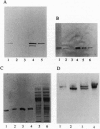
Abstract
NAD(P)H:quinone oxidoreductase (NQOR; EC 1.6.99.2) is a homodimeric enzyme which catalyzes the reduction of quinones, azo dyes, and other electron acceptors by NADPH or NADH. To pursue subunit functional studies, we expressed a wild-type/mutant heterodimer of NQOR in Escherichia coli. The wild-type subunit of the heterodimer was tagged with polyhistidine and the other subunit contained a His-194-->Ala mutation (H194A), a change known to dramatically increase the Km for NADPH. This approach enabled us to efficiently purify the heterodimer (H194A/HNQOR) from the homodimers by stepwise elution with imidazole from a nickel nitrilotriacetate column under nondenaturing conditions. The composition of the purified heterodimer was confirmed by SDS and nondenaturing polyacrylamide gel electrophoresis and immunoblot analysis. The enzyme kinetics of the purified heterodimer were studied with two two-electron acceptors, 2,6-dichloroindophenol and menadione, and a four-electron acceptor, methyl red, as the substrates. With two-electron acceptors, the Km(NADPH) and Km(NADH) values of the heterodimer H194A/HNQOR were virtually identical to those of the wild-type homodimer, but the kcat-(NADPH) and kcat(NADH) values were only about 50% those of the wild-type homodimer. With the four-electron acceptor, the Km and kcat values of H194A/HNQOR for NADPH and NADH were similar to those of the low-efficiency mutant homodimer. These results suggest that the subunits of NQOR function independently with two-electron acceptors, but dependently with a four-electron acceptor. This heterodimer approach may have general applications for studying the functional and structural relationships of subunits in dimeric or oligomeric proteins.
Full text
PDF
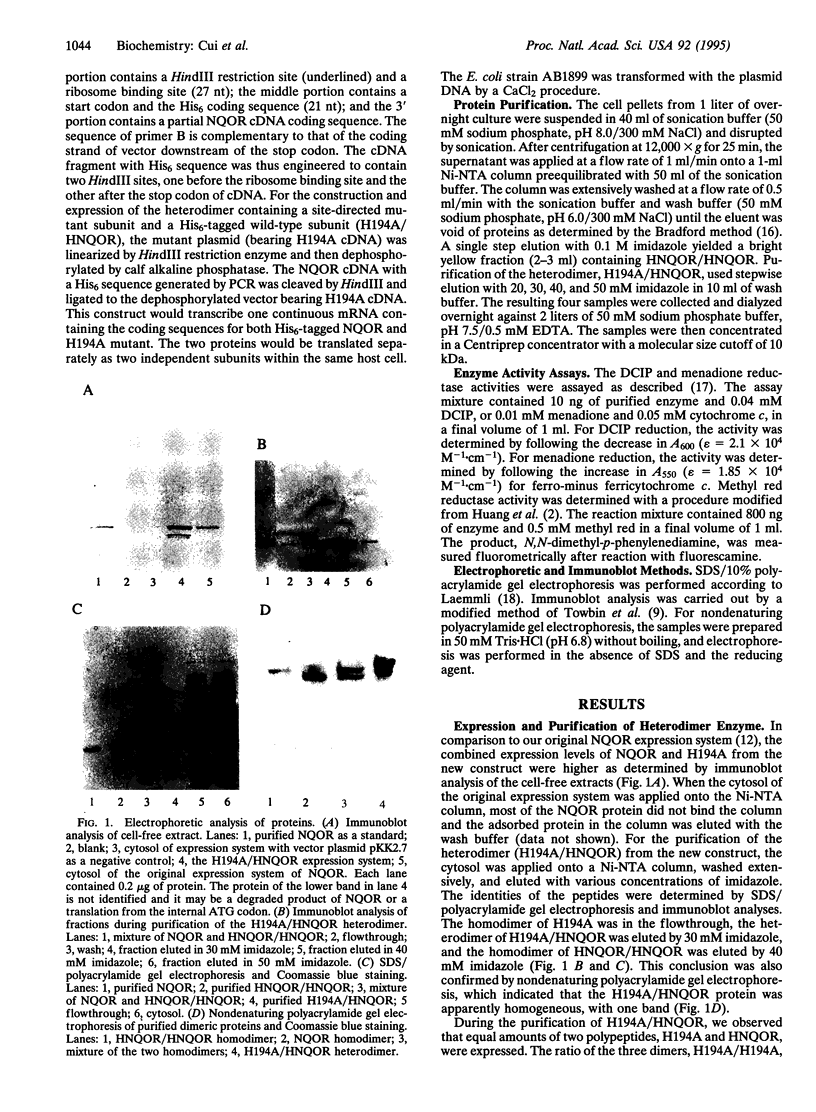
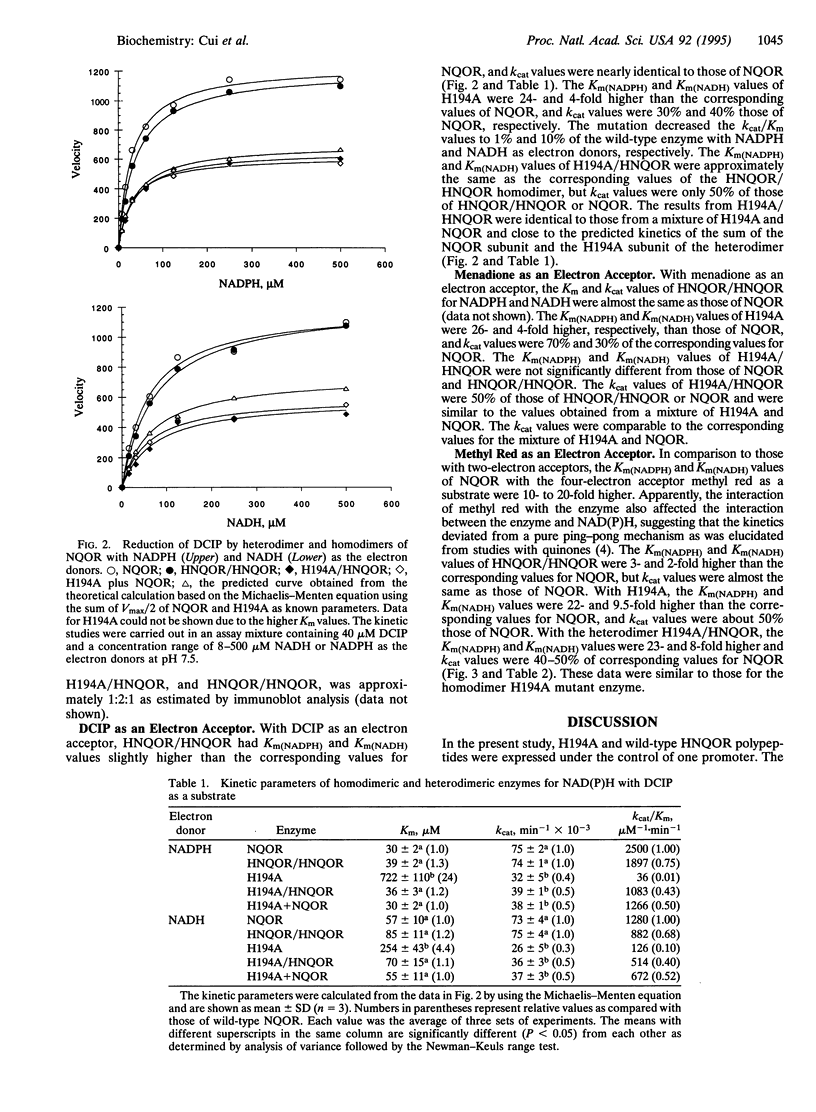
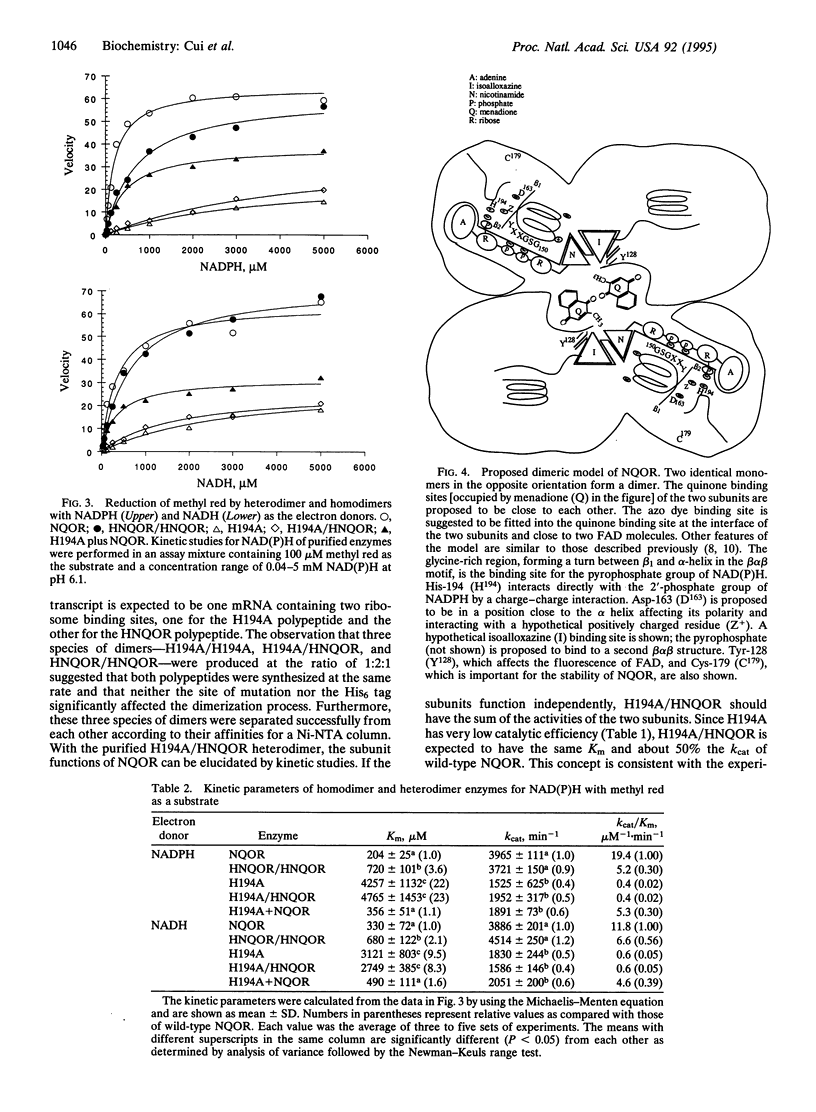

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayney R. M., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver NAD(P)H: quinone reductase nucleotide sequence analysis of a quinone reductase cDNA clone and prediction of the amino acid sequence of the corresponding protein. J Biol Chem. 1987 Jan 15;262(2):572–575. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- ERNSTER L., DANIELSON L., LJUNGGREN M. DT diaphorase. I. Purification from the soluble fraction of rat-liver cytoplasm, and properties. Biochim Biophys Acta. 1962 Apr 9;58:171–188. doi: 10.1016/0006-3002(62)90997-6. [DOI] [PubMed] [Google Scholar]
- Haniu M., Yuan H., Chen S. A., Iyanagi T., Lee T. D., Shively J. E. Structure-function relationship of NAD(P)H:quinone reductase: characterization of NH2-terminal blocking group and essential tyrosine and lysine residues. Biochemistry. 1988 Sep 6;27(18):6877–6883. doi: 10.1021/bi00418a033. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Miwa G. T., Cronheim N., Lu A. Y. Rat liver cytosolic azoreductase. Electron transport properties and the mechanism of dicumarol inhibition of the purified enzyme. J Biol Chem. 1979 Nov 25;254(22):11223–11227. [PubMed] [Google Scholar]
- Huang M. T., Miwa G. T., Lu A. Y. Rat liver cytosolic azoreductase. Purification and characterization. J Biol Chem. 1979 May 25;254(10):3930–3934. [PubMed] [Google Scholar]
- Jaiswal A. K., McBride O. W., Adesnik M., Nebert D. W. Human dioxin-inducible cytosolic NAD(P)H:menadione oxidoreductase. cDNA sequence and localization of gene to chromosome 16. J Biol Chem. 1988 Sep 25;263(27):13572–13578. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larimer F. W., Lee E. H., Mural R. J., Soper T. S., Hartman F. C. Intersubunit location of the active site of ribulose-bisphosphate carboxylase/oxygenase as determined by in vivo hybridization of site-directed mutants. J Biol Chem. 1987 Nov 15;262(32):15327–15329. [PubMed] [Google Scholar]
- Le Grice S. F., Grüninger-Leitch F. Rapid purification of homodimer and heterodimer HIV-1 reverse transcriptase by metal chelate affinity chromatography. Eur J Biochem. 1990 Jan 26;187(2):307–314. doi: 10.1111/j.1432-1033.1990.tb15306.x. [DOI] [PubMed] [Google Scholar]
- Ma Q., Cui K., Wang R. W., Lu A. Y., Yang C. S. Site-directed mutagenesis of rat liver NAD(P)H: quinone oxidoreductase: roles of lysine 76 and cysteine 179. Arch Biochem Biophys. 1992 May 1;294(2):434–439. doi: 10.1016/0003-9861(92)90708-5. [DOI] [PubMed] [Google Scholar]
- Ma Q., Cui K., Xiao F., Lu A. Y., Yang C. S. Identification of a glycine-rich sequence as an NAD(P)H-binding site and tyrosine 128 as a dicumarol-binding site in rat liver NAD(P)H:quinone oxidoreductase by site-directed mutagenesis. J Biol Chem. 1992 Nov 5;267(31):22298–22304. [PubMed] [Google Scholar]
- Ma Q., Wang R., Yang C. S., Lu A. Y. Expression of mammalian DT-diaphorase in Escherichia coli: purification and characterization of the expressed protein. Arch Biochem Biophys. 1990 Dec;283(2):311–317. doi: 10.1016/0003-9861(90)90648-i. [DOI] [PubMed] [Google Scholar]
- Rase B., Bartfai T., Ernster L. Purification of DT-diaphorase by affinity chromatography. Occurrence of two subunits and nonlinear Dixon and Scatchard plots of the inhibition by anticoagulants. Arch Biochem Biophys. 1976 Feb;172(2):380–386. doi: 10.1016/0003-9861(76)90089-8. [DOI] [PubMed] [Google Scholar]
- Robertson J. A., Chen H. C., Nebert D. W. NAD(P)H:menadione oxidoreductase. Novel purification of enzyme cDNA and complete amino acid sequence, and gene regulation. J Biol Chem. 1986 Nov 25;261(33):15794–15799. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente S. R., Schachman H. K. Shared active sites in oligomeric enzymes: model studies with defective mutants of aspartate transcarbamoylase produced by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1987 Jan;84(1):31–35. doi: 10.1073/pnas.84.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]