Abstract
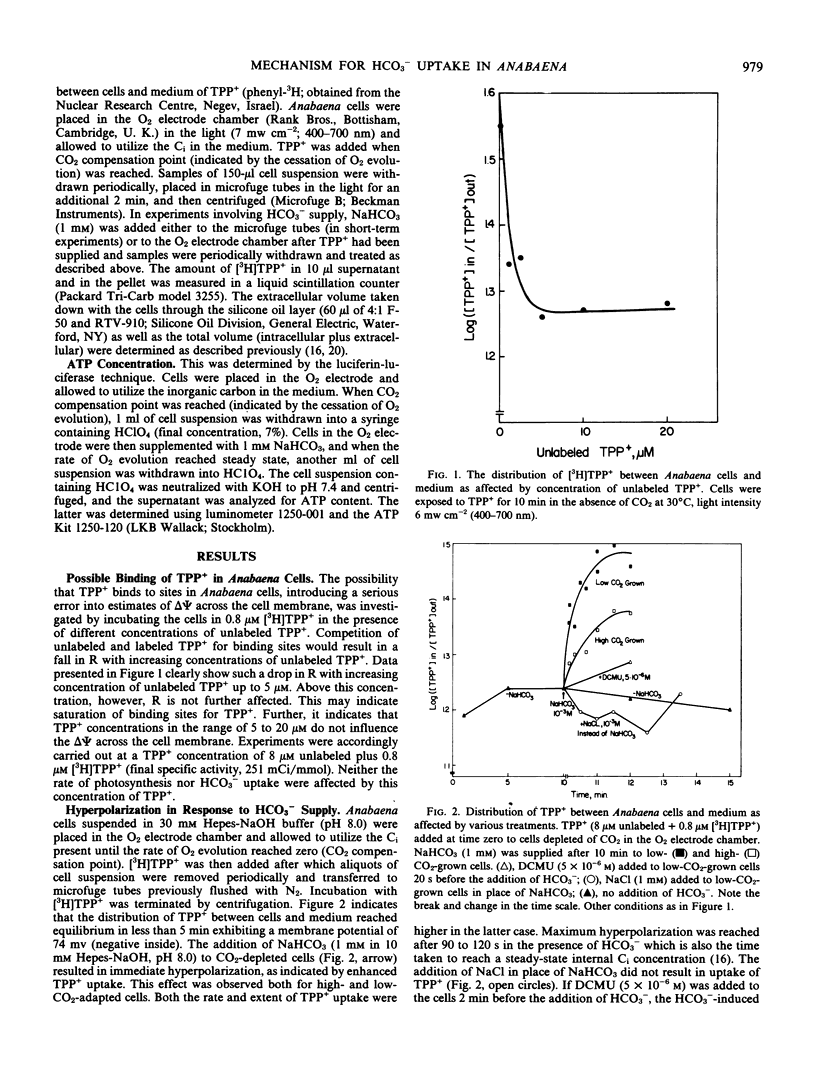
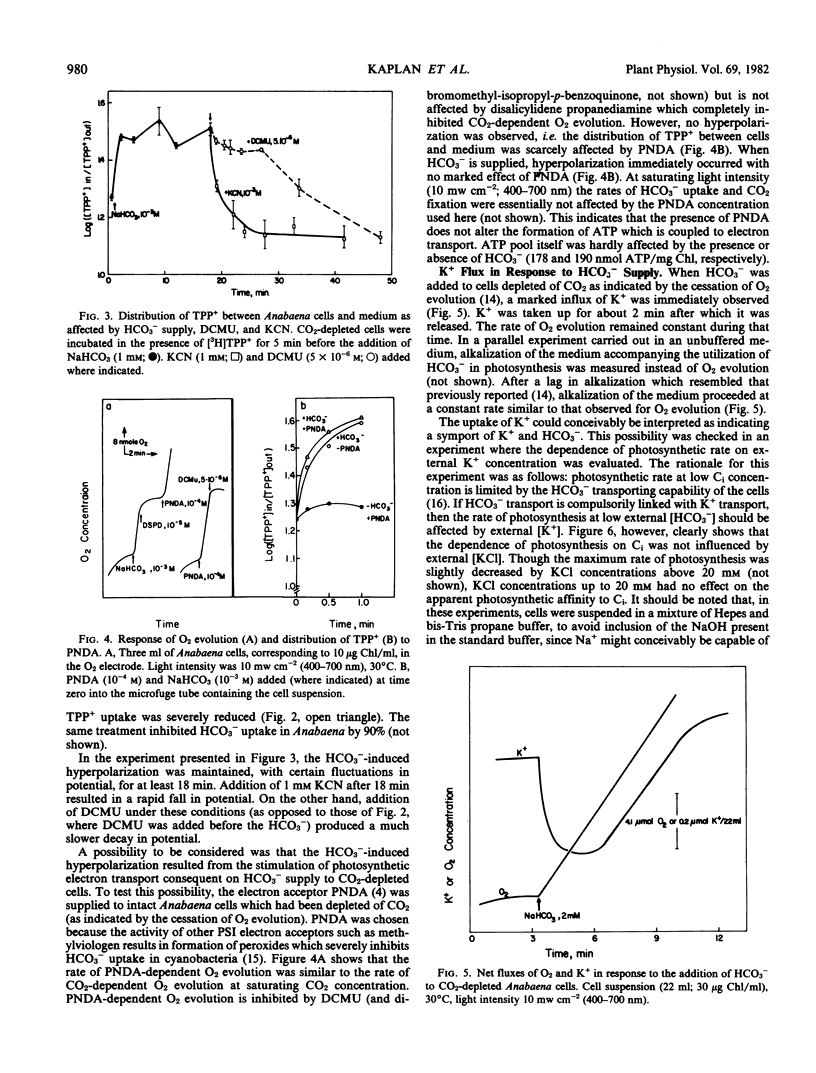
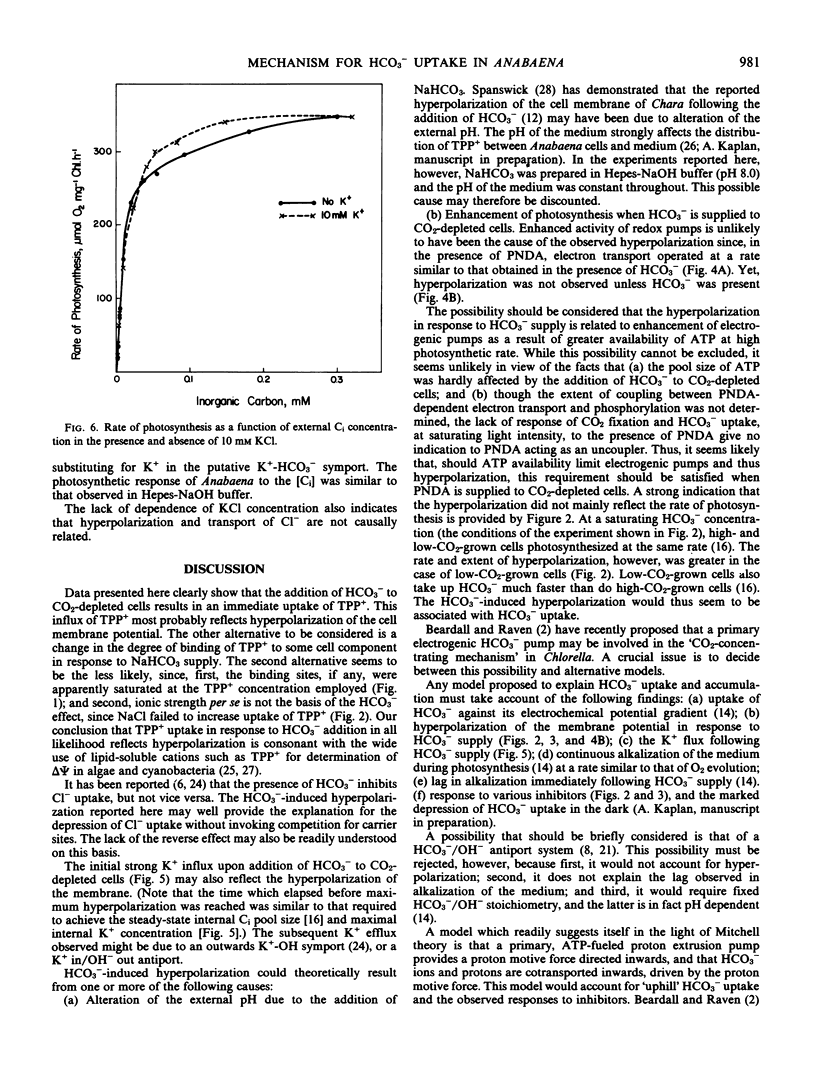
The response of the membrane potential to HCO3− supply has been studied in the cyanobacterium Anabaena variabilis strain M-3 under various conditions. Changes in potential were followed with the aid of the lipophilic cation tetraphenyl phosphonium bromide.
Addition of HCO3− to CO2-depleted cells resulted in rapid hyperpolarization. The rate and extent of hyperpolarization were greater in low-CO2-adapted than in high-CO2-adapted cells. Addition of the electron acceptor p-nitrosodimethylaniline which resulted in O2 evolution in CO2-depleted cells did not cause hyperpolarization. The hyperpolarization was not attributable to a change in pH or in ionic strength of the medium. Pretreatment with 3-(3,4-dichlorophenyl)-1,1-dimethylurea prevented the hyperpolarization. KCN depolarized hyperpolarized cells. Addition of HCO3− also brought about immediate K+ influx which was succeeded after about 2 minutes by K+ efflux.
Two of the models considered would be capable of explaining these and previous findings: (a) a primary electrogenic pump for transporting HCO3− ions; (b) proton-HCO3− contransport, the driving force for which is generated by a proton pump which is sensitive to the HCO3− concentration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier J. M. Apparent Bicarbonate Uptake and Possible Plasmalemma Proton Efflux in Chara corallina. Plant Physiol. 1980 Dec;66(6):1198–1199. doi: 10.1104/pp.66.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Berry J. A. Glycolate Excretion and the Oxygen to Carbon Dioxide Net Exchange Ratio during Photosynthesis in Chlamydomonas reinhardtii. Plant Physiol. 1981 Feb;67(2):229–232. doi: 10.1104/pp.67.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. Photosynthetic Response to Alkaline pH in Anabaena variabilis. Plant Physiol. 1981 Feb;67(2):201–204. doi: 10.1104/pp.67.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Evidence for HCO(3) Transport by the Blue-Green Alga (Cyanobacterium) Coccochloris peniocystis. Plant Physiol. 1980 Feb;65(2):397–402. doi: 10.1104/pp.65.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P., Boswell K. H., Mian A. M., Meyer R. B., Jr, Robins R. K., Khwaja T. A. 2' Derivatives of guanosine and inosine cyclic 3',5'-phosphates. Synthesis, enzymic activity, and the effect of 8-substituents. Biochemistry. 1976 Jan 13;15(1):217–223. doi: 10.1021/bi00646a033. [DOI] [PubMed] [Google Scholar]
- Raven J. A. Nutrient transport in microalgae. Adv Microb Physiol. 1980;21:47–226. doi: 10.1016/s0065-2911(08)60356-2. [DOI] [PubMed] [Google Scholar]
- Reed R. H., Rowell P., Stewart W. D. Components of the proton electrochemical potential gradient in Anabaena variabilis. Biochem Soc Trans. 1980 Dec;8(6):707–708. doi: 10.1042/bst0080707. [DOI] [PubMed] [Google Scholar]


