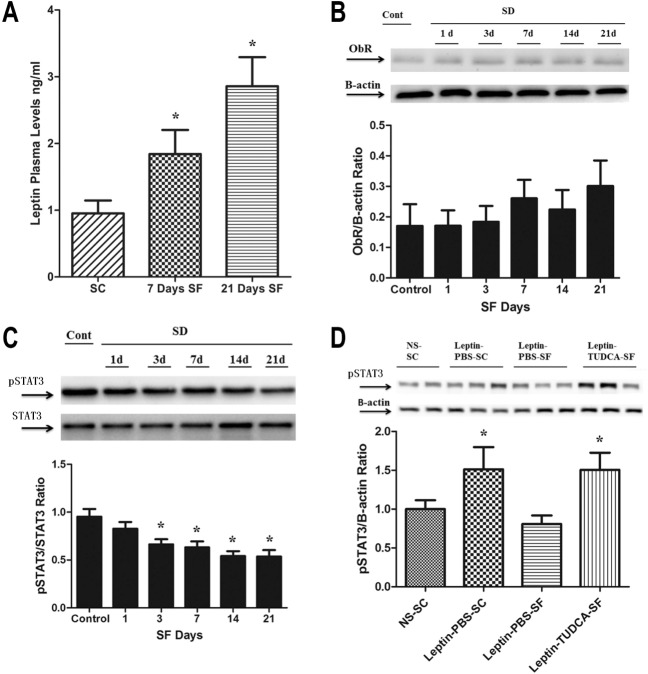
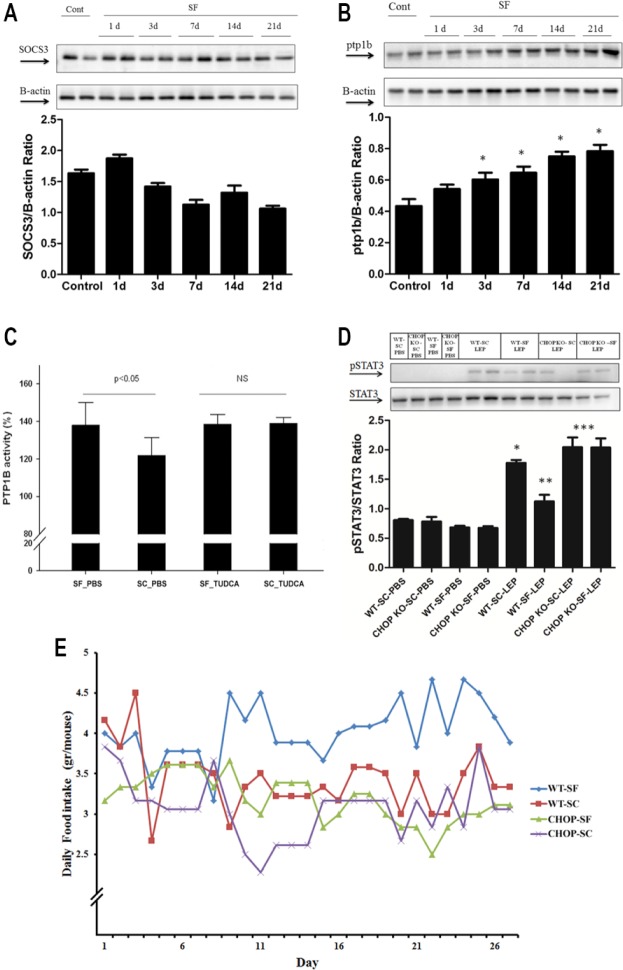
Leptin levels in plasma were increased after the first week of SF and were highest at 21 days of SF (2.86 ± 0.44 ng/mL, versus 0.96 ± 0.19 ng/mL in SC; n = 11 per group; P < 0.001) in a contextual setting of increased food intake by SF-exposed mice (Figure 3A), thereby suggesting altered ObR signaling. Indeed, ObR expression remained unaltered in the hypothalami of SF-exposed mice (Figure 3B), and was accompanied by reduced p-STAT3/STAT3 ratios in fasting conditions (P < 0.03) (Figure 3C). We therefore performed leptin injections, and found that SF-exposed mice exhibited significantly reduced STAT3 phosphorylation as compared to SC exposed mice that were injected with either leptin or vehicle (Figure 3D). Treatment of SF-exposed mice with the chemical chaperone TUDCA completely abrogated the emergence of hyperphagic behaviors, and preserved leptin receptor signaling in hypothalamus despite SF exposures (Figure 3D). Of note, TUDCA (Intra peritoneal) IP administration was found to attenuate the early increase in ER stress induced by SF, with attenuation of ATF6 and GRP78 after 12 h of IP TUCDA administration (data not shown). To further explore potential downstream signaling mechanisms that may underlie ObR resistance during SF, SOCS3 and PTP1B expression were assessed. Although SOCS3 expression remained unaltered by SF (Figure 4A), significant increases in PTP1B expression emerged over time (P < 0.04; Figure 4B). PTP1B activity assays further confirmed increases in PTP1B activity in SF-exposed mice (P < 0.05; Figure 4C). Furthermore, SF-exposed mice failed to develop increases in PTP1B expression and activity when treated with TUDCA, suggesting that the anticipated increases in PTP1b activity did not occur after TUDCA treatment in SF-exposed mice.
Figure 3.
Sleep fragmentation-exposed mice develop hyperleptinemia over time and attenuation of leptin receptor (ObR) signaling in the hypothalamus. (A) Leptin levels were measured in plasma drawn from fasting mice, and when compared to mice undergoing sleep control (SC) showed increasing levels starting after 1-w exposure to sleep fragmentation (SF), and further increasing at 21 days of SF exposure (n = 11 per group; P < 0.001). (B) Leptin receptor (ObR) expression showed a nonstatistically significant trend toward increases in expression. In parallel, (C) p-STAT3/STAT3 in SF decreased over time when compared with SC (P < 0.03), suggesting reduced ObR receptor signaling. (D) SF mice showed low levels of p-STAT3 expression after leptin injection when compared to baseline (saline injection) or to SC conditions. SF-induced ObR resistance was abrogated when mice were treated with the chemical chaperone TUDCA. PBS, phosphate buffered saline; STAT3, signal transducer and activator of transcription 3; TUDCA, tauroursodeoxycholic acid.
Figure 4.
Potential pathways underlying leptin receptor resistance in CHOP +/+ and CHOP −/+ mice in response to SF exposures. (A) Although SOCS3 expression remained unaltered by sleep fragmentation (SF), significant increases in PTP1B expression (B) emerged (P < 0.04) overtime, implicating upregulation of PTP1B as a putative mechanism underling attenuation of leptin receptor signaling in SF. Not only protein levels were high, but PTP1B activity was also increased (C) after 3-w SF exposures in mice (i.e., before weight increases occur) (P < 0.05); this increased PTP1B activity following SF was abrogated by treatment with the chemical chaperone TUDCA. (D) Representative Western blot of STAT3 phosphorylation in hypothalamic tissues from CHOP −/+ and CHOP +/+ mice treated with saline or leptin and exposed to either SF or sleep control (SC) for 3 w. CHOP −/+ mice exposed to SF for 3 w exhibit unaltered leptin receptor sensitivity compared to the reductions in STAT3 phosphorylation responses to leptin injections in wild-type littermates exposed to SF (n = 6/group; CHOP −/+ SF versus CHOP +/+ SF: P < 0.01; CHOP −/+ SF versus CHOP +/+ SF: P < 0.01) PBS, phosphate buffered saline. (E) CHOP −/+ mice exposed to SF exhibited no significant differences in their eating behaviors compared to SC-exposed littermates (3.19 ± 0.06 g/mouse/day versus 3.07 ± 0.07 g/mouse/ day in SC; n = 6 per group; P = not significant). In contrast, wild-type littermates exposed to SF (WT-SF) demonstrated increased food intake behaviors during SF (4.08 ± 0.06 g/mouse/ day versus 3.36 ± 0.07 g/mouse/day in SC; n = 6 per group; P < 0.001). CHOP, CCAAT/ enhancer-binding protein (C/EBP) homologous protein; PTP1B, protein-tyrosine phosphatase 1B; SOCS3, suppressor of cytokine signaling-3; TUDCA, tauroursodeoxycholic acid.
Effect of Sleep Fragmentation on Leptin Signaling in CHOP Hemizygous Null Mice
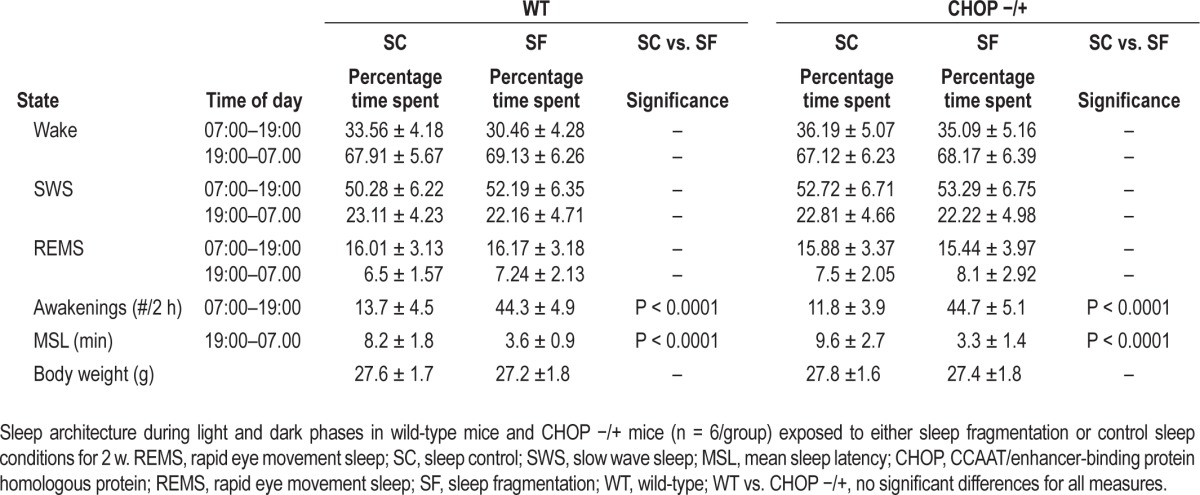
CHOP −/+ KO mice exposed to SF displayed similar eating behaviors than SC-exposed CHOP −/+ mice (Figure 4E; SF: 3.19 ± 0.06 g chow/mouse/day versus 3.07 ± 0.07 g chow/ mouse/day in SC conditions; n = 6/group; P > 0.05). In parallel experiments, wild-type littermates exposed to SF showed increased food intake compared to control conditions (Figure 4D; SF: 4.08 ± 0.06 g chow/mouse/day versus 3.36 ± 0.07 g chow/ mouse/day in SC; n = 6 per group; P < 0.001). However, both wild-type mice and CHOP −/+ KO mice exposed to SF displayed similar alterations in sleep structure, i.e., increased arousals in the absence of sleep duration curtailment or sleep state distribution (Table 1). To further confirm that the differences in leptin receptor sensitivity between SF and SC in wild-type mice were indeed ascribable to SF-induced UPR responses, we performed leptin injections in all four treatment groups. As anticipated, wild-type mice exposed to SF exhibited the same reductions in leptin injection-induced STAT3 phosphorylation. In contrast, CHOP −/+ mice injected with leptin showed not only higher phosphorylation of STAT3 in SC conditions, but also exhibited no alterations in STAT3 phosphorylation responses to leptin injections when exposed to SF (Figure 4D). Furthermore, no changes in PTP1B activity occurred in the hypothalamus of CHOP −/+ mice exposed to SF for 3 w (n = 6/group; SF: 112.3 ± 13% versus SC: 110 ± 11.7%; P, not significant).
Table 1.
Sleep recordings in wild-type and CHOP −/+ mice exposed to sleep fragmentation and sleep control conditions.
DISCUSSION
Obesity is an escalating problem that constitutes a major threat to global human health. In this context, altered sleep has emerged as a potentially important contributor, even though the mechanisms linking fragmented sleep patterns and obesity have not been elucidated. In the current study, we show that mice exposed to chronic sleep fragmentation during their natural sleep period exhibit increased daily food intake and reduced leptin receptor signaling in the hypothalamus that appear to be mediated by ER stress and activation of the UPR despite the absence of sleep curtailment. Furthermore, administration of TUDCA, a chemical chaperone, to wild-type mice undergoing SF exposures, or alternatively SF exposures of CHOP −/+ mice revealed abrogation of the orexigenic behaviors and preservation of hypothalamic ObR sensitivity despite similar disruption of sleep and resultant sleepiness by the SF paradigm. These findings implicate ER stress and activation of the UPR response in the hypothalamus as an important determinant of the altered energy homeostasis that ultimately results in obesity in the context of fragmented sleep.
Several studies in humans have shown that shortened sleep duration in otherwise healthy individuals is associated with alterations in hedonic food preferences, overall increases in food intake, in the context of globally preserved energy expenditure, thereby promoting increased obesogenic tendencies.29–33 Furthermore, such changes appear to also occur in the context of acute, short-lasting sleep fragmentation/deprivation.34,35 The current study focuses on chronic sleep fragmentation, a more prevalent pathological condition of sleep that is present in multiple common disorders (e.g., sleep apnea, depression, asthma), all of which have been associated with obesity and metabolic dysfunction. Recently, Wang et al. showed that chronic SF in mice induces an increased weight gain and an increase in both visceral and subcutaneous adipose tissue volumes as assessed by MRI approaches.36 Of note, our current study focuses on the early hyperphagic phase, and investigates the early changes in hypothalamic function that eventually will promote weight gain and metabolic dysfunction.
The presence of increased markers of ER stress in the context of disrupted and deprived sleep is not novel, and has been reported previously.37–41 However, all of these studies addressed the acute effects of sleep deprivation (usually a few hours) rather than sleep fragmentation, and primarily, if not exclusively, focused on cortical structures. In a recent review, Naidoo summarized published studies on ER stress and sleep and concluded that during acute sleep deprivation there are adaptive mechanisms that are upregulated and serve to protect neurons from injury.41 However, in the context of chronic sleep disturbances such as the sleep disruption/fragmentation that occurs with aging or sleep apnea, it is likely that the sustained nature of ER stress associated with chronic sleep perturbations that can develop in these disorders and others may result in extensive neural dysfunction or cellular losses.37 We now present initial evidence that ER stress peaks after several days of SF, and is associated with not only activation of the UPR response, but also with evidence of leptin resistance and consequent functional deficits in homeostatic regulatory functions such as energy balance, ultimately leading to obesity.
In our current experiments, SF induced ER stress and activated all three major pathways of the UPR response, ultimately leading to increased expression of molecular chaperones that can rescue misfolded proteins by breaking up aggregates and assisting in the refolding process, while proteins that cannot be rescued by refolding are delivered to the ubiquitin-proteasome pathway by other chaperones for recycling.42,43 As such, earlier activation of the UPR pathways followed by delayed increases in GRP 78 and HSP70 and HSP90 were anticipated, because among the cellular pathways involved in protection against oxidative and nitrosative stress, the heat shock proteins family plays a key role, particularly in brain cells.44,45 It will be important in future studies to explore the specific proteins that are targeted for degradation by the proteasome in the context of the sustained ER stress induced by SF, and which among those proteins plays a leading role in hypothalamic neurons to induce the deregulation of food-seeking behaviors and increased food intake observed herein.
The hypothalamus plays an essential role in the control of appetite and energy metabolism, via tightly regulated sensing of fluctuations in body energy levels through hormones, nutrients, and the autonomic nervous system, and under complex feedback signaling loops dictates coordinated adaptive responses to preserve energy balance.46 The adipocyte-derived hormone, leptin, is viewed as one of the most critical factors relaying peripheral energy state information to the hypothalamus, and the absence of leptin and its receptors leads to hyperphagia and morbid obesity in both rodents and humans.47–49 Conversely, leptin administration successfully suppressed appetite and reduced excessive fat in obese rodents and humans with genetic leptin deficiency.50 However, obese individuals generally exhibit elevated circulating leptin concentrations, indicating the presence of leptin resistance rather than deficits in leptin.51 Current results show that mice exposed to SF develop hyperphagic behaviors and leptin resistance (high leptin levels and reduced STAT3 phosphorylation after leptin injection), and that such SF-induced effects were abrogated by treatment with a chemical chaperone or via transgenic ablation of CHOP. The mechanistic link between ER stress and leptin resistance has been previously demonstrated in the context of high-fat diet,17 but this is the first study to link sleep perturbations to leptin resistance via ER stress-mediated pathways. Further exploration of potential mechanisms underlying the negative regulators of ObR signaling such as SOCS3 and PTP1B revealed that although SOCS3 expression and phosphorylation remained unaltered, progressive increases in PTP1B expression and PTP1B activity occurred in SF-exposed mice, but not in TUDCA-treated mice or in CHOP −/+ mice exposed to SF. Increases in PTP1B expression and activity as elicited by SF exposures further suggest this signaling pathway as a major mechanism promoting increased food intake, weight gain, and metabolic dysfunction in the context of sleep perturbations. We therefore believe that it will be interesting in future studies to assess the effects of SF using organ- or even cell-specific PTP1B null mice to dissect in greater detail the mechanisms underlying the effects of SF. In this context, the feed-forward and feed-back mechanisms associated with PTP1B activity and ER stress need to be incorporated into the experimental approaches, especially when considering that PTP1b can directly elicit ER stress, as shown by Agouni et al. in the context of liver ER stress.52 This is in contrast with the effects of high caloric diets, which activate the ER stress and the UPR, but seem to induce leptin resistance via upregulation of SOCS3.53 However, we should also be cautious about the extrapolation of current findings from a murine model to human sleep disorders, particularly in light of the large discrepancies reported for the transition of a priori effective therapies from mouse to patients.54
In summary, the current study provides compelling evidence supporting a novel pathway involving ER stress and the UPR that links sleep integrity, rather than just sleep duration to obesogenic behaviors and leptin resistance in mice. Figure 5 illustrates this putative relationship, showing that SF induces ER stress, activates the UPR, and down-regulates leptin receptor signaling in the hypothalamus, most probably through the PTP1B signaling pathway. Current findings further highlight the role of preserved and intact sleep architecture in the hypothalamic regulation of energy balance.
Figure 5.
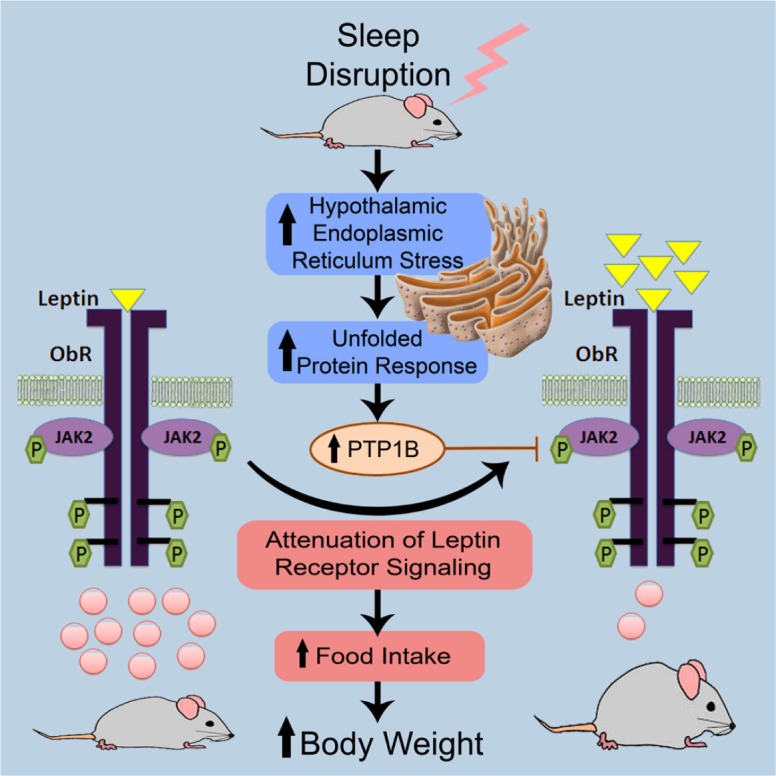
Schematic diagram illustrating the putative pathways underlying the emergence of increased food consumption in mice subjected to sleep fragmentation during their circadian sleep period. As illustrated on the left side, under control conditions, leptin (yellow triangles) activates the leptin receptor signaling via the JAK/STAT cascade, which elicits STAT3 (pink circles) binding and translocation into the nucleus to act as a transcription factor by binding to specific response elements in the promoter of its target genes, aiming to inhibit food desire. Upon the introduction of sleep fragmentation, endoplasmic reticulum (ER) stress is induced, driving the activation of the unfolded protein response (UPR) ultimately leading to increased expression and activity of PTPB1. As shown on the right side, those changes are accompanied by decreased leptin receptor sensitivity in the hypothalamus, which is evidenced by the accumulation of leptin (yellow triangles), along with lower expression of STAT3 (pink circles), manifesting as increased food consumption, the latter ultimately promoting the emergence of obesity. JAK2, Janus kinase 2; ObR, leptin receptor; P, phosphorylation; PTP1B, protein-tyrosine phosphatase 1B; STAT3, signal transducer and activator of transcription 3.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Gozal is supported by National Institutes of Health grants HL-065270 and HL-086662. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Dr. Hakim performed experiments, analyzed data, and drafted components of the manuscript. Dr. Wang analyzed data, drafted portions of the manuscript, and served as blinded observer. Dr Carrereas, Ms. Hirotsu, and Dr. Zhang performed the sleep studies in mice including scoring and analyses, and also performed subset of the Western blots. Mr. Peris performed some of the enzyme-linked immunosor-bent assays. Dr. Gozal provided the conceptual design of the project, analyzed data, drafted the manuscript, and is responsible for the financial support of the project and the manuscript content. All authors have reviewed and approved the final version of the manuscript.
ABBREVIATIONS
- α-MSH
alpha melanocyte stimulating hormone
- ATF6
activating transcription factor 6
- CHOP
CCAAT/enhancer-binding protein (C/EBP) homologous protein
- CHOP +/+
wild type mice
- CHOP −/+
CHOP heterozygous null mice
- CHOP −/−
CHOP homozygous null mice
- ER
endoplasmic reticulum
- eIF2α
eukaryotic initiation factor 2
- FAT10
HLA-F adjacent transcript 10
- GRP78
glucose-regulated protein of 78 kDa
- HSP70
70 kilodalton heat shock protein
- HSP90
90 kilodalton heat shock protein
- IRE1α
inositol-requiring transmembrane kinase-endoribonuclease-1α
- JAK2
janus kinase 2
- NPY
neuropeptide Y
- ObR
leptin receptor
- PERK
protein kinase-like ER kinase
- PTP1B
protein-tyrosine phosphatase 1B
- STAT3
signal transducer and activator of transcription3
- SOCS3
suppressor of cytokine signaling-3
- TUDCA
tauroursodeoxycholic acid
- UPR
unfolded protein response
- XBP1
x-box binding protein-1
SUPPLEMENTAL MATERIAL
Surgical Procedures for Sleep Recordings
All surgical procedures were performed under sterile conditions and isoflurane general anesthesia: induction, 3% isoflurane and 1 L per min of O2 and maintenance, 2% isoflurane and 0.5 L per min of O2. First, the animals were positioned in sternal recumbency, and a dorsal neck incision of 2–3 cm was made through the skin along the dorsal midline and covered with a sterile bandage, after which a 1.5–2 cm incision was performed through the skin and abdominal wall along the ventral midline. A telemetric transmitter weighing 3.5 g, F20-EET (DSI, St. Paul, MN, USA), which allows simultaneous monitoring of two biopotential channels, temperature, and locomotor activity was inserted, biopotential leads were exteriorized, and the abdominal wall was closed using 4-0 nonabsorbable suture with a simple interrupted pattern. The two pairs of biopotential leads were then advanced subcutaneously from the ventral abdomen incision to the dorsal neck incision using a trocar. Animals were then fixed in a stereotaxic apparatus for implantation of electroencephalographic (EEG) electrodes, with the first pair of biopotential leads being fixed to the skull above the frontal area (1 mm anterior to bregma and 2 mm lateral to midsagittal suture for one of the leads, and 1 mm anterior to lambda and 2.5 mm lateral to mid-sagittal suture for the other lead). The other pair of biopotential leads was placed within the same bundle of dorsal neck muscles for the recording of nuchal electromyography (EMG).
Western Blotting
Hypothalamic tissues were homogenized at 4°C with a tissue bullet blender (Next Advance Inc.; Averill Park, NY, USA) in 1 vol. of 1% SDS (Sodium dodecyl sulfate) at 90°C with 0.5 mm diameter glass beads. The homogenate was centrifuged for 5 min at 15,000 × g at 4°C to pellet beads and debris. Protein content was measured in each soluble fraction using the Pierce BCA Protein Assay kit (Thermoscientific; Rockford, IL, USA), and samples were frozen at −80°C until analysis. Homogenate proteins (20 μg) were buffered with 5 × Laemli buffer and 2-mercaptoethanol as a reducing agent, concentration was adjusted with 1% SDS to 1 mg/mL and heated for 5 min at 90°C. Samples were loaded onto 6-10% polyacrylamide gel electrophoresis gels. Electrophoresis was performed at 120 V over 90 min and then transferred electrophoretically (60 min) onto a nitrocellulose membrane (Whatmann; Florham Park, NJ, USA). Membranes were blocked in 5% skimmed milk in Tris-buffered saline solution/0.05% Tween (TBST) at room temperature for 1 h, and incubated overnight with primary antibody at the optimized dilution in 5% skim milk in TBST at 4°C. After incubation, membranes were washed three times in TBST for 10 min and incubated at room temperature with the secondary antibody in 5% skim milk in TBST for 1 h at room temperature. Immunoreactive bands were visualized using an enhanced chemiluminescence detection system (Chemidoc XRS+, BioRad; Hercules, CA, USA). Blots were normalized using β-actin as a housekeeper.
Quantification of X-Box Protein 1 splicing
Real-time reverse transcription-polymerase chain reaction analysis was used to assess the splicing of the X-Box Protein 1 (XBP-1) and was performed using ABI PRISM 7500 System (Applied Biosystems, Foster City, CA, USA). RNA from hypothalami that were harvested from mice exposed to both sleep fragmentation (SF) and sleep control (SC) were prepared with Qiazol (Qiagen, Valencia, CA, USA). Complementary DNA (cDNA) synthesis was performed using a high-capacity cDNA archive kit (Applied Biosystems). β-actin was used as a reference gene to normalize the expression ratios for the gene of interest. Taqman primer and probes for XBP-1 were purchased from Applied Biosystems (assay #: 4351372- Mm00457360_ g1). One μg of total RNA was used to generate cDNA templates and TaqMan® Master Mix Reagent Kit (Applied Biosystems) was used to amplify and quantify the transcripts in 20 μL reactions. Duplicate polymerase chain reactions were performed in 96 well in parallel with the β-actin ribosomal RNA. The steps involved in the reaction program included: the initial step of 2 min at 50°C; denaturation at 95°C for 10 min, followed by 45 thermal cycles of denaturation (15 sec at 95°C) and elongation (1 min at 60°C). Expression values were obtained from the cycle number (Ct value) using the Biosystems analysis software. These Ct values were averaged and the difference between the β-actin Ct (Avg) and the gene of interest Ct (Avg) was calculated (Ct-diff). The relative expression of XPB-1 splicing was analyzed using the 2-ΔΔCT method. Quantitative results were expressed as the mean ± standard deviation (SF).
Protein Extraction, Immunoprecipitation, and PTP1b Activity Assay
Hypothalami from SF and SC conditions were homogenized using bullet blender (Next Advance Inc; Averill Park, NY, USA) in fresh lysis buffer [50 mM Tris-HCl (pH 7.5), 5mM EDTA, 10 mM EGTA, protease inhibitor, 50 mM PMSF (Phenylmethylsulphonyl fluoride), 10 mM benzamidine]. After homogenization, the lysates were centrifuged for 30 min at 4°C in microcentrifuge. The supernatants were collected and stored at -80°C until analysis. In order to perform immunoprecipitation, anti-PTP1B antibody (1:10; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was added with Dynabead protein G (Invitrogen, Oslo, Norway). Each sample (200 μg of total protein) containing the PTP1B antigen (Ag) was added to the Dynabeads-Antibody (Ab) complex, and incubated with rotation for 10 min at room temperature to allow antigen to bind to the Dynabeads-Ab complex. Then, the Dynabeads-Ab-Ag complex was washed for three times by using 200 μL washing buffer for each wash. After removal of supernatant, the complex was resuspended again with 100 μL washing buffer and the bead suspension was transferred to a clean tube before proceeding to elution. After removal of the supernatant in the tube placed on the magnet, 20 μL elution buffer was added into the tube, and the Dynabeads-Ab-Ag complex was resuspended by gentle pipetting. PTP1B activity assay was conducted using commercial PTP1B assay kit (Calbiochem, EMD Millipore, Billerica, MA, USA) according to manufacturer's protocol. The protein concentration of supernatants was determined by (Detergent Compatible) DC protein assay (Biorad, Hercules, CA, USA).
Antibodies used in western blotting experiments.
REFERENCES
- 1.Li Z, Bowerman S, Heber D. Health ramifications of the obesity epidemic. Surg Clin North Am. 2005;85:681–701. v. doi: 10.1016/j.suc.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab. 2010;24:687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pejovic S, Vgontzas AN, Basta M, et al. Leptin and hunger levels in young healthy adults after one night of sleep loss. J Sleep Res. 2010;19:552–8. doi: 10.1111/j.1365-2869.2010.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 6.Seetho IW, Wilding JP. Screening for obstructive sleep apnoea in obesity and diabetes - potential for future approaches. Eur J Clin Invest. 2013;43:640–55. doi: 10.1111/eci.12083. [DOI] [PubMed] [Google Scholar]
- 7.Paspala I, Katsiki N, Kapoukranidou D, Mikhailidis DP, Tsiligiroglou-Fachantidou A. The role of psychobiological and neuroendocrine mechanisms in appetite regulation and obesity. Open Cardiovasc Med J. 2012;6:147–55. doi: 10.2174/1874192401206010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houseknecht KL, Portocarrero CP. Leptin and its receptors: regulators of whole-body energy homeostasis. Domest Anim Endocrinol. 1998;15:457–75. doi: 10.1016/s0739-7240(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 9.Wunderlich CM, Hovelmeyer N, Wunderlich FT. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. Jak-Stat. 2013;2:e23878. doi: 10.4161/jkst.23878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahu A. Intracellular leptin-signaling pathways in hypothalamic neurons: the emerging role of phosphatidylinositol-3 kinase-phosphodiesterase-3B-cAMP pathway. Neuroendocrinology. 2011;93:201–10. doi: 10.1159/000326785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matarazzo V, Schaller F, Nedelec E, et al. Inactivation of Socs3 in the hypothalamus enhances the hindbrain response to endogenous satiety signals via oxytocin signaling. J Neurosci. 2012;32:17097–107. doi: 10.1523/JNEUROSCI.1669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitam Horm. 2013;91:405–24. doi: 10.1016/B978-0-12-407766-9.00017-1. [DOI] [PubMed] [Google Scholar]
- 13.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 14.Xue B, Pulinilkunnil T, Murano I, et al. Neuronal protein tyrosine phosphatase 1B deficiency results in inhibition of hypothalamic AMPK and isoform-specific activation of AMPK in peripheral tissues. Mol Cell Biol. 2009;29:4563–73. doi: 10.1128/MCB.01914-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bence KK, Delibegovic M, Xue B, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–24. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 16.White CL, Whittington A, Barnes MJ, Wang Z, Bray GA, Morrison CD. HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and -independent mechanisms. Am J Physiol Endocrinol Metab. 2009;296:E291–9. doi: 10.1152/ajpendo.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Martinez de Morentin PB, Varela L, Ferno J, Nogueiras R, Dieguez C, Lopez M. Hypothalamic lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:350–61. doi: 10.1016/j.bbalip.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair D, Zhang SX, Ramesh V, et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184:1305–12. doi: 10.1164/rccm.201107-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hummasti S, Hotamisligil GS. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ Res. 2010;107:579–91. doi: 10.1161/CIRCRESAHA.110.225698. [DOI] [PubMed] [Google Scholar]
- 22.Won JC, Jang PG, Namkoong C, et al. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 2009;17:1861–5. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- 23.Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005;92:1150–7. doi: 10.1111/j.1471-4159.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- 24.Naidoo N. Cellular stress/the unfolded protein response: relevance to sleep and sleep disorders. Sleep Med Rev. 2009;13:195–204. doi: 10.1016/j.smrv.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramesh V, Nair D, Zhang SX, et al. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-alpha pathway. J Neuroinflammation. 2012;9:91. doi: 10.1186/1742-2094-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samali A, Fitzgerald U, Deegan S, Gupta S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int J Cell Biol. 2010;2010:830307. doi: 10.1155/2010/830307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banno R, Zimmer D, De Jonghe BC, et al. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720–34. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 30.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–44. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–24. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benedict C, Brooks SJ, O'Daly OG, et al. Acute sleep deprivation enhances the brain's response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97:E443–7. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]
- 35.Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr. 2012:1–9. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Carreras A, Lee S, et al. Chronic sleep fragmentation promotes obesity in young adult mice. Obesity (Silver Spring) 2013;22:758–62. doi: 10.1002/oby.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown MK, Naidoo N. The UPR and the anti-oxidant response: relevance to sleep and sleep loss. Mol Neurobiol. 2010;42:103–13. doi: 10.1007/s12035-010-8114-8. [DOI] [PubMed] [Google Scholar]
- 38.Chou YT, Zhan G, Zhu Y, et al. C/EBP homologous binding protein (CHOP) underlies neural injury in sleep apnea model. Sleep. 2013;36:481–92. doi: 10.5665/sleep.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim DC, Veasey SC. Neural injury in sleep apnea. Curr Neurol Neurosci Rep. 2010;10:47–52. doi: 10.1007/s11910-009-0078-6. [DOI] [PubMed] [Google Scholar]
- 40.Lungato L, Gazarini ML, Paredes-Gamero EJ, Tersariol IL, Tufik S, D'Almeida V. Sleep deprivation impairs calcium signaling in mouse splenocytes and leads to a decreased immune response. Biochim Biophys Acta. 2012;1820:1997–2006. doi: 10.1016/j.bbagen.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Naidoo N. Roles of endoplasmic reticulum and energetic stress in disturbed sleep. Neuromolecular Med. 2012;14:213–9. doi: 10.1007/s12017-012-8179-9. [DOI] [PubMed] [Google Scholar]
- 42.Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–9. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- 43.Banhegyi G, Mandl J, Csala M. Redox-based endoplasmic reticulum dysfunction in neurological diseases. J Neurochem. 2008;107:20–34. doi: 10.1111/j.1471-4159.2008.05571.x. [DOI] [PubMed] [Google Scholar]
- 44.Lehotsky J, Urban P, Pavlikova M, Tatarkova Z, Kaminska B, Kaplan P. Molecular mechanisms leading to neuroprotection/ischemic tolerance: effect of preconditioning on the stress reaction of endoplasmic reticulum. Cell Mol Neurobiol. 2009;29:917–25. doi: 10.1007/s10571-009-9376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mancuso C, Scapagini G, Curro D, et al. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci. 2007;12:1107–23. doi: 10.2741/2130. [DOI] [PubMed] [Google Scholar]
- 46.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–32. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 47.Clement K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 48.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 49.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 50.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Considine RV, Caro JF. Leptin: genes, concepts and clinical perspective. Horm Res. 1996;46:249–56. doi: 10.1159/000185096. [DOI] [PubMed] [Google Scholar]
- 52.Agouni A, Mody N, Owen C, et al. Liver-specific deletion of protein tyrosine phosphatase (PTP) 1B improves obesity- and pharmacologically induced endoplasmic reticulum stress. Biochem J. 2011;438:369–78. doi: 10.1042/BJ20110373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olofsson LE, Unger EK, Cheung CC, Xu AW. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc Natl Acad Sci U S A. 2013;110:E697–706. doi: 10.1073/pnas.1218284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perrin S. Preclinical research: make mouse studies work. Nature. 2014;507:423–5. doi: 10.1038/507423a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antibodies used in western blotting experiments.