Abstract
Objective
Impaired adaptive response to oxidative injuries is a fundamental mechanism central to the pathogenesis of chronic hepatitis C (CHC). Glycogen synthase kinase (GSK) 3β is an indispensable regulator of the oxidative stress response. However, the exact role of GSK3β in CHC is uncertain and was examined.
Design
GSK3β and Nrf2 signaling pathways were examined in JFH1 hepatitis C virus (HCV) infected Huh 7.5.1 hepatocytes and also in liver biopsy specimens from CHC patients.
Results
HCV infection elicited prominent Nrf2 antioxidant response in hepatocytes, marked by elevated expression of the Nrf2 dependent molecule heme oxygenase-1 and subsequent protection from apoptotic cell death. Inhibitory phosphorylation of GSK3β seems to be essential and sufficient for HCV induced Nrf2 response. Mechanistically, GSK3β physically associated and interacted with Nrf2 in hepatocytes. In silico analysis revealed that Nrf2 encompasses multiple GSK3β phosphorylation consensus motifs, denoting Nrf2 as a cognate substrate of GSK3β. In the presence of TGFβ1, the HCV induced GSK3β phosphorylation was blunted via a protein phosphatase 1-dependent mechanism and the cytoprotective Nrf2 response drastically impaired. Lithium, a selective inhibitor of GSK3β, counteracted the effects of TGFβ1. In liver biopsy specimens from CHC patients, the expression of phosphorylated GSK3β positively correlated with Nrf2 expression and was inversely associated with the degree of liver injury. Moreover, CHC patients who received long-term lithium carbonate therapy primarily for concomitant psychiatric disorders exhibited much less liver injury, associated with enhanced hepatic expression of Nrf2.
Conclusions
Inhibition of GSK3β exerts hepatoprotection in CHC possibly through its direct regulation of Nrf2 antioxidant response.
Keywords: glycogen synthase kinase 3β, chronic hepatitis C, NF-E2 related factor-2, transformation growth factor β1, antioxidant response
INTRODUCTION
Persistent Hepatitis C virus (HCV) infection inexorably progresses to liver fibrosis, cirrhosis and end-stage liver disease and potentially leads to hepatocellular carcinoma1. Although the exact molecular mechanisms underlying the HCV related liver injury are not fully understood, a growing body of evidence suggests that HCV replication causes accumulation of massive of reactive oxygen species (ROS) and induces oxidative stress in hepatic cells, followed by hepatic cell death, hepatic inflammation and fibrogenesis, a final common pathway for perpetuation of liver damage regardless of the original etiology2,3. Viral molecules of HCV could elicit the production of ROS via multiple mechanisms, including alteration of calcium homeostasis4, mitochondrial perturbation, induction of NADPH oxidase expression5, and activation of endoplasmic reticulum oxidoreductases6. Upon oxidative stress, an adaptive antioxidant response is harnessed by multiple organ systems including the liver to sustain redox homeostasis and cellular integrity. Central to this self-protective antioxidant mechanism is NF-E2-related factor (Nrf2), a cap’n’collar basic-region leucine zipper nuclear transcription factor that mediates the primary cellular defense against the cytotoxic effects of oxidative stress, including pathways for xenobiotic detoxification, antioxidants, anti-inflammatory response, DNA repair, molecular chaperones, and proteasome systems. In its inactive state, Nrf2 is sequestered in the cytoplasm and associated with the actin anchored Kelch-like ECH-associated protein 1 (Keap1)7,8. However, upon its activation triggered by oxidative stress, Nrf2 dissociates from Keap1 and subsequently translocates into the nucleus7,8. In the nucleus, Nrf2 recognizes and binds to a conserved antioxidant response element (ARE) and induces transcription of a battery of chemoprotective antoxidant genes9,10, including those encoding antioxidant proteins like heme oxygenase (HO-1)11.
How the Nrf2/ARE pathway reacts to HCV infection in hepatic cells remains largely obscure. In an HCV replicating cell culture model, HCV blunted Nrf2 activation and inhibited the induction of ARE-regulated genes12. By contrast, HCV or HCV proteins were found by another studies13,14 to induce ROS production and activate Nrf2/ARE pathway, which subsequently protected hepatic cells from oxidative stress. This result is, however, directly contradictory to the findings made in human liver biopsy specimens15: Nrf2 expression is evident at a high level in hepatic cells in normal liver but is strikingly repressed in a variety of liver diseases including chronic hepatitis C (CHC). Further in-depth studies are merited to define the exact response and the mechanistic role of Nrf2 directed antioxidant pathway in the pathogenesis of HCV induced liver injury.
The Nrf2 dependent self protective antioxidant response is a complex and highly orchestrated pathophysiological process that is regulated by a myriad of signaling pathways. Of many of these pathways, glycogen synthase kinase (GSK) 3β has emerged as the integration point and plays a crucial role in controlling the Nrf2 activity. GSK 3β is a ubiquitously expressed, constitutively active, proline-directed serine/threonine kinase involved in diverse biophysiological functions that include glycogen metabolism, embryo development, tissue injury, repair and regeneration, immunomodulation, and redox homeostasis16. Recent studies demonstrated that GSK3β is also involved in the regulation of Nrf217,18,19. A multitude of evidence suggests that GSK3β regulation of Nrf2 is implicated in ageing20, type 2 diabetes21, hepatotoxicity22, and neurological degeneration23–25. Very little, however, was known about how GSK3β regulates Nrf2 antioxidant response in HCV related liver injury. This study examined the regulatory effect of GSK3β on Nrf2 antioxidant response in HCV-replicating hepatic cells. The effect of TGFβ1, an important profibrotic cytokine implicated in liver cirrhosis, as well as lithium, a selective inhibitor of GSK3β and FDA approved mood stabilizer26, on GSK3β regulated Nrf2 response and hepatic injury in hepatitis C was delineated.
Materials and Methods
Cell Culture
Huh 7.5.1 cells were grown in Dulbecco's modified Eagle's medium supplemented with and 10% fetal bovine serum27. JFH1 HCV (genotype 2a infectious HCV isolate) was employed to infect Huh7.5.1 cells as previously reported28,29. Lithium chloride, trigonelline, tautomycetin and recombinant TGFβ1 were purchase from Sigma (St. Louis, MO) and used to treated the cells.
Plasmids and Transient Transfection
The eukaryotic expression plasmids for the Nrf2, including pWXL-Nrf2-V5 and pEYFG-Nrf2-V5 were a kind gift from Dr Ana Rojo30. The expression plasmids encoding the hemagglutinin (HA)-tagged wild (WT) type (WT-GSK3β-HA/pcDNA3), constitutively active (S9A) mutant (S9A-GSK3β-HA/pcDNA3) and kinase dead (KD) mutant (K85R-GSK3β-HA/pcDNA3) of GSK3β were applied in this study as previously described31. Transient transfection of Huh7.5.1 cells was carried out by using the Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA).
RNA interference (RNAi)
GSK3β specific siRNA sequence (5’-GAGCAAAUCAGAGAAAUGAtt-3’) was designed according to the complete coding sequence of human GSK3β gene (GenBank accession number: EU302497.1). A scrambled siRNA sequence (5’-GCGAGUAGCGCUAGGAAGUtt-3’) without sequence similarity to any known gene sequences from mouse, rat, or human was designed as control for RNAi. Predesigned siRNA duplex was chemically synthesized by Ambion (Austin, TX). Efficiency of Lipofectamine (Invitrogen)-mediated gene-silencing efficiency was assessed by immunoblot analysis for GSK3β. Near-complete (>90%) suppression of GSK3β protein expression was achieved.
Subcellular Protein Fractionation and Western Immunoblot Analysis
The cytoplasmic and nuclear fractions were prepared by using the NE-PER kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instruction. Samples were processed for immunoblot analysis by using the anti-Nrf2 antibody.
Western Immunoblot Analysis and Immunoprecipitation
Total cell lysates or subcellular protein fractionations were prepared and processed for immunoprecipitation or immunoblot analysis as described previously28,29. The antibodies against pGSK3β, Nrf2, GSK3β, heme oxygenase (HO)-1 and HA were from Santa Cruz Biotechnology (Santa Cruz, CA) or Cell Signaling Technology (Beverly, MA).
Patients
This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of the Second Xiangya Hospital of Central South University in China. Written informed consent was obtained from each subject. Discarded or excess liver biopsy specimens from 24 patients with chronic hepatitis C (CHC) were included. All patients had serology and biopsy-proven CHC with varying severity of hepatic pathology features. No clinical, morphologic or serologic evidence of other specific type of liver injuries was observed in these cases. In addition, 6 CHC patients receiving lithium carbonate therapy for concomitant psychiatric disorders and 20 CHC control patients matched on age, gender, and duration of CHC but never treated with lithium carbonate were include in this study and excess liver biopsy specimens examined.
Morphology and Immunohistochemistry Studies
The paraffin-embedded formalin-fixed liver tissues were prepared into 3-μm sections. For general histology, sections were processed for hematoxylin and eosin staining. Liver injury was assessed by Knodell scoring system. Immunoperoxidase staining of pGSK3β and Nrf2 was performed with a Vectastain ABC kit (Vector Laboratories, Burlingame, CA). As a negative control, the primary antibody was replaced by preimmune IgG from the same species.
Semi-quantitative Morphometric Analysis
Morphology analysis of all sections was performed by an independent pathologist in a blinded manner as described previously. In brief, immunohistochemistry images were captured and digitized using a computerized image analysis system consisting of a high-resolution digital camera (MicroPublisher 3.3 RTV, QImaging, Burnaby, BC, Canada) attached to a microscope (Olympus BX41, Japan) and to a computer. Images were displayed at a pixel resolution of 1024 × 768 pixels (spatial resolution = 0.11 µm per pixel). The Image Pro Plus version 5.1 software (Media Cybernetics, MD, USA) was used for image segmentation, threshold establishment and signal intensity measurements. Manual corrections were performed as needed. For each biopsy specimen, the entire section was digitally scanned at a magnification of ×400. All areas on the sections were sampled for morphometrical analysis and final data expressed as the value of integrated pixel density for Nrf2 or pGSK3β staining. The final result was reported as low expression if the value of integrated pixel density was less than 50% of the mean value of normal liver samples, or otherwise reported as high expression.
Fluorescent Immunocytochemistry
Huh 7.5.1 cells cultured on coverslips were fixed using freshly prepared 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. After washing in PBS, the cells were blocked with 20% normal donkey serum in PBS at room temperature and incubated with the specific primary antibody and then the Alexa Fluor labeled secondary antibody (Molecular Probes, Eugene, OR, USA). Finally, cells were counter-stained with 4,6-diamidino-2-phenylindole (DAPI; Invitrogen) and observed under a fluorescence microscope.
Measurement of Cell Apoptosis
Fixed cells were subjected to terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining by using a cell apoptosis detection kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions followed by nuclear counterstaining with propidium iodide (PI; Invitrogen). Apoptotic cells were visualized by fluorescence microscopy.
Statistical Analysis
For immunoblot analysis, bands were scanned and the integrated pixel density was determined using a densitometer and the NIH image analysis program. All data are expressed as means ± S.D. Unless otherwise indicated, all experimental observations were made in triplicate. Statistical analysis of the data from multiple groups was performed by ANOVA followed by Student-Newman-Keuls tests. Data from two groups were compared by t-test. Linear regression analysis was applied to examine possible relationships between two parameters. P<0.05 was considered significant.
Results
HCV infection induces nuclear accumulation of Nrf2 in Huh7.5.1 cells
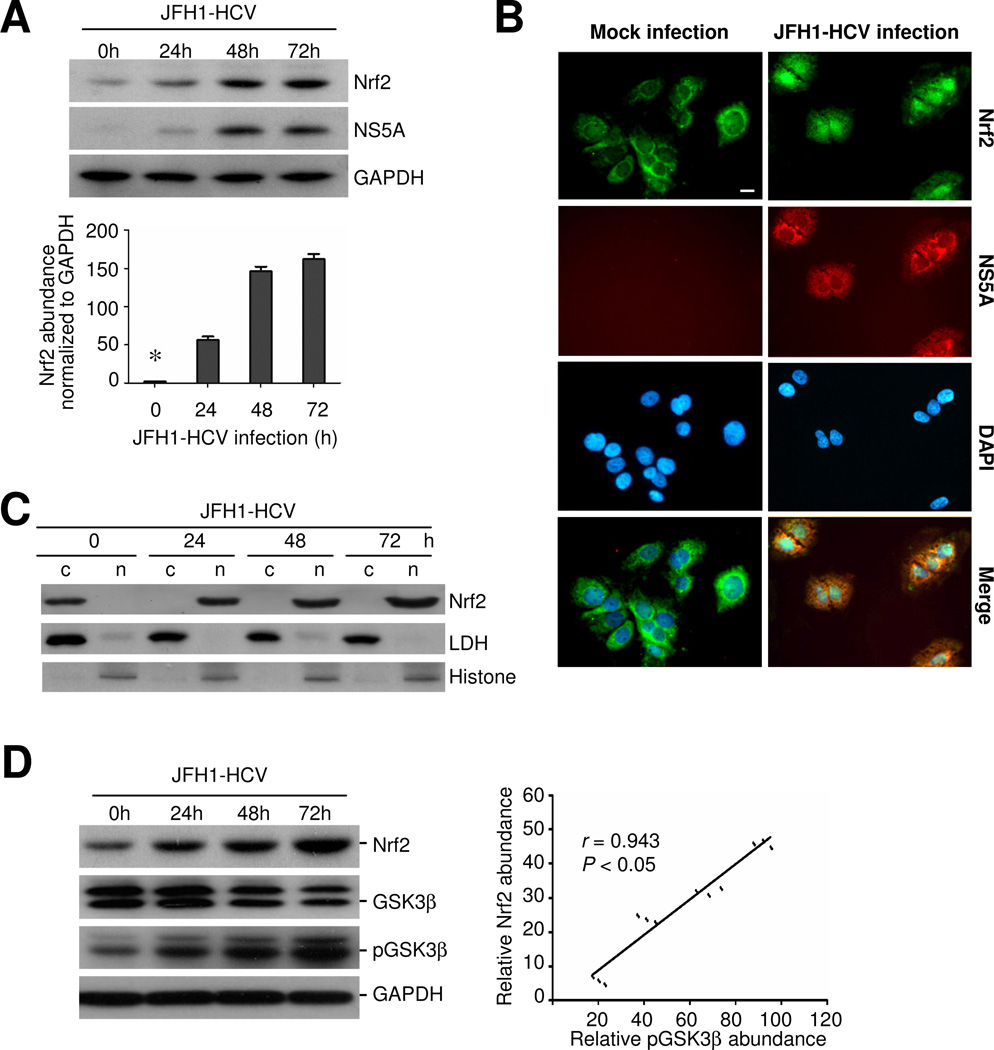
Huh7.5.1 cells were infected with JFH-1 HCV at a multiplicity of infection (m.o.i) of 0.5. Mock-infected cells served as control. Cells were collected at various time points (0, 24, 48, 72 h) after infection and cellular lysates were subjected to western immunoblot analysis. Shown in Figure 1, NS5A, a viral molecule of HCV, was evidently detected in hepatic cells in 24 h, denoting a successful infection. Under basal condition, Nrf2 was faintly expressed in Huh7.5.1 cells and JFH-1 HCV infection remarkably induced the expression of Nrf2 in a time-dependent manner (Figure 1A). By contrast, mock infection barely exhibited any effect on Nrf2 expression (data not shown). To further define the subcellular distribution of the HCV induced Nrf2 expression in Huh7.5.1 cells, fluorescent immunocytochemistry was carried out. In normal cells or mock-infected cells with no NS5A expression, Nrf2 staining was basally expressed in the cytoplasm (Figure 1B). In cells infected by HCV for 24 h and expressing evident NS5A, Nrf2 staining was abundantly induced and exclusively localized in the nuclei (Figure 1B). To avoid imaging bias, immunoblot analysis was performed on the cytoplasmic and nuclear fractions (Figure 1C). Consistent with the morphologic findings, immunoblot analysis demonstrated an evident cytoplasmic expression of Nrf2 in Huh7.5.1 cells under basal condition. The Nrf2 expression was drastically enhanced in the nuclei in a time dependent fashion following HCV infection but barely detected in the cytoplasm (Figure 1C). The quality of nuclear or cytosol extractants was assured by immunoblot analysis for the cytoplasmic molecule lactate dehydrogenase (LDH) or the nuclear molecule histone respectively: LDH was barely measured in the nuclear fractions and histone was not detected in the cytosol fractions (Figure 1C).
Figure 1. HCV infection induces Nrf2 antioxidant response, characterized by nuclear accumulation of Nrf2 in human hepatic cells, associated with inhibitory phosphorylation of GSK3β.
Huh7.5.1 cells were mock infected or infected with JFH-1 at a multiplicity of infection (m.o.i.) of 0.5. (A) At different time points, cellular lysates were prepared and analyzed by western immunoblot (WB) for Nrf2, NS5A and GAPDH; Relative abundance of Nrf2 normalized by GAPDH was determined by densitometric analysis of immunoblots; *P<0.05 versus all other groups, (n=3); (B) Fluorescent immunocytochemistry staining for Nrf2 (green signals) and NS5A (red signals) in mock infected or JFH-1 infected Huh7.5.1 cells, which were counterstained with DAPI (blue signals); Bar = 10 μm. (C) Cytoplasmic(c) and nuclear(n) protein fractions were prepared from JFH-1 infected cells and subjected to immunoblot analysis for Nrf2 or for nuclear protein histone and cytoplasmic protein lactate dehydrogenase (LDH), which served as quality control respectively for cytoplasmic and nuclear protein extraction. (D) Cell lysates were analyzed by immunoblot analysis for Nrf2, GSK3β, p-GSK3β (Ser9) and GAPDH; Relative abundance of Nrf2 and pGSK3β normalized by GAPDH and GSK3β respectively was determined by densitometric analysis and linear regression analysis indicates that inhibitory phosphorylation of GSK3β positively correlates with the expression of Nrf2 (P < 0.05, n=3).
HCV infection induced Nrf2 expression is associated with inhibitory phosphorylation of GSK3β
Recent evidence suggests that GSK3β is a key regulator of the Nrf2 pathway. To elucidate the molecular mechanisms accounting for the HCV induced Nrf2 accumulation, GSK3β signaling was examined next. Immunoblot analysis indicated that the HCV elicited Nrf2 induction was accompanied with inhibitory phosphorylation of the serine 9 of GSK3β (Figure 1D). Densitometric analysis of immunoblots revealed a significantly positive correlation between induced Nrf2 expression and inhibitory phosphorylation of GSK3β (Figure 1D).
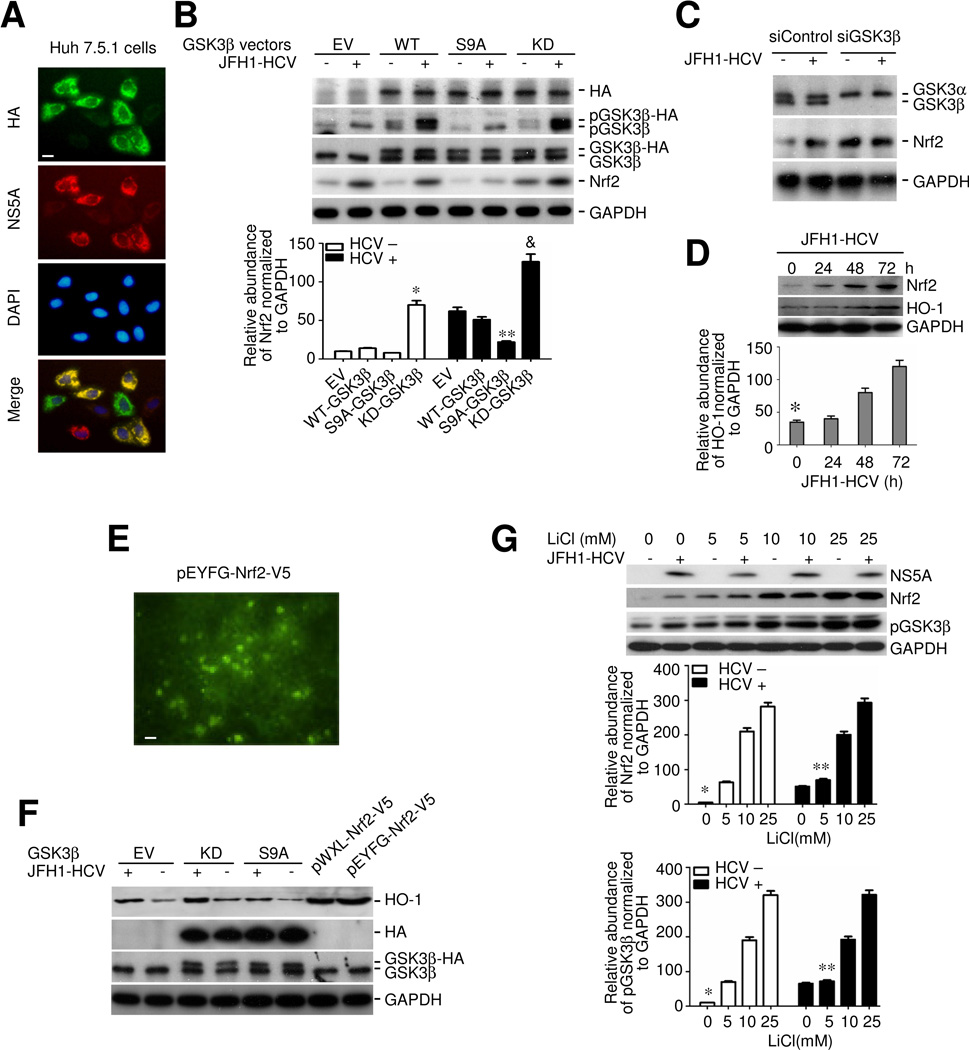
GSK3β inhibition is necessary and sufficient for HCV induced Nrf2 response in hepatic cells
To explore the possibility of a causal relationship between inhibitory phosphorylation of GSK3β and the HCV induced Nrf2 expression, the activity of GSK3β was artificially manipulated in Huh7.5.1 cells by forced expression of vectors encoding empty vector (EV), or HA conjugated wild type (WT), constitutively active (S9A) or dominant negative kinase dead (KD) GSK3β followed by HCV or mock infection. Immunofluorescent staining (Figure 2A) as well as immunoblot analysis (Figure 2B) using an antibody against the HA epitope revealed that ~80% of the cells expressed the HA-tagged constructs and that ~60% of the HCV infected cells were double positive for both HCV (NS5A) and the HA-tagged constructs, denoting a satisfactory transfection efficiency. Shown in Figure 2B, HCV infection induced serine 9 phosphorylation of both endogenous GSK3β and the ectopic WT or KD mutant. However, the phosphorylated form of the ectopic S9A, where the serine 9 was substituted by alanine, was undetectable. Ectopic expression of the KD mutant substantially boosted the basal Nrf2 expression and enhanced the HCV induced Nrf2 expression (Figure 2B), inferring that GSK3β inhibition is sufficient for HCV induced Nrf2 expression. In contrast, forced expression of the S9A mutant repressed the basal Nrf2 expression and largely abrogated the HCV induced Nrf2 expression (Figure 2B). To further ascertain if GSK3β is essential for the HCV promoted Nrf2 response, RNAi was applied to specifically silence the GSK3β expression in Huh7.5.1 cells. Shown in Figure 2C, RNAi with GSK3β-specific siRNA (siGSK3β) drastically knocked down GSK3β expression as validated by immunoblot analysis. By contrast, RNAi with scramble siRNA (siControl) barely affected its expression. GSK3β knockdown considerably up regulated the basal expression of Nrf2. In cells transfected with scrambled siRNA, augmented Nrf2 expression was apparently observed upon HCV infection. However, in GSK3β silenced cells, HCV infection barely had any enhancing effect on Nrf2 expression (Figure 2C), suggesting that GSK3β is indispensable for the HCV stimulated Nrf2 response.
Figure 2. Inhibition of GSK3β mediates the HCV induced Nrf2 response and heme oxygenase-1 expression in hepatocytes.
(A) Huh7.5.1 cells were transiently transfected with vectors encoding empty vector (EV), or hemagglutinin (HA) conjugated wild type (WT), constitutively active (S9A) or dominant negative kinase dead (KD) GSK3β and then subjected to JFH-1 HCV infection at a m.o.i of 0.5 for 24 h. Representative micrographs of fluorescent immunocytochemistry staining for HA (green signals) and NS5A (red signals) in JFH-1 infected Huh7.5.1 cells, which were counterstained with DAPI (blue signals); Bar = 10 μm. (B) Cell were treated as stated in (A) and cell lysates were collected and subjected to immunoblot analysis for Nrf2, HA, pGSK3β, GSK3β and GAPDH. Relative abundance of Nrf2 normalized by GAPDH was determined by densitometric analysis of immunoblots and suggests that ectopic expression of the S9A mutant of GSK3β abrogated the HCV infection induced Nrf2 expression, while forced expression of the KD mutant of GSK3β was sufficient for Nrf2 expression and enhanced the HCV infection induced Nrf2 expression. *P<0.05 versus all other mock infected groups; **P<0.05 versus all other HCV infected groups; (C) Huh7.5.1 cells were transiently transfected with a scrambled siRNA (siControl) or GSK3β specific siRNA (siGSK3β) and then subjected to JFH-1 HCV infection at a m.o.i of 0.5 for 24 h. cell lysates were collected and subjected to immunoblot analysis for GSK3β, Nrf2 and GAPDH. (D) JFH1-HCV infected Huh7.5.1 cells were harvested at different time points and cell lysates probed for heme oxygenase-1 (HO-1), Nrf2 and GAPDH by immunoblot analysis. Relative abundance of HO-1 normalized by GAPDH was determined by densitometric analysis of immunoblots; *P<0.05 versus all other groups. (E) Huh7.5.1 cells were transiently transfected with the plasmid of pEYFG-Nrf2-V5 for 24 h. Ectopic expression of EYFG-Nrf2-V5 fusion protein was visualized by fluorescent microscopy and mainly situated in the nuclei in addition to the cytoplasm; Bar = 25 μm (F) Huh7.5.1 cells were transiently transfected with vectors encoding EV, KD, and S9A and subjected to JFH1 HCV infection or mock infection for 24 h. Huh7.5.1 cells transiently transfected with the plasmid of pWXL-Nrf2-V5 and pEYFG-Nrf2-V5 served as Nrf2 expressing cells. Cell lysates were analyzed by immunoblot analysis for HO-1, HA, GSK3β and GAPDH. (G) Lithium, a selective inhibitor of GSK3β, mimics the effect of KD mutant of GSK3β and promotes HCV induced Nrf2 expression. Huh7.5.1 cells were subjected to JFH-1 HCV infection or mock-infection for 48 h. Cells were treated with different doses of LiCl (0, 5, 10, and 25mM) for 6 h. Cell lysate were analyzed by immunoblot analysis for NS5A, Nrf2, pGSK3β and GAPDH; Relative abundance of Nrf2 and pGSK3β normalized by GAPDH was determined by densitometric analysis of immunoblots; *P<0.05 versus all other mock infected groups; **P<0.05 versus all other HCV infected groups (n=3).
GSK3β regulates the Nrf2 dependent antioxidant response to HCV infection
To define the functional consequence of GSK3β regulation of Nrf2, the expression of HO-1, a prototypical detoxifying/antioxidant enzyme and an Nrf2 dependent molecule, was examined. HCV infection evidently induced HO-1 production, associated with an elicited Nrf2 response (Figure 2D). Nrf2 seems to be sufficient for the HCV induced HO-1 expression, because ectopic expression of vectors (pWXL-Nrf2-V5 and pEYFG-Nrf2-V5) encoding the constitutively active (undegradable) Nrf2-V5 mutants, which were largely detected in the nuclei of Huh7.5.1 cells (Figure 2E), strongly boosted up HO-1 expression even in the absence of HCV infection (Figure 2F). Moreover, consistent with GSK3β regulation of Nrf2, artificial manipulation of GSK3β activity also dictated the levels of HO-1 expression (Figure 2F). Thus, forced expression of the KD mutant promoted while ectopic expression of the S9A mutant blunted the HCV elicited HO-1 expression. Lithium is a selective inhibitor of GSK3β and its effect on Nrf2 expression was also evaluated. Huh7.5.1 cells were treated with vehicle or lithium following HCV or mock infection. Shown in Figure 2G, lithium consistently induced inhibitory phosphorylation of GSK3β and Nrf2 expression in a dose dependent fashion in mock transfected cells. In HCV infected cells, lithium treatment reinforced the inhibitory phosphorylation of GSK3β and enhanced the Nrf2 response, reminiscent of the effect of ectopic expression of the KD mutant of GSK3β.
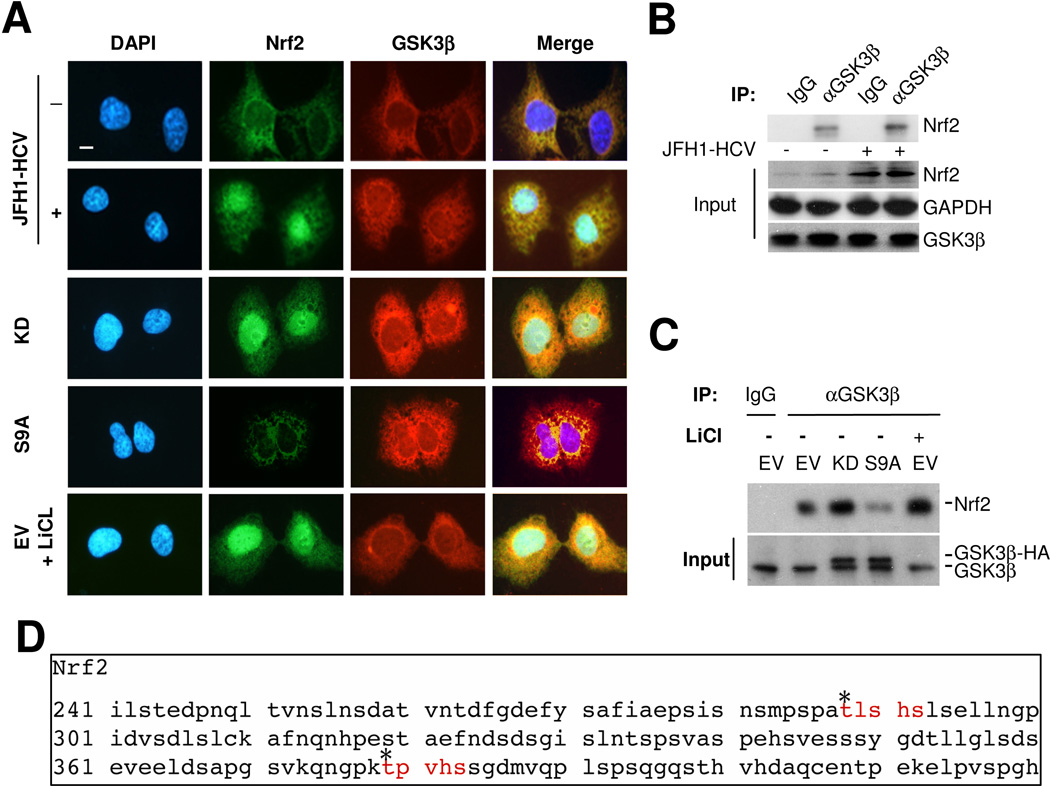
Nrf2 physically associates and interacts with GSK3β as its putative substrates in hepatic cells
To understand the molecular essence of the GSK3β regulated expression of Nrf2, the physical relationship between Nrf2 and GSK3β was explored. Dual fluorescent immunocytochemistry staining indicated a potential physical association between GSK3β and Nrf2 in the cytoplasm of HCV or mock infected Huh7.5.1 cells (Figure 3A). To validate this morphologic finding, immunoprecipitation was performed and revealed that Nrf2 evidently coprecipitated with GSK3β in both HCV and mock infected Huh7.5.1 cells (Figure 3B). More Nrf2 coprecipitated with GSK3β in HCV infected cells as compared with mock infected cells, suggesting that more Nrf2 was expressed in HCV infected cells and associated with GSK3β. Furthermore, in HCV uninfected cells, ectopic expression of the KD mutant of GSK3β enhanced the morphologic association between Nrf2 and GSK3β (Figure 3A) and resulted in more Nrf2 coprecipitation with GSK3β (Figure 3C), whereas forced expression of S9A mutant diminished the subcellular association between Nrf2 and GSK3β (Figure 3A) and reduced Nrf2 coprecipitation with GSK3β (Figure 3C), suggesting that the activity of GSK3β dictates the physical interaction between Nrf2 and GSK3β. Evidence suggests that upon phosphorylation, Nrf2 undergoes nuclear exit and subsequent proteasomal degradation. Indeed, GSK3β has been found to be responsible for nuclear exclusion of Nrf2 in multiple cell types, including N2A neuroblasts and human embryo kidney 293T cells, in which Nrf2 was excluded from the nucleus once it binds to GSK3β. This might explain why there was little coexpression of the two proteins in the nuclei in our hands. To further explore the mechanism by which GSK3β regulates the expression of Nrf2, the amino acid sequence of Nrf2 was subjected to computational active site analysis for putative GSK3β consensus motifs. In silico analysis revealed that residues Thr288 and Thr379 of Nrf2 reside in consensus motifs for phosphorylation by GSK3β (Figure 3C), denoting that Nrf2 is a putative cognate substrate for GSK3β.
Figure 3. Nrf2 physically interacts and associates with GSK3β as its cognate substrate.
(A) Huh7.5.1 cells were transiently transfected with vectors encoding EV, KD, and S9A in the presence or absence of LiCl (10mM) treatment or were subjected to JFH-1 HCV infection or mock-infection for 24 h, followed by fluorescent immunocytochemistry staining for Nrf2 and GSK3β. Nuclei were counterstained with DAPI; Bar = 10 μm. (B) Huh7.5.1 cells were subjected to JFH-1 HCV infection or mock-infection for 24 h and cell lysates were subjected to immunoprecipitation by using anti-GSK3β antibody or control IgG. Immunoprecipitates were probed for Nrf2 by immunoblot analysis. (C) Another portion of cell lysates served as input controls for immunoprecipitation and demonstrated equal amounts of input GAPDH or GSK3β. (D) In Silico analysis of the amino acid sequence of human Nrf2 for the putative GSK3β consensus motifs, which are highlighted in red.
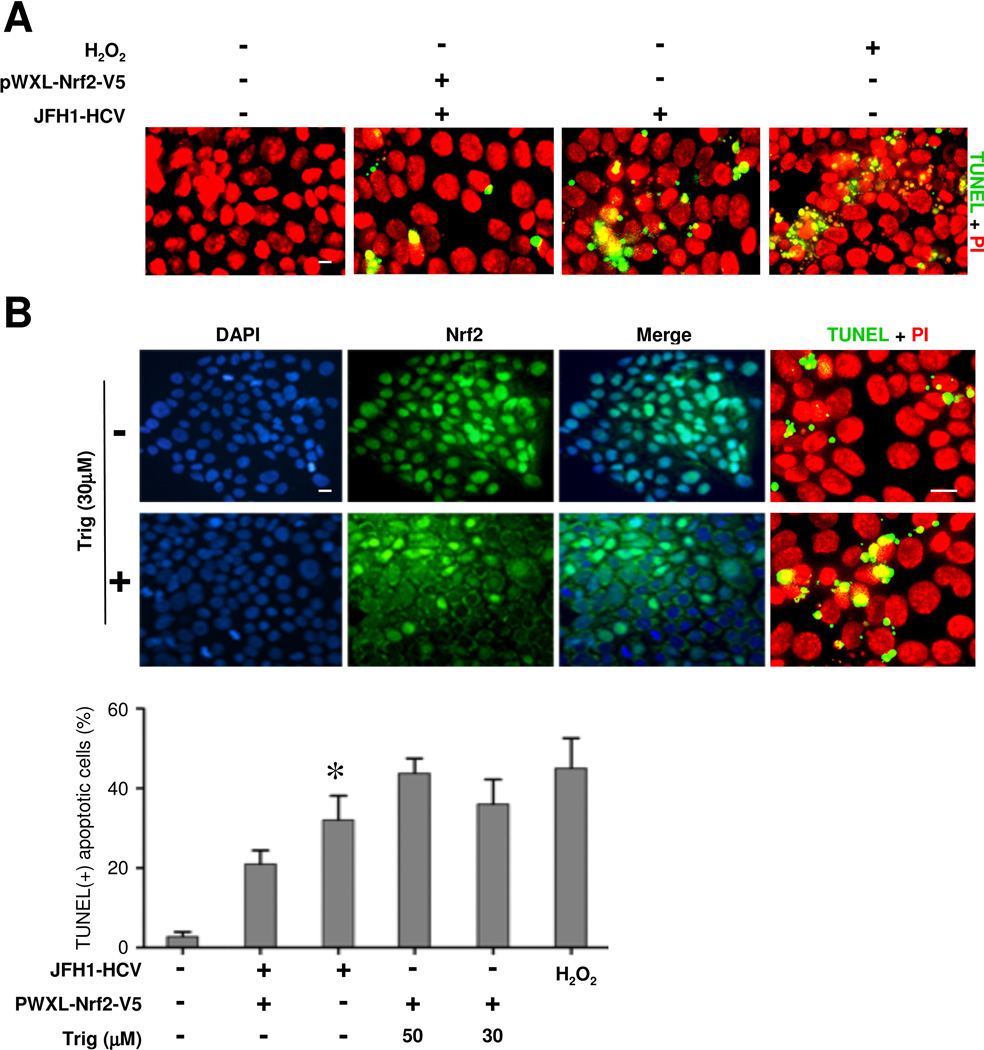
Nrf2 antioxidant response mediates hepatic cytopretection against HCV induced apoptosis
To discern the biological significance of the HCV induced Nrf2 expression, apoptotic cell death was evaluated in Huh7.5.1 cells. As a positive control, treatment with a low dose of hydrogen peroxide (100μM) for 12 h induced considerable apoptosis as indicated by TUNEL staining (Figure 4A, 4B). Likewise, HCV infection also elicited evident apoptosis (Figure 4A). Ectopic expression of a constitutively active Nrf2-V5 mutant markedly blunted this effect (Figure 4B). Conversely, trigonelline (Trig), a highly selective small molecule inhibitor of Nrf2, obliterated nuclear accumulation of Nrf2 and significantly potentiated the HCV induced apoptosis in a dose dependent fashion (Figure 4B), suggesting a cytoprotective role of Nrf2 in HCV induced hepatic cell death.
Figure 4. The Nrf2 dependent antioxidant response protects hepatic cells from HCV induced apoptosis.
(A) Huh7.5.1 cells were transfected with the plasmid of pWXL-Nrf2-V5 or empty vector followed by JFH1 HCV or mock infection for 24 h. Cell apoptotic death was probed by immunofluorescence and in situ terminal nick end-labeling (TUNEL, green signals). Nuclei were counterstained with propidium iodide (PI, red signals); Bar = 10 μm. Cells stimulated with hydrogen peroxide (100μM) served as positive control for apoptosis. (B) Huh7.5.1 cells were infected with JFH1 HCV for 48 h in the presence or absence of trigonelline (Trig, 30μM or 50μM), a highly selective small molecule inhibitor of Nrf2. Cells were fixed and subjected to immunofulrescent staining for Nrf2 or TUNEL staining (green signals). Cells were counterstained with DAPI (blue signals) or PI (red signals); Bar = 10 μm. TUNEL-positive cells were counted in five random fields in each culture well, and expressed in percentages of the total number of cells. *P<0.05 versus all other groups (n=3).
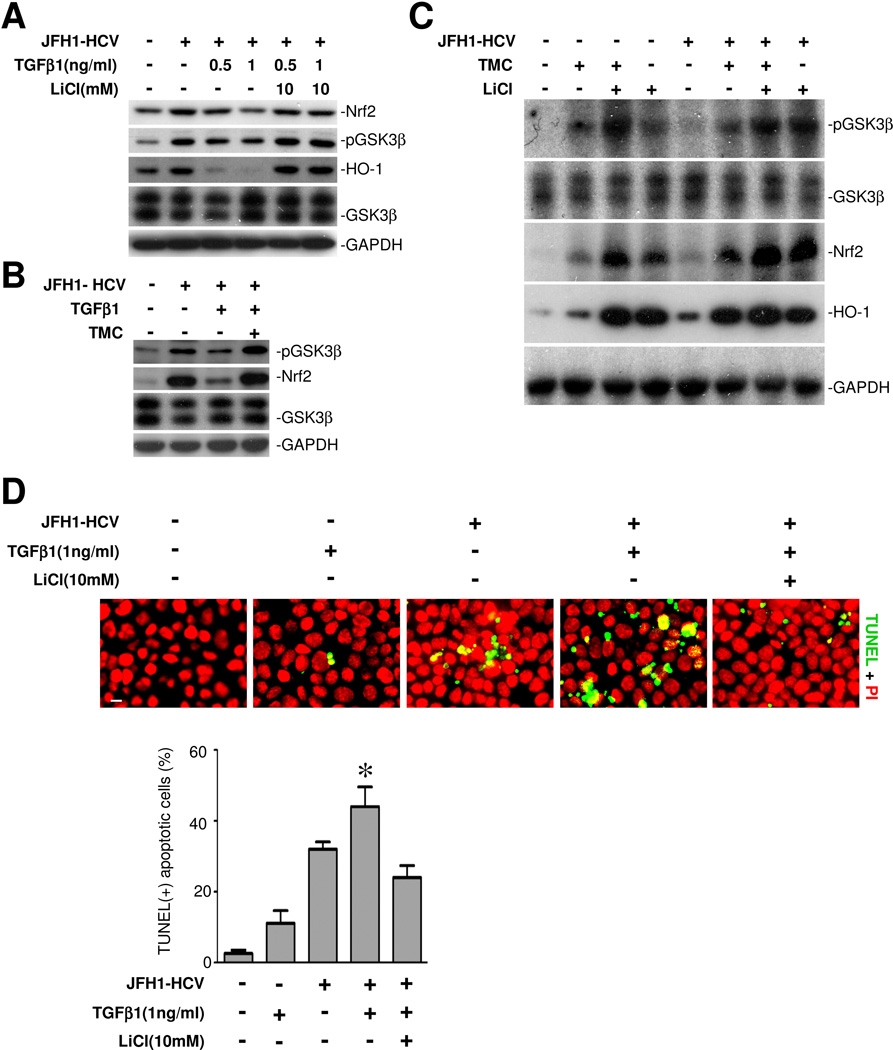
Inhibition of GSK3β by lithium reinstates the TGF-β1 impaired Nrf2 antioxidant response to HCV infection
TGF-β1 has been widely regarded as a pathogenic factor that plays a key role in liver destruction, hepatic cell dropout and progression to liver cirrhosis in CHC and other liver diseases. Indeed, TGFβ1 overrode the HCV induced Nrf2 response and HO-1 expression in a dose dependent manner (Figure 5A) and exacerbated the HCV induced hepatic cell apoptosis (Figure 5D), associated with diminished phosphorylation of GSK3β (Figure 5A). This effect of TGFβ1 is likely to be mediated by the protein phosphatase 1 directed signaling pathway because tautomycetin (TMC, 5μM), a specific inhibitor of protein phosphatase 1, largely counteracted the suppressive effect of TGFβ1 on the HCV induced GSK3β phosphorylation and the subsequent Nrf2 response (Figure 5B). In the absence of TGFβ1, TMC alone minimally induced GSK3β phosphorylation and Nrf2 expression in both HCV infected and uninfected cells, associated with a modest increase in HO-1 production; whereas lithium treatment markedly phosphorylated GSK3β and prominently reinforced the Nrf2 antioxidant response. Pharmacological inhibition of GSK3β by lithium markedly counteracted the suppressive effect of TGFβ1 on HCV induced GSK3β phosphorylation, reinstated the ensuing Nrf2 response, restored the induction of HO-1 expression (Figure 5A) and strikingly mitigated hepatic cell apoptosis (Figure 5D).
Figure 5. Inhibition of GSK3β by lithium reinstates the TGFβ1 impaired Nrf2 antioxidant response to HCV infection.
(A) Huh7.5.1 cells were infected with JFH1 HCV in the presence or absence of TGFβ1 or LiCl (10mM) treatment for 48 h. Cell lysates were probed for Nrf2, pGSK3β, HO-1, GSK3β and GAPDH. (B) Huh7.5.1 cells were infected with JFH1 HCV in the presence or absence of TGFβ1 (1ng/ml) or tautomycetin (TMC, 5μM), a specific inhibitor of protein phosphatase 1, for 48 h. Cell lysates were probed for pGSK3β, Nrf2, GSK3β and GAPDH. (C) JFH1 HCV infected or uninfected Huh7.5.1 cells were treated with LiCl (10mM) and/or tautomycetin (TMC, 5μM) in the absence of TGFβ1 for 48 h. Cell lysates were subjected to immunoblot analysis for Nrf2, pGSK3β, HO-1, GSK3β and GAPDH. (D) Cells were treated as stated in (A) and then subjected to TUNEL staining (green signals). Nuclei were counterstained with propidium iodide (PI, red signals); Bar = 10 μm. TUNEL-positive cells were counted in five random fields in each culture well, and expressed in percentages of the total number of cells. *P<0.05 versus all other groups (n=3).
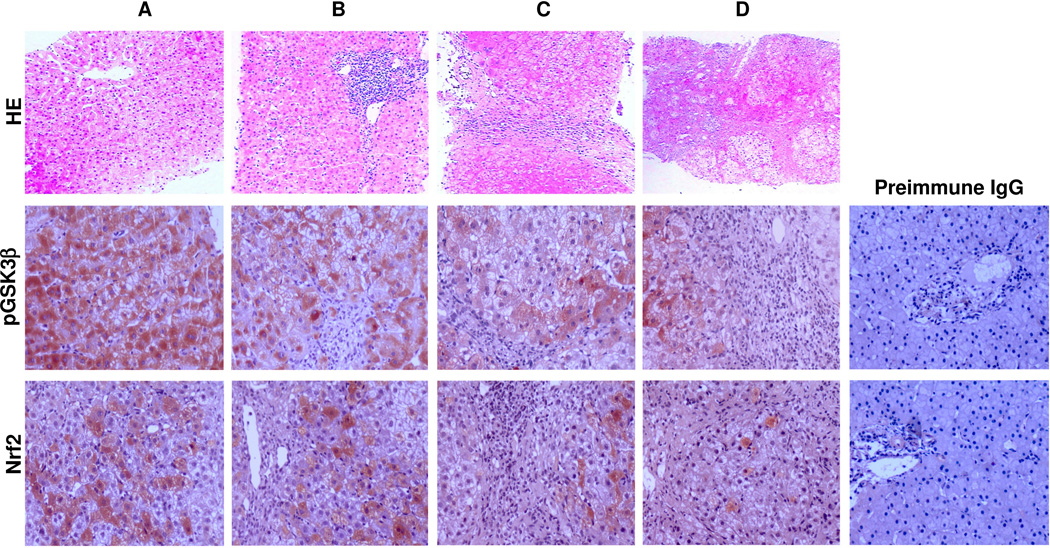
Association of inhibitory phosphorylation of GSK3β and Nrf2 expression in hepatitis C
To explore the role of the GSK3β directed Nrf2 response in CHC, liver biopsy specimens from 24 patients with varying severity of CHC were studied. Liver specimens from 6 unusable allograft livers served as normal control. Peroxidase immunohistochemistry staining indicated that immunoreacivity of phosphorylated GSK3β was primarily detected in hepatic cytoplasm while focal nuclear expression was noted in some hepatocytes in CHC (Figure 6). In normal liver tissues (Figure 6A), Nrf2 expression was evidently noted mainly in nuclei and to a lesser extent in cytoplasm of hepatic cells. In liver specimens from CHC patients (Figure 6B~D), Nrf2 expression was significantly diminished. As a negative control, the primary antibodies were replaced by preimmune IgG from the same species and no specific staining was noted (Figure 6). Semi-quantitative morphometric analysis revealed that intense staining of phosphorylated GSK3β was significantly associated with stronger Nrf2 staining as well as low grade histological hepatitis C liver injury (less inflammation and fibrosis) as assessed by Knodell scoring system (Table 1). In contrast, weak staining of phosphorylated GSK3β was significantly associated with weaker Nrf2 staining and high grade histological liver damage (prominent inflammation and fibrosis) (Table 1). Thus, high levels of phosphorylated GSK3β expression were observed in liver specimens from patients with low-grade or subsiding hepatitis C, associated with high levels of Nrf2 expression; whereas, low phosphorylated GSK3β correlated with lower hepatic Nrf2 expression and more severe histological liver injury (Table 1 and Figure 6).
Figure 6. GSK3β inactivation as denoted by phosphorylation of GSK3β correlates with induced Nrf2 expression and less hepatic injury in the livers with chronic hepatitis C.
Representative micrographs of liver biopsy specimens from a healthy donor (A) and patients with mild (B), moderate (C) or severe (D) chronic hepatitis C, which were subjected to hematoxylin and eosin staining and peroxidase immunohistochemistry staining for pGSK3β and Nrf2. As a negative control, the primary antibody was replaced by preimmune IgG and no specific staining was noted.
Table 1.
Relationship between between hepatic expression of pGSK3β and Nrf2 in human livers with chronic hepatitis C.
| Nrf2 |
||||
|---|---|---|---|---|
| Low | High | p value | ||
| pGSK3β | ||||
| Low | 8 | 2 | ||
| High | 3 | 11 | ||
| p<0.01 | ||||
| Liver injury grades (Knodell) | ||||
| Mild | 2 | 8 | ||
| Moderate | 3 | 1 | ||
| Severe | 9 | 1 | ||
| p<0.01 | ||||
The expression patterns of the two molecules in biopsy specimens form CHC by immunoperoxidase staining as shown in Figure 6. The correlation between p-GSK3β and Nrf2 or severity of liver injuries (Knodell grades) were analyzed by Fisher’s exact probability test.
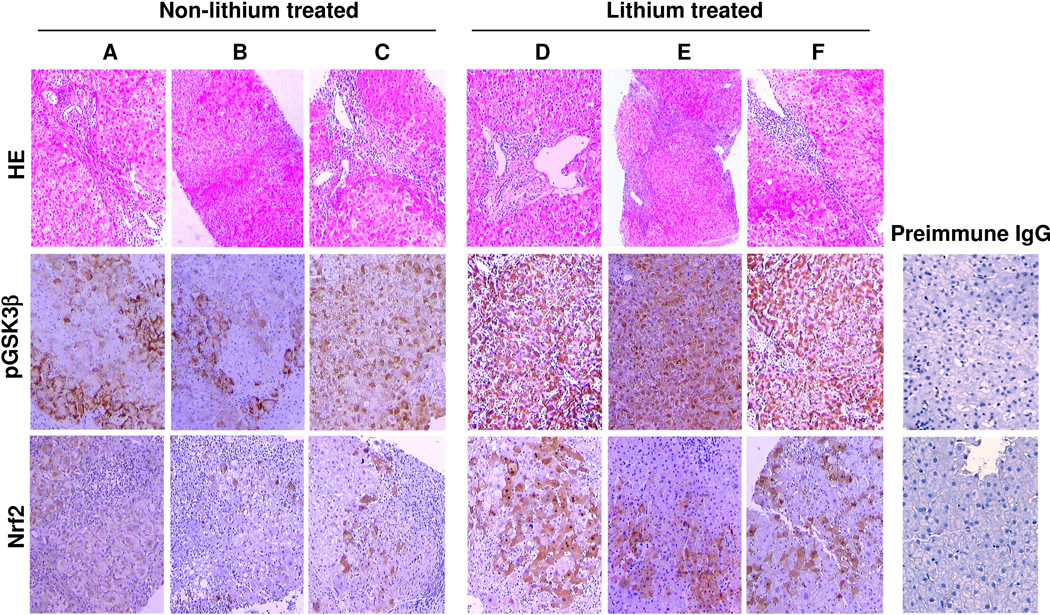
Lithium therapy inhibits GSK3β in liver and improves liver histopathology in patients with CHC
Lithium has been an FDA-approved first line treatment for bipolar affective disorders for over a half-century. To examine the effect of lithium as a selective inhibitor of GSK3β on hepatitis C, a group of 6 CHC patients who received long-term lithium carbonate therapy primarily for concomitant psychiatric disorders was studied. Another group of 20 CHC patients that were matched on age, gender, and duration of CHC but never received lithium carbonate therapy, was adopted and served as control. Shown in Table 2, CHC patients treated with long-term lithium therapy demonstrated a favorable trend in improvement of liver function as indicated by lesser serum levels of hepatic transaminase and higher serum levels of albumin. More importantly, lithium treated patients versus control patients exhibited a significantly lower grade hepatitis C histological injury as estimated by the Knodell scoring system, despite comparable age, gender, duration of hepatitis C, HCV viral load and genotype status. Consistent with the GSK3β inhibitory activity of lithium, liver biopsy specimens from lithium treated patients revealed strikingly more expression of phosphorylated GSK3β on immunohistochemistry (Figure 7B). This was associated with more intense Nrf2 expression (Figure 7B), as compared with those specimens from control patients (Figure 7A).
Table 2.
Clinical and biochemical characteristics of patients with chronic hepatitis C treated with or without lithium carbonate.
| CHC Non-lithium treated |
CHC Lithium treated |
p value | |
|---|---|---|---|
| Patient numbers | 20 | 6 | |
| Age, median (range) | 46(19–71) | 45(20–58) | 0.1 |
| Male:Female | 7:13 | 2:4 | 0.1 |
| Course of disease (years) mean (±SD) | 17.83(±0.68) | 17.65 (±0.71) | 0.7 |
| Serum ALT (IU/L) mean (±SD) | 71.36(±7.34) | 58.17 (±8.53) | 0.2 |
| Serum TBIL (mmol/L) mean (±SD) | 37.64(±2.75) | 36.72(±4.61) | 0.6 |
| Serum ALB (g/L) mean (±SD) | 44.56(±3.78) | 46.91(±2.13) | 0.3 |
| HCV genotype | 0.4 | ||
| 1a | 6 | 2 | |
| 1b | 13 | 4 | |
| 6 | 1 | 0 | |
| Viral load (Log10IU/mL) mean (±SD) | 5.98(±0.71) | 6.23(±0.64) | 0.7 |
| Liver injury Knodell score mean (±SD) | 17.41(±0.62) | 6.63(±0.17) | <0.01 |
CHC, chronic hepatitis C; ALT, alanine transaminase; TBIL, total bilirubin; ALB, albumin.
Figure 7. Long-term lithium carbonate therapy primarily for the concomitant psychiatric disorders inhibits GSK3β activity in the liver and improves liver histopathology in patients with chronic hepatitis C.
Representative micrographs of liver biopsy specimens from chronic hepatitis C patients treated without (A, B, C) or with (D, E, F) lithium chloride. The liver biopsy specimens were subjected to hematoxylin and eosin staining and immunoperoxidase staining for pGSK3β and Nrf2. As a negative control, the primary antibody was replaced by preimmune IgG and no specific staining was noted.
DISCUSSION
Approximately 2~3% of the population worldwide are infected with HCV. HCV infection involves prominent induction of excessive reactive oxygen species in liver cells leading to oxidative stress32,33. Upon oxidative stress, mammalian cells will immediately initiate a highly efficient cytoprotective machinery to protect cells against the harmful effects of ROS. Much of this cytoprotective machinery stems from the Nrf2 antioxidant response pathway, which is the primary cellular defense signaling against the cytotoxic effects of oxidative stress. The transcription factor Nrf2 is constitutively expressed in all tissues, although levels may vary among organs, with the key detoxification organs (such as kidney and liver) exhibiting highest levels15. The high levels of Nrf2 expression by liver cells may protect liver from oxidative stress generated by basal hepatic metabolism or by hepatotoxins or microorganisms. This Nrf2 effect seems to be essential for cellular survival because trigonelline, a highly selective small molecule inhibitor of Nrf2, abrogated HCV infection induced nuclear accumulation of Nrf2 and significantly potentiated the HCV induced apoptosis. In agreement with our data, Ivanov et al13 found that HCV proteins core, E1, E2, NS4B, and NS5A induced ROS production and activated Nrf2/ARE pathway. Mechanistically, inhibitory phosphorylation of GSK3β was found by the present study to be sufficient and essential for HCV induced Nrf2 response. As suggested by a study by Street et al, the HCV elicited GSK3β phosphorylation might involve the activation of phosphoinositide 3-kinase. Besides Nrf2, other transcription factors like β-catenin are likewise under the control of this signaling. The molecular essence of the GSK3β regulated Nrf2 response is likely attributable to a putative GSK3β directed control of phosphorylation of Nrf2 as its cognate substrate, followed by Nrf2 nuclear exit and degradation. Of note, activation of the Nrf2 antioxidant response subsequent to GSK3β inhibition seems not particular to HCV infection, as it has been found as a general mechanism for self protection in multiple organ systems upon injury.
In contrast to the HCV induced Nrf2 response in cultured human hepatic cells in vitro, Nrf2 expression has been previously reported to drastically subside in diseased liver in a variety of liver diseases, including CHC15. Consistently, our study indicated that the level of Nrf2 expression was inversely correlated with the severity of liver injury in a cohort of CHC patients. The disparity between the in vitro and the in vivo observations suggest that the Nrf2 antioxidant response in vivo in the liver is substantially impaired under the disease state. The mechanism accounting for this impaired Nrf2 response in diseased liver has barely been investigated, but our study suggests that TGF-β1, a widely regarded pathogenic factor for chronic liver diseases, might serve in this role and intercept the Nrf2 antioxidant response in the diseased liver. Indeed, concomitant TGF-β1 treatment profoundly obliterated the Nrf2 response in a dose dependent manner. Our finding is in agreement with a study by Michaeloudes et al34, in which TGF-β was found to inhibit Nrf2-antioxidant response in cultured airway smooth muscle cells (ASMC). In addition, the Nrf2-antioxidant response was also found to be impaired in ASMC procured from patients with severe asthma compared with ASMC from patients with nonsevere asthma and normal subjects. In congruent with the role of GSK3β in control of Nrf2 response, TGF-β1 remarkably abrogated the inhibitory phosphorylation of GSK3β, potentially via activating protein phosphatase 1 because tautomycetin, a specific inhibitor of protein phosphatase 1, largely counteracted the suppressive effect of TGF-β1. TGF-β1 has been confirmed to be able to activate protein phosphatase 135, which is highly involved in dephosphorylation of GSK3β at serine 9 residual36. Thus, it is conceivable that TGF-β1 induces GSK3β dephosphorylation and reduces inhibitory phosphorylation of GSK3β via activating protein phosphatase 1, thereby augments the activity of GSK3β, amplifies the phosphorylation of GSK3β’s substrates like Nrf2, ultimately promotes nuclear exit and degradation of Nrf2 and diminishes the Nrf2 response.
Another important finding made by our study is that lithium, a selective inhibitor of GSK3β, could induce inhibitory phosphorylation of GSK3β and enhance the HCV induced Nrf2 response. More importantly, lithium could counteract the suppressive effect of TGF-β1 on GSK3β phosphorylation as well as Nrf2 response in hepatic cells. Of note, lithium has been extensively, safely and successfully used for decades as an FDA approved first line mood stabilizer to treat many psychiatric diseases, including mania, depression and biopolar disorder26. As a matter of fact, lithium has been demonstrated to have a general protective effect in multiple organ systems37,38, including the liver39,40. Actually, long time ago, lithium orotate was used to treat patients with hepatitis and exhibited a great beneficial effect39,40. In the present study, a group of CHC patients who received long-term lithium carbonate therapy primarily for psychiatric disorders demonstrated better liver function and significantly better liver histology than a group of CHC control patients who were not treated with lithium, despite comparable age and gender profiles, duration of CHC and HCV viral load and genotype status. This was associated with more intense staining of phosphorylated GSK3β and much greater expression of Nrf2 in the liver, suggesting that long-term treatment with lithium might ameliorate liver injury in CHC possibly through reinforcing the Nrf2 antioxidant response in the hepatic cells.
In conclusion, HCV infection elicits the cytoprotective Nrf2 antioxidant response in hepatic cells through inhibitory phosphorylation of GSK3β. In diseased liver with CHC, this hepatoprotective Nrf2 response is, however, largely impaired by TGF-β1 likely via a protein phosphatase 1-dependent mechanism. Lithium, a selective inhibitor of GSK3β, could counteract the suppressive effect of TGF-β1, reinstate the hepatoprotective Nrf2 response and ameliorate liver injury in CHC. Our findings suggest that pharmacological targeting of GSK3β by either novel selective small-molecule inhibitors or existing FDA-approved drugs with GSK3β inhibitory activity, such as lithium, represents a novel therapeutic strategy for CHC.
Summary Box.
What is already known about this subject?
Approximately 2~3% of the population worldwide are infected with hepatitis C virus (HCV).
Impaired adaptive response to HCV induced oxidative stress is a fundamental mechanism central to the pathogenesis of chronic hepatitis C.
Nrf2 detoxification/antioxidant response is the primary cellular defense against the cytotoxic effects of oxidative stress.
What are the new findings?
Inhibitory phosphorylation of glycogen synthase kinase (GSK) 3β is necessary and sufficient for HCV induced self-protective Nrf2 antioxidant response.
TGFβ1 blunts the HCV induced inhibitory phosphorylation of GSK3β via a protein phosphatase 1-dependent mechanism and impairs the Nrf2 antioxidant response in hepatic cells. This effect is counteracted by lithium, a selective inhibitor of GSK3β that enhances the inhibitory phosphorylation of GSK3β.
In patients with chronic hepatitis C, the expression of phosphorylated GSK3β positively correlates with Nrf2 expression in liver biopsy specimens and is inversely associated with the severity of liver injury.
Chronic hepatitis C patients who receive long-term lithium carbonate therapy primarily for concomitant psychiatric disorders have attenuated liver injuries, associated with stronger Nrf2 expression in the liver.
How might it impact on clinical practice in the foreseeable future?
Pharmacological targeting of GSK3β by the existing FDA-approved drugs with GSK3β inhibitory activity, such as lithium, promotes Nrf2 antioxidant response in the liver and represents a novel and feasible therapeutic strategy for chronic hepatitis C.
Acknowledgements
The authors are indebted to Drs. Weiwei Xu, Hui Bao and Bo Jin for their technical assistance with the plasmid transfection experiments.
Funding This study was made possible in part by the funding from the US National Institutes of Health grant R01DK092485 and the Scientific Research Projects (2013-1132 and 2012-1493) from the Development and Reform Commission of Hunan Province, China.
Footnotes
Contributors Data acquisition/analysis: YJ, HB, YG, WT, DC, KL, GG, RG. Material support: YG, YJ, WT, DC, KL, GG. Study concept and design: YJ, RG. Analysis and interpretation of data: YJ, HB, YG, RG. Manuscript revision: YJ, RG. Funding: YJ, RG. Paper authorship: RG.
Competing interests No competing interests.
References
- 1.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 2.Tanikawa K, Torimura T. Studies on oxidative stress in liver diseases: important future trends in liver research. Med Mol Morphol. 2006;39:22–27. doi: 10.1007/s00795-006-0313-z. [DOI] [PubMed] [Google Scholar]
- 3.Clement S, Pascarella S, Negro F. Hepatitis C virus infection: molecular pathways to steatosis, insulin resistance and oxidative stress. Viruses. 2009;1:126–143. doi: 10.3390/v1020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong G, Waris G, Tanveer R, et al. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci U S A. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Mochel NS, Seronello S, Wang SH, et al. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology. 2010;52:47–59. doi: 10.1002/hep.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos CX, Tanaka LY, Wosniak J, et al. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 7.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 9.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 10.Chan K, Lu R, Chang JC, et al. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam J, Stewart D, Touchard C, et al. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 12.Carvajal-Yepes M, Himmelsbach K, Schaedler S, et al. Hepatitis C virus impairs the induction of cytoprotective Nrf2 target genes by delocalization of small Maf proteins. J Biol Chem. 2011;286:8941–8951. doi: 10.1074/jbc.M110.186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanov AV, Smirnova OA, Ivanova ON, et al. Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PLoS One. 2011;6:e24957. doi: 10.1371/journal.pone.0024957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burdette D, Olivarez M, Waris G. Activation of transcription factor Nrf2 by hepatitis C virus induces the cell-survival pathway. J Gen Virol. 2010;91:681–690. doi: 10.1099/vir.0.014340-0. [DOI] [PubMed] [Google Scholar]
- 15.Kurzawski M, Dziedziejko V, Urasinska E, et al. Nuclear factor erythroid 2-like 2 (Nrf2) expression in end-stage liver disease. Environ Toxicol Pharmacol. 34:87–95. doi: 10.1016/j.etap.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 17.Jain AK, Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 18.Rada P, Rojo AI, Evrard-Todeschi N, et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axis. Mol Cell Biol. 2012;32:3486–3499. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rada P, Rojo AI, Chowdhry S, et al. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomobe K, Shinozuka T, Kuroiwa M, et al. Age-related changes of Nrf2 and phosphorylated GSK-3beta in a mouse model of accelerated aging (SAMP8) Arch Gerontol Geriatr. 2012;54:e1–e7. doi: 10.1016/j.archger.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Bitar MS, Al-Mulla F. A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am J Physiol Endocrinol Metab. 2011;301:E1119–E1129. doi: 10.1152/ajpendo.00047.2011. [DOI] [PubMed] [Google Scholar]
- 22.Kay HY, Kim YW, Ryu da H, et al. Nrf2-mediated liver protection by sauchinone, an antioxidant lignan, from acetaminophen toxicity through the PKCdelta-GSK3beta pathway. Br J Pharmacol. 2011;163:1653–1665. doi: 10.1111/j.1476-5381.2010.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espada S, Rojo AI, Salinas M, et al. The muscarinic M1 receptor activates Nrf2 through a signaling cascade that involves protein kinase C and inhibition of GSK-3beta: connecting neurotransmission with neuroprotection. J Neurochem. 2009;110:1107–1119. doi: 10.1111/j.1471-4159.2009.06208.x. [DOI] [PubMed] [Google Scholar]
- 24.Correa F, Mallard C, Nilsson M, et al. Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: restoring effects of inhibitors of HDACs, p38 MAPK and GSK3beta. Neurobiol Dis. 2011;44:142–151. doi: 10.1016/j.nbd.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojo AI, Rada P, Egea J, et al. Functional interference between glycogen synthase kinase-3 beta and the transcription factor Nrf2 in protection against kainate-induced hippocampal cell death. Mol Cell Neurosci. 2008;39:125–132. doi: 10.1016/j.mcn.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Muller-Oerlinghausen B, Berghofer A, Ahrens B. The antisuicidal and mortality-reducing effect of lithium prophylaxis: consequences for guidelines in clinical psychiatry. Can J Psychiatry. 2003;48:433–439. doi: 10.1177/070674370304800702. [DOI] [PubMed] [Google Scholar]
- 27.Moradpour D, Evans MJ, Gosert R, et al. Insertion of green fluorescent protein into nonstructural protein 5A allows direct visualization of functional hepatitis C virus replication complexes. J Virol. 2004;78:7400–7409. doi: 10.1128/JVI.78.14.7400-7409.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T, Date T, Miyamoto M, et al. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology. 2003;125:1808–1817. doi: 10.1053/j.gastro.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Kato T, Furusaka A, Miyamoto M, et al. Sequence analysis of hepatitis C virus isolated from a fulminant hepatitis patient. J Med Virol. 2001;64:334–339. doi: 10.1002/jmv.1055. [DOI] [PubMed] [Google Scholar]
- 30.Rojo AI, Sagarra MR, Cuadrado A. GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J Neurochem. 2008;105:192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- 31.Gong R, Ge Y, Chen S, et al. Glycogen synthase kinase 3beta: a novel marker and modulator of inflammatory injury in chronic renal allograft disease. Am J Transplant. 2008;8:1852–1863. doi: 10.1111/j.1600-6143.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- 32.Di Bona D, Cippitelli M, Fionda C, et al. Oxidative stress inhibits IFN-alpha-induced antiviral gene expression by blocking the JAK-STAT pathway. J Hepatol. 2006;45:271–279. doi: 10.1016/j.jhep.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 33.Nishina S, Hino K, Korenaga M, et al. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226–238. doi: 10.1053/j.gastro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Michaeloudes C, Sukkar MB, Khorasani NM, et al. TGF-beta regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L295–L304. doi: 10.1152/ajplung.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gruppuso PA, Mikumo R, Brautigan DL, et al. Growth arrest induced by transforming growth factor beta 1 is accompanied by protein phosphatase activation in human keratinocytes. J Biol Chem. 1991;266:3444–3448. [PubMed] [Google Scholar]
- 36.King TD, Gandy JC, Bijur GN. The protein phosphatase-1/inhibitor-2 complex differentially regulates GSK3 dephosphorylation and increases sarcoplasmic/endoplasmic reticulum calcium ATPase 2 levels. Exp Cell Res. 2006;312:3693–3700. doi: 10.1016/j.yexcr.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Culman J, Blume A, et al. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2003;34:1287–1292. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]
- 38.Petrini M, Azzara A. Lithium in the treatment of neutropenia. Curr Opin Hematol. 19:52–57. doi: 10.1097/MOH.0b013e32834da93b. [DOI] [PubMed] [Google Scholar]
- 39.Nieper HA. The clinical applications of lithium orotate. A two years study. Agressologie. 1973;14:407–411. [PubMed] [Google Scholar]
- 40.Sartori HE. Lithium orotate in the treatment of alcoholism and related conditions. Alcohol. 1986;3:97–100. doi: 10.1016/0741-8329(86)90018-2. [DOI] [PubMed] [Google Scholar]