Abstract
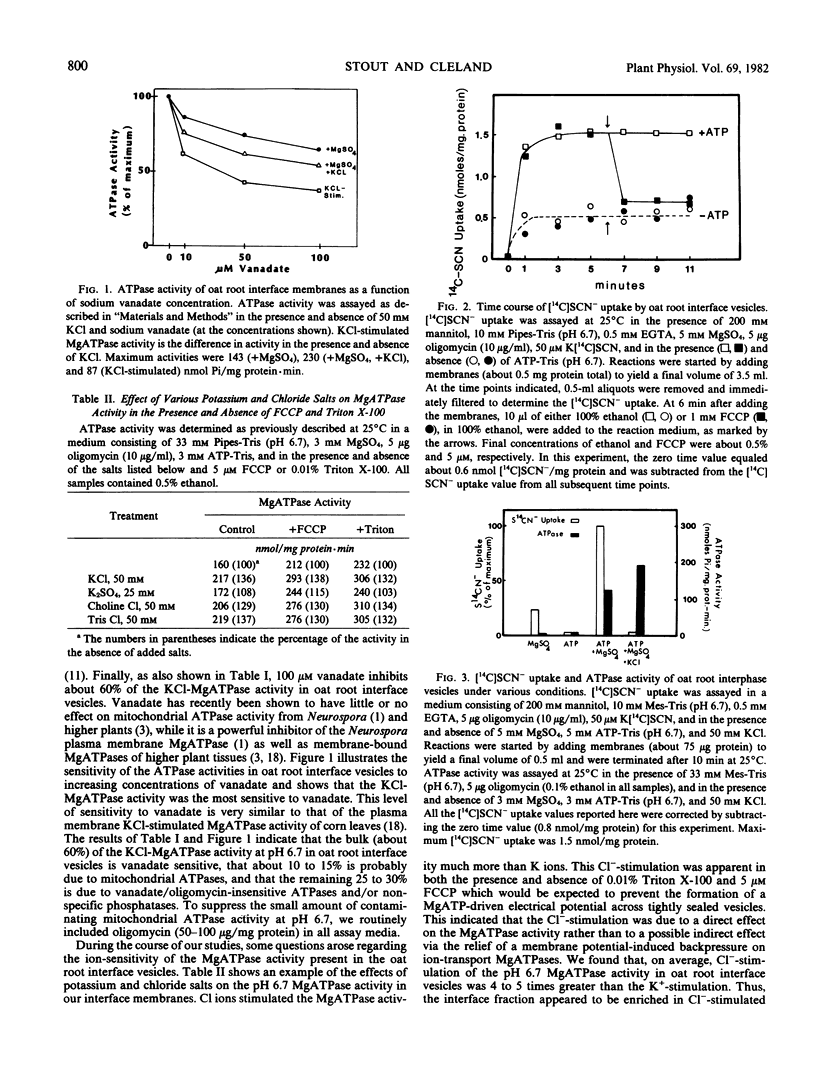
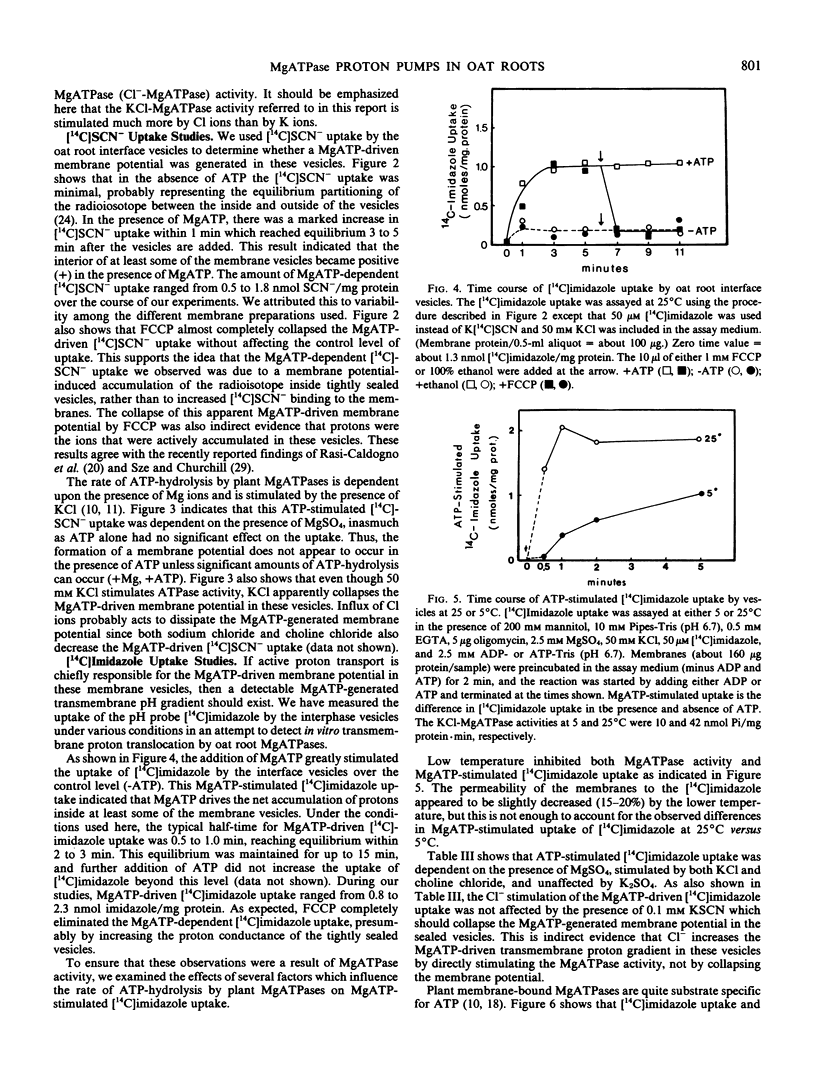
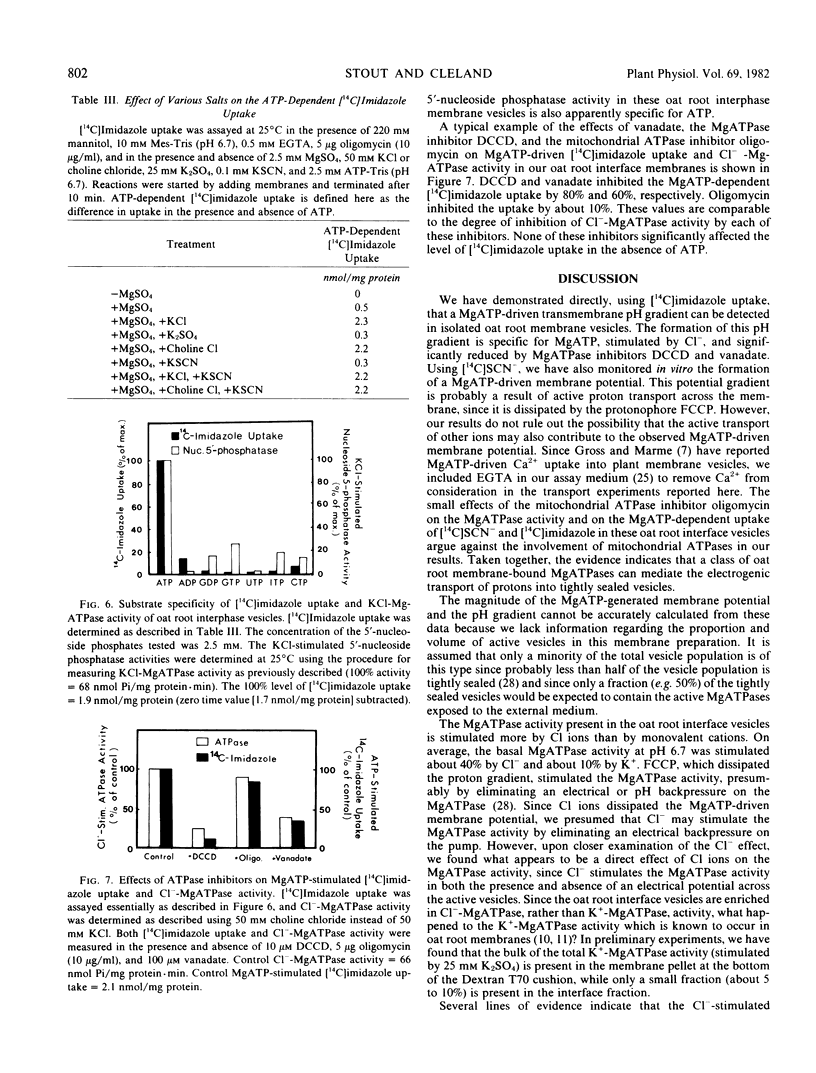
The possibility that plant membrane-bound MgATPases may act as electrogenic proton pumps has been investigated. Using an oat (Avena sativa L. cv. Victory) root membrane preparation which is partially enriched in tightly sealed vesicles, we have shown that MgATP stimulates the uptake of the membrane-permeable anion [14C]SCN− by the vesicles; this indicates that an electrical potential (interior positive) is generated across the membrane. Both Cl− ions and the proton ionophore trifluoromethoxy(carbonyl-cyanide)phenylhydrazone inhibit the MgATP-driven [14C]SCN− uptake, presumably by collapsing the MgATP-generated membrane potential. The uptake of the pH gradient probe [14C]imidazole into the vesicles is also greatly stimulated by MgATP, indicating the presence of a transmembrane proton gradient (interior acid). MgATP-driven [14C]imidazole uptake is temperature sensitive, Cl−-stimulated, substrate specific for MgATP, sensitive to the MgATPase inhibitors vanadate and N,N′-dicyclohexylcarbodiimide, and completely eliminated by trifluoromethoxy(carboxyl-cyanide)phenylhydrazone. The mitochondrial ATPase inhibitor oligomycin has little effect on the MgATPase activity and on the MgATP-dependent [14C]SCN− and [14C]imidazole uptake. These data indicate that a class of oat root membrane-bound MgATPases, stimulated primarily by Cl ions, is capable of using the free energy of ATP-hydrolysis to generate an apparent electrochemical proton gradient in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Gross J., Marmé D. ATP-dependent Ca uptake into plant membrane vesicles. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1232–1236. doi: 10.1073/pnas.75.3.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A., Frenzel R., Laible D. ATP-dependent proton transport into vesicles of microsomal membranes of Zea mays coleoptiles. Z Naturforsch C. 1980 Sep-Oct;35(9-10):783–793. doi: 10.1515/znc-1980-9-1021. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Perlin D. S., Spanswick R. M. Characterization of ATPase activity associated with corn leaf plasma membranes. Plant Physiol. 1981 Sep;68(3):521–526. doi: 10.1104/pp.68.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., de Michelis M. I., Pugliarello M. C. Evidence for an electrogenic ATPase in microsomal vesicles from pea internodes. Biochim Biophys Acta. 1981 Mar 20;642(1):37–45. doi: 10.1016/0005-2736(81)90135-8. [DOI] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- Sachs G. H+ transport by a non-electrogenic gastric ATPase as a model for acid secretion. Rev Physiol Biochem Pharmacol. 1977;79:133–162. doi: 10.1007/BFb0037090. [DOI] [PubMed] [Google Scholar]
- Scarborough G. A. Proton translocation catalyzed by the electrogenic ATPase in the plasma membrane of Neurospora. Biochemistry. 1980 Jun 24;19(13):2925–2931. doi: 10.1021/bi00554a017. [DOI] [PubMed] [Google Scholar]
- Scarborough G. A. The neurospora plasma membrane ATPase is an electrogenic pump. Proc Natl Acad Sci U S A. 1976 May;73(5):1485–1488. doi: 10.1073/pnas.73.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. G., Cleland R. E. Partial characterization of fusicoccin binding to receptor sites on oat root membranes. Plant Physiol. 1980 Sep;66(3):353–359. doi: 10.1104/pp.66.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Churchill K. A. Mg/KCl-ATPase of plant plasma membranes is an electrogenic pump. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5578–5582. doi: 10.1073/pnas.78.9.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Hodges T. K. Characterization of passive ion transport in plasma membrane vesicles of oat roots. Plant Physiol. 1976 Sep;58(3):304–308. doi: 10.1104/pp.58.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H. Nigericin-stimulated ATPase activity in microsomal vesicles of tobacco callus. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5904–5908. doi: 10.1073/pnas.77.10.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]


