Abstract
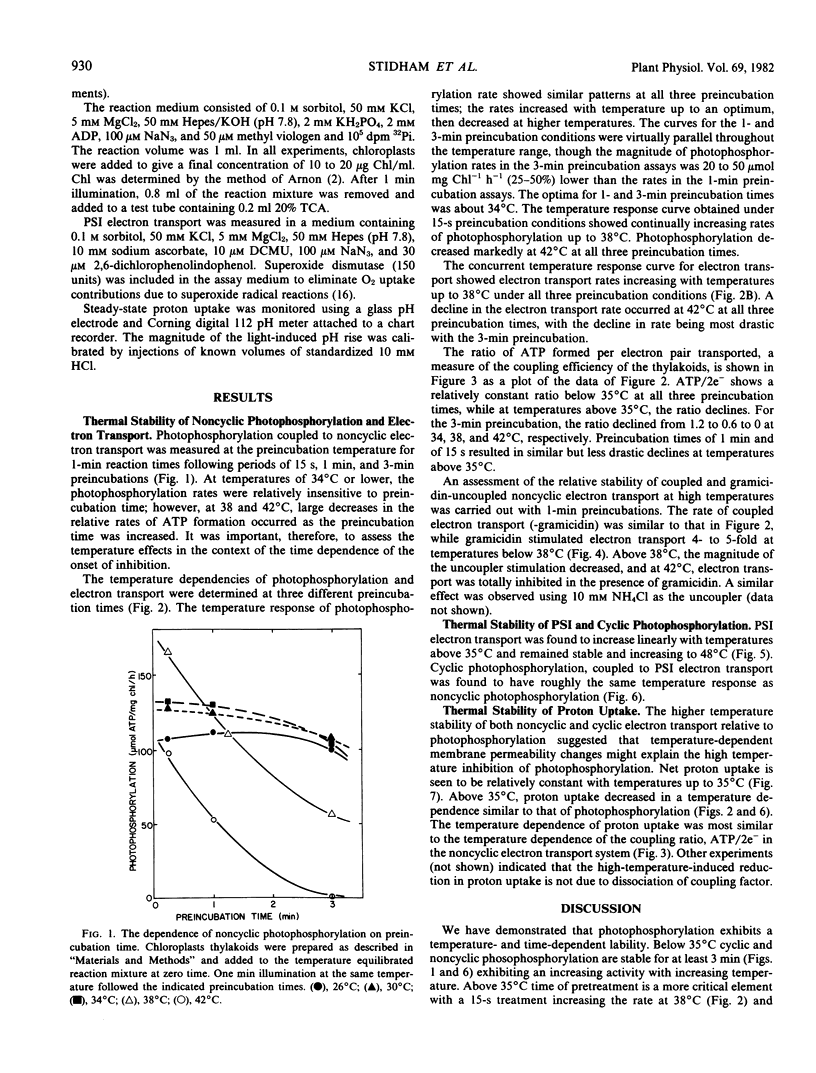
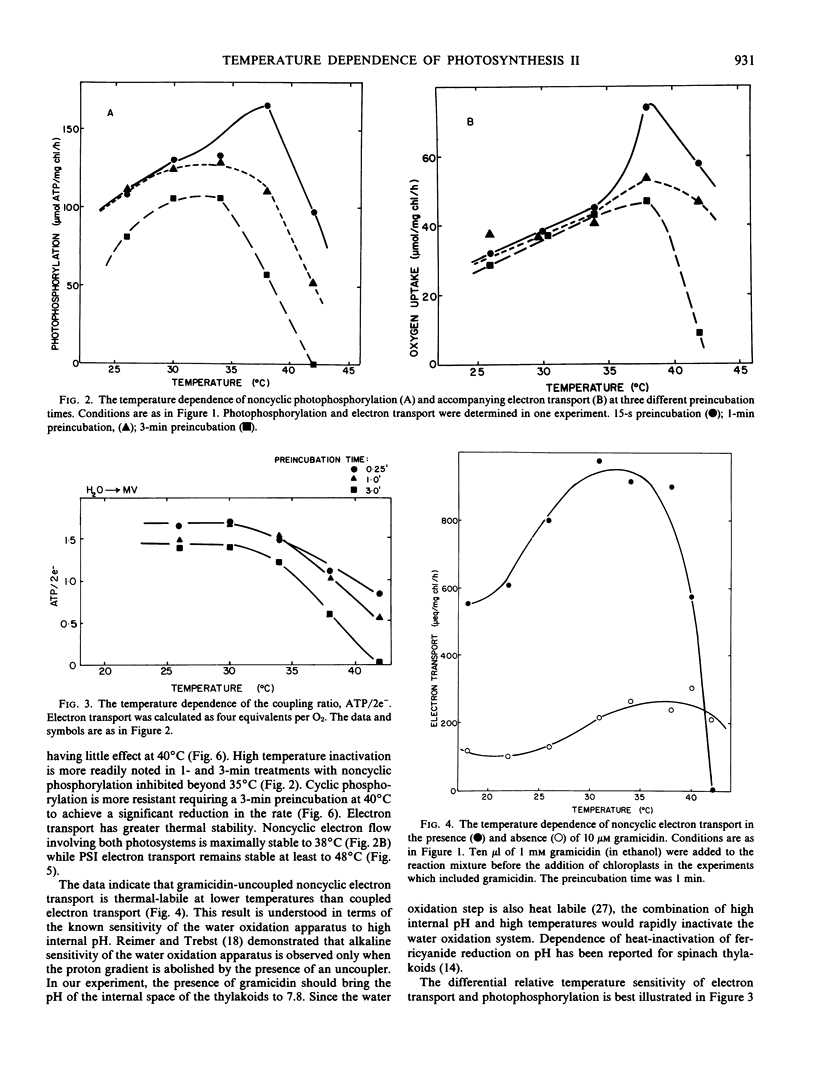
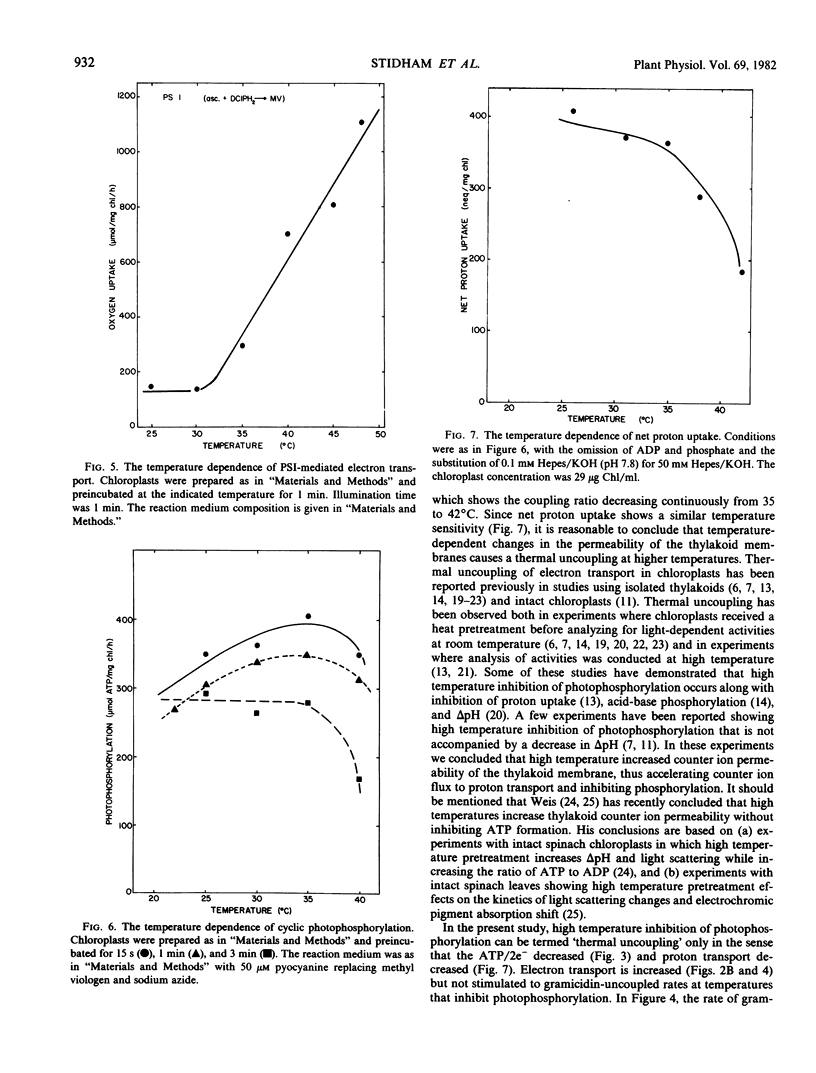
As part of an analysis of the factors regulating photosynthesis in Agropyron smithii Rydb., a C3 grass, the response of electron transport and photophosphorylation to temperature in isolated chloroplast thylakoids has been examined. The response of the light reactions to temperature was found to depend strongly on the preincubation time especially at temperatures above 35°C. Using methyl viologen as a noncyclic electron acceptor, coupled electron transport was found to be stable to 38°C; however, uncoupled electron transport was inhibited above 38°C. Photophosphorylation became unstable at lower temperatures, becoming progressively inhibited from 35 to 42°C. The coupling ratio, ATP/2e−, decreased continuously with temperature above 35°C. Likewise, photosystem I electron transport was stable up to 48°C, while cyclic photophosphorylation became inhibited above 35°C. Net proton uptake was found to decrease with temperatures above 35°C supporting the hypothesis that high temperature produces thermal uncoupling in these chloroplast thylakoids. Previously determined limitations of net photosynthesis in whole leaves in the temperature region from 35 to 40°C may be due to thermal uncoupling that limits ATP and/or changes the stromal environment required for photosynthetic carbon reduction. Previously determined limitations to photosynthesis in whole leaves above 40°C correlate with inhibition of photosynthetic electron transport at photosystem II along with the cessation of photophosphorylation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armond P. A., Schreiber U., Björkman O. Photosynthetic Acclimation to Temperature in the Desert Shrub, Larrea divaricata: II. Light-harvesting Efficiency and Electron Transport. Plant Physiol. 1978 Mar;61(3):411–415. doi: 10.1104/pp.61.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett J. M., Walker D. A. Thermal uncoupling in chloroplasts. Inhibition of photophosphorylation without depression of light-induced pH change. Arch Biochem Biophys. 1973 Jul;157(1):106–113. doi: 10.1016/0003-9861(73)90395-0. [DOI] [PubMed] [Google Scholar]
- Emmett J. M., Walker D. A. Thermal uncoupling in chloroplasts. Biochim Biophys Acta. 1969 Jun 24;180(2):424–425. doi: 10.1016/0005-2728(69)90129-7. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Hirano M., Satoh K., Katoh S. The effect on photosynthetic electron transport of temperature-dependent changes in the fluidity of the thylakoid membrane in a thermophilic blue-green alga. Biochim Biophys Acta. 1981 May 13;635(3):476–487. doi: 10.1016/0005-2728(81)90107-9. [DOI] [PubMed] [Google Scholar]
- Katoh S., San Pietro A. Ascorbate-supported NADP photoreduction by heated Euglena chloroplasts. Arch Biochem Biophys. 1967 Oct;122(1):144–152. doi: 10.1016/0003-9861(67)90133-6. [DOI] [PubMed] [Google Scholar]
- Monson R. K., Stidham M. A., Williams G. J., Edwards G. E., Uribe E. G. Temperature Dependence of Photosynthesis in Agropyron smithii Rydb. : I. FACTORS AFFECTING NET CO(2) UPTAKE IN INTACT LEAVES AND CONTRIBUTION FROM RIBULOSE-1,5-BISPHOSPHATE CARBOXYLASE MEASURED IN VIVO AND IN VITRO. Plant Physiol. 1982 Apr;69(4):921–928. doi: 10.1104/pp.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan W. G., Smillie R. M. Multi-temperature effects on Hill reaction activity of barley chloroplasts. Biochim Biophys Acta. 1976 Sep 13;440(3):461–475. doi: 10.1016/0005-2728(76)90034-7. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Izawa S. Studies on the Energy-coupling Sites of Photophosphorylation: V. Phosphorylation Efficiencies (P/e(2)) Associated with Aerobic Photooxidation of Artificial Electron Donors. Plant Physiol. 1974 Mar;53(3):370–376. doi: 10.1104/pp.53.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr, Heldt H. W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim Biophys Acta. 1976 Dec 6;449(3):434–436. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Inhibition of chloroplasts by UV-irradiation and heat-treatment. Plant Physiol. 1968 Dec;43(12):2037–2040. doi: 10.1104/pp.43.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]


