Abstract
Despite the ubiquity of sleep across phylogeny, its function remains elusive. In this review, we consider one compelling candidate: brain plasticity associated with memory processing. Focusing largely on hippocampus-dependent memory in rodents and humans, we describe molecular, cellular, network, whole-brain and behavioral evidence establishing a role for sleep both in preparation for initial memory encoding, and in the subsequent offline consolidation ofmemory. Sleep and sleep deprivation bidirectionally alter molecular signaling pathways that regulate synaptic strength and control plasticity-related gene transcription and protein translation. At the cellular level, sleep deprivation impairs cellular excitability necessary for inducing synaptic potentiation and accelerates the decay of long-lasting forms of synaptic plasticity. In contrast, NREM and REM sleep enhance previously induced synaptic potentiation, although synaptic de-potentiation during sleep has also been observed. Beyond single cell dynamics, large-scale cell ensembles express coordinated replay of prior learning-related firing patterns during subsequent sleep. This occurs in the hippocampus, in the cortex, and between the hippocampus and cortex, commonly in association with specific NREM sleep oscillations. At the whole-brain level, somewhat analogous learning-associated hippocampal (re)activation during NREM sleep has been reported in humans. Moreover, the same cortical NREM oscillations associated with replay in rodents also promote human hippocampal memory consolidation, and this process can be manipulated using exogenous reactivation cues during sleep. Mirroring molecular findings in rodents, specific NREM sleep oscillations before encoding refresh human hippocampal learning capacity, while deprivation of sleep conversely impairs subsequent hippocampal activity and associated encoding. Together, these cross-descriptive level findings demonstrate that the unique neurobiology of sleep exert powerful effects on molecular, cellular and network mechanism of plasticity that govern both initial learning and subsequent long-term memory consolidation.
Introduction
Sleep appears to be a universal phenomenon. It has been observed across a vast array of phylogeny, from fruit flies to humans. Furthermore, it occupies a non-trivial amount of the lifespan in all such species. Considering that sleep is a state that negates gathering of nutritional resources, contravenes reproduction, precludes active social interaction, and renders vulnerability to predation, it is perhaps the most perplexing of all organismic behaviors. On these grounds, there should have been significant evolutionary pressure to select against such a brain and behavioral state of apparent inactivity. Yet, sleep has persisted, suggesting that there must be considerable functional benefits that outweigh the obvious detriments. In this review, we consider one such adaptive process that may have provided an evolutionary driving force for the emergence and/or persistence of sleep: neural plasticity underlying memory processing.
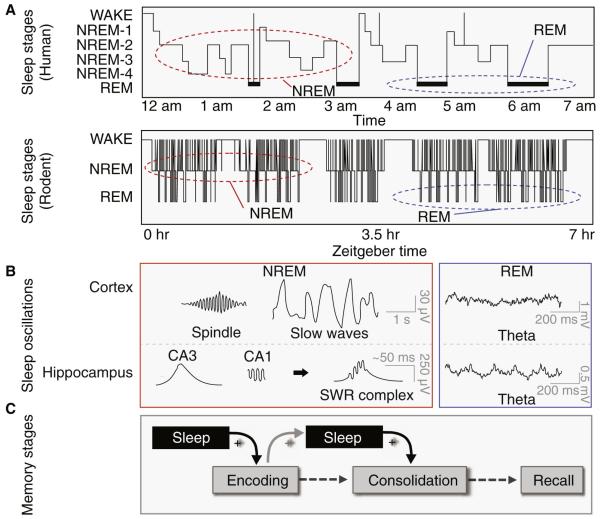
Figure 1A and 1B provide a synopsis of the stages and structure of non-rapid eye movement (NREM) and rapid eye movement (REM) sleep, and associated electrophysiological properties most relevant to the ensuing discussion of learning and memory. Figure 1C additionally describes the key stages of memory that sleep has been shown to interact with: the initial encoding or learning of information and the subsequent offline consolidation of that information, long-term. In the current review, we focus largely on learning, memory and plasticity that is dependent upon the hippocampus, across rodents and humans. We examine the role of sleep before learning, in determining initial encoding, and sleep after learning, in governing offline memory consolidation. Furthermore, this analysis is performed at the molecular, cellular, network and whole-brain human level. For reviews of sleep and its role in other forms of memory, and other stages of memory, see [1-3].
Figure 1. The Stages of Sleep and Memory.
A. Hypnograms demonstrate sleep architecture in humans (upper panel)_and mice (lower panel). In mammals, sleep is classified as rapid eye movement (REM) sleep and non-rapid eye movement (NREM)) sleep. In humans, NREM is further subdivided into 4 stages (corresponding to increasing depth of sleep), with Stages 3 and 4 often collectively termed “Slow Wave Sleep” (SWS). NREM and REM progress in cycles, varying in length across species. In humans, sleep is organized into a single nocturnal bout with approximately 90-minute NREM-REM cycles. SWS dominates the early night, while Stage 2 and REM sleep dominate later in the night. In contrast, rodent sleep is organized into many short bouts. Compared to humans, rodent NREM-REM cycles are compressed, lasting approximately 15 minutes. Both humans and rodents demonstrate periods of microarousal indicative of short arousals to waking EEG activity that do not grossly disrupt sleep architecture. B. During NREM, cortical EEG “slow waves” (0.5 – 4 Hz) result from cell populations switching between hyperpolarized “down-states” and depolarized “up-states”, accompanied by synchronized phasic “sleep spindles” (<2 seconds, 11 –15 Hz in frequency). At the level of the hippocampus, high-amplitude “sharp wave” deflections originating in the CA3 subfield give rise to corresponding fast “ripples” in CA1, which collectively form sharp wave ripple (SWR) complexes (~200ms, 100 – 250 Hz) [143]. SWRs are temporally coupled to the slow wave and spindles in neocortex, forming a potential oscillatory substrate for hippocampus-neocortex communication during NREM. During REM, both the neocortex and the hippocampus express faster, tonic, patterns of theta activity (~ 4 – 8 Hz, varying in frequency across species [130,144,145]). C. Memories develop in several stages over time. A memory is first encoded by engaging with an experience. Resulting in a neural “memory representation”. Following encoding, this representation can undergo several stages of development [1,3], the most commonly recognized being consolidation wherein memories become increasingly resistant to forgetting overtime. Following successful consolidation, memories can be retrieved long-term. Both sleep before, and after learning seems to be modulated by, and capable of modulating, these distinct phases of memory.
The molecular impact of sleep and sleep deprivation
Studies in the 1970’s and 80’s reported that transcription, the process of making ribonucleic acid (RNA) from deoxyribonucleic acid (DNA), was accelerated by sleep [4]. The observation that sleep modulates gene transcription was confirmed using more sophisticated microarray analyses, which allow for the simultaneous examinationof thousands of individual transcripts. These studies revealed that approximately 5% of gene transcripts in the rat cortex are modulated by sleep and wakefulness [5]. Wakefulness was associated with increased expression of genes in the cortex that support high-energy demand, elevated transcriptional activity and synaptic potentiation. Interestingly, sleep was reported to elevate cortical messenger RNA (mRNA) levels of genes associated with protein synthesis [5,6], a process known to be critical for the building of new synapses and the strengthening of existing synapses. Although much of this research has focused on the cortex, recent work has revealed similar patterns of gene regulation in the hippocampus [7]. Thus, the general consensus is that sleep promotes mRNA translation, while extended wakefulness caused by sleep deprivation negatively impacts clusters of genes critical for translational processes, including those known to be essential for memory encoding and consolidation [5-10]. Extending these findings, recent work in the cortex has demonstrated that sleep-dependent consolidation of visual experience also involves the regulation of protein translation, similar to that observed in studies of the impact of sleep deprivation on hippocampal function [7,11] (Figure 2A). Thus, sleep deprivation may impair memory consolidationin part by reducing the synthesis of proteins needed to support synaptic plasticity (for review see [12]).
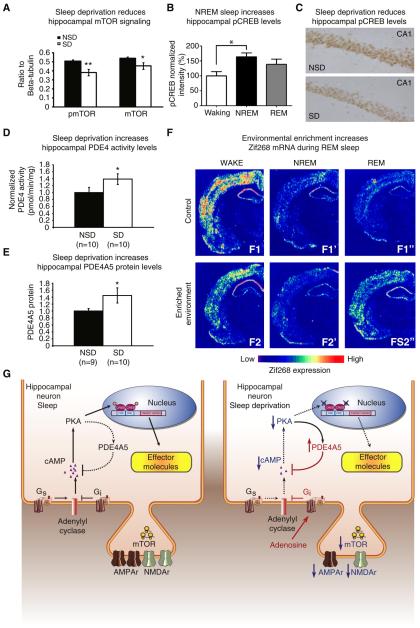
Figure 2. The molecular impact of sleep and sleep deprivation.
A. Five hours of total sleep deprivation reduces total protein levels of mTOR and mTOR phosphorylation in the hippocampus (n = 9 per group, **P < 0.005, *P < 0.01). All error bars denote s.e.m. Adapted from Vecsey, et al., 2012. [7] B. pCREB immunoreactivity in area CA1 of the hippocampus is elevated during NREM sleep (n = 5-8 per group, P< 0.05).Adapted from Luo, et al., 2013. [16] C. Representative images of pCREB immunoreactivity in area CA1 of the hippocampus is reduced after 5 hours of sleep deprivation. D. PDE4 activity was significantly upregulated in hippocampi from mice deprived of sleep for 5 hours (P = 0.039). E. The PDE4 isoform PDE4A5 was significantly upregulated by sleep deprivation in the hippocampus (P = 0.033). C-D Adapted from Vecsey, et al., 2009. [17] F. Effect of previous novelty experience on zif-268 brain expression during waking and sleep states. Shown are autoradiograms of brain sections whose gene expression levels best represent the means for each group studied. In controls, zif-268 expression decreased from WK (F1) to SW (F1′) and REM (F1″). In enriched environment animals, zif-268 levels decreased from WK (F2) to SW (F2′), but increased from the latter to REM (F2″). This effect was particularly noticeable in the cerebral cortex and the hippocampus. Adapted from Riberio, et al., 1999. [146] G. A schematic overview of hippocampal signaling pathways whose modulation by sleep deprivation may contribute to the effects of sleep deprivation on memory encoding and consolidation. Left Panel, signaling pathways under conditions of sleep. Right Panel, sleep deprivation has been reported to reduce glutamatergic signaling and attenuates cAMP signaling and CREB-mediated gene transcription. All of these molecular events are shown in a single connected pathway in order to demonstrate how the effects of sleep deprivation could potentially interact to impact learning and memory. Dashed black lines and blue arrows pointing down indicate attenuation of the signaling pathway. Red labels and lines indicate an increase of the signaling pathway.
Studies using genetically modified mice have established that transcriptional regulatory proteins such as the cAMP-response element binding protein (CREB) are critical for long-lasting forms of synaptic plasticity and associated memory consolidation. Moreover, they are activated by multiple signaling cascades including the cAMP-PKA signaling pathway [13,14]. Although the impact of sleep deprivation on gene expression is not uniform throughout the brain, most genes that are affected by sleep deprivation contain a cAMP-responsive element [15]. This indicates that sleep and sleep deprivation may specifically modulate the function of transcription factors such as CREB that bind to the cAMP-responsive element. Consistent with this view, CREB phosphorylation within the hippocampus is elevated during REM sleep (Figure 2B) [16]and reduced after 5-6 hours of total sleep deprivation or longer periods of REM sleep deprivation (Figure 2C) [17-20], but see [21]. This compromised CREB signaling caused by sleep deprivation could be a critical component of memory deficits associated with loss of sleep.
The cAMP signaling pathway regulates CREB activity, and this pathway is critical for long-lasting forms of hippocampal synaptic plasticity and memory storage [22,23]. Hippocampal cAMP levels are elevated during REM sleep [16] and cAMP signaling is impaired by sleep deprivation [17]. Long-lasting, cAMP-dependent forms of long-term potentiation (LTP), a cellular model of learning that relies on molecular mechanisms that also underlie memory consolidation [24], are impaired by 5 hours of total sleep deprivation. Importantly, the deficits in LTP were unrelated to the mild increase in corticosterone levels observed after sleep deprivation indicating that sleep loss, rather than accrued stress, were underlying these LTP impairments [17]. In line with these observations, adrenalectomized rodents still show memory deficits after sleep deprivation [25]further emphasizing that while sleep deprivation can be stressful, it is sleep loss itself, rather than stress induced by sleep loss, that perturbs synaptic plasticity underlying memory consolidation.
Biochemical analyses indicated that such sleep deprivation results in the elevated activity of cAMP-degrading phosphodiesterases (PDE), particularly of the PDE4 family (Figure 2D, 1E). Moreover, deficits in synaptic plasticity and memory caused by short periods of total sleep deprivation are prevented by blocking PDE4 activity. Together, these observations indicate that cAMP signaling is negatively impacted by sleep deprivation that may in turn perturb memory consolidation processes through impairments in CREB-mediated gene transcription.
Only a few studies have conversely examined how gene expression profiles are altered by the presence (rather than absence) of sleep after a waking experience. Ribeiro et al [26] found that expression of the immediate early gene zif268 is upregulated during both REM sleep and SWS in animals that were exposed to an enriched environment prior to the sleep episodes relative to the sleep of control animals not exposed to the enriched environment (Figure 2F). Because zif268, like CREB, is critical for memory storage, the upregulation of zif268 RNA levels during REM sleep and SWS following exposure to the enriched environment may reflect ongoing consolidation of prior waking information encoded in the enriched environment. In a follow up study, Ribeiro et al [27] induced LTP in the hippocampus during wake and examined expression of zif268 during subsequent periods of REM sleep. LTP-induction in the hippocampus during wake led to the upregulation of zif268 mRNA levels in REM sleep. Interestingly, the upregulation of zif268 mRNA levels were not limited to the hippocampus where LTP was induced, but was also observed in the amygdala, entorhinal, and auditory cortices. These findings suggest that the hippocampus may be involved in a larger scale (re)activation of prior learning-dependent networks during subsequent REM sleep, thereby facilitating the consolidation of memory traces and their transformation throughout other brain regions.
In summary, key molecular mechanisms known to be involved in memory encoding and consolidation, including cAMP-CREB-mediated gene transcription and de novo protein synthesis through mTOR signaling, are beneficially regulated by sleep, and disrupted by sleep deprivation (Figure 2G). From these data emerge the question of how such molecular changes alter cellular properties in neuronal circuits, to which we now turn our attention.
The cellular impact of sleep and sleep deprivation
Electrophysiological recordings in vivo allow researchers to follow the same neurons over time and measure firing properties of individual neurons or local field potentials. These techniques have been used to examine how cellular activity, including aspects related to learning and memory, change during wake, sleep, and throughout the course of sleep deprivation. Work in the 1980’s suggested that REM sleep was critical for memory consolidation (for review, [28]). Recent studies have revealed that local field potentials are enhanced after a period of NREM slow wave sleep, suggesting that the strength of synaptic connections between neurons increases during NREM sleep (Figure 3A) [29]. In vitro analyses indicated that the enhancement required activation of both AMPA and NMDA receptors as does neocortical postsynaptic LTP. Such findings suggest that sleep, and in particular slow wave sleep, may act to maintain cortical synaptic plasticity through LTP-like phenomena that are critical for the consolidation of memories.
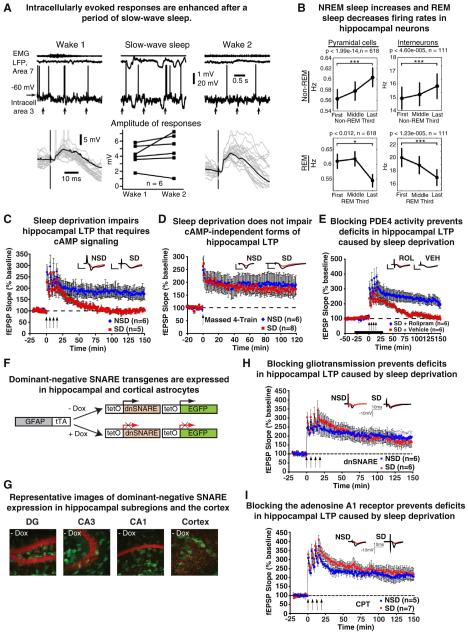
Figure 3. The cellular impact of sleep and sleep deprivation.
A. Upper panels: EMG form neck muscle, surface LFP from area 7, and intracellular recording from somatosensory cortex in consecutive states of vigilance, as indicated. Vertical arrows indicate the time of medial lemniscus stimulation. Bottom panels, left and right, superimposition of 20 individual responses (gray traces) and the averaged response (black traces) during the first (left panel) and the second (right panel) episode of wake. Note that responses are ampler in the second episode of wake. Middle panel, paired comparison of intracellular response amplitude of six neurons during two consecutive wake episodes separated by a slowwave sleep episode. Each symbol represents the averaged response amplitude of one neuron during either wake 1 (left) or wake 2 (right).Adapted fromChauvette, et al., 2012. [29] B. Firing rate increases of pyramidal cells and interneurons from the first to last third of non-REM episodes (top row) and rate decreases from the first to last third of REM episodes (bottom row). Adapted from Grosmark, et al., 2012. [46] C. The maintenance of cAMP-PKA-dependent spaced 4-train LTP was significantly disrupted in slices from sleep-deprived (SD) mice (P = 0.03); NSD, non-sleep deprived. D. cAMP-PKA-independent massed 4-train LTP was unaffected in hippocampal slices from sleep deprived mice (P = 0.67). E. Rolipram (ROL) treatment rescued deficits in spaced 4-train LTP due to sleep deprivation (P = 0.003). The black bar represents the time of rolipram treatment.C-EAdapted from Vecsey, et al., 2009. [17] F. Schematic of the generation of the bitransgenic mice expressing dnSNARE in glia using two different lines of transgenic mice. One is a line of mice in which the human GFAP promoter drives the expression of tTA. The second, tetO.dnSNARE contains a tet operator (tetO)-regulated dnSNARE domain and EGFP reporter gene. By crossing these two lines, the dnSNARE domain is expressed only in astrocytes. All mice were maintained on +Dox until the beginning of the experiments. Three to 4 weeks before experiments, mice were put off doxycycline food (−Dox) to induce dnSNARE expression. G. Confocal images showing expression of the reporter transgene EGFP (green) throughout the different hippocampal subfields [dentate gyrus (DG), CA3, and CA1] and cortex after 4–5 weeks off doxycycline food (−Dox). Propidium iodide (red) was used as a nuclear marker. Magnification x20. H. In hippocampal slices from dnSNARE mice, L-LTP is maintained after SD at the same level as in NSD mice. dnSNARE expression does not affect the maintenance of LTP under non-sleep deprivation conditions (data not shown). I, the L-LTP impairment is prevented in SD mice treated with a chronic infusion of the adenosine A1R antagonist CPT. CPT treatment alone does not affect the maintenance of LTP (data not shown). Insets show representative recordings from an animal from each group taken during the first and last 5 min of the recording. fEPSPs, field excitatory postsynaptic potentials. All error bars denote s.e.m. F-I Adapted from Florian, et al., 2011. [51].
The synaptic homeostasis hypothesispostulates that experience and learning during wakefulness leads to a widespread synaptic potentiation. This hypothesis, developed by Tononi and Cirelli [30,31], derives support from experiments that have identified increases in neuronal activity during prolonged wakefulness at the cellular and molecular level. Counteracting this, sleep is associated with synaptic downscaling [30,31]. Further, synaptic downscaling is proposed to occur during SWS, based on the homeostatic regulation observed with these slow oscillations. The hypothesis, which focuses on sleep as a synapticre-balancing mechanism, has received support from a number of integrated experimental findings [32-37], has stimulated much research, and has led to constructive discussion [38-40]. How results consistent with the synaptic homeostasis hypothesis may be reconciled with the cellular results demonstrated here indicative of sleep-dependent maintenance of LTP, remains unknown. However, the work by Chauvetteand colleagues [29] along with the work of Tononi and otherssuggeststhat perhaps synaptic downscaling and upscaling may occur in parallel, but in different neural circuits within the same cortical brain region. Future studies are required to address the mechanism(s) that may simultaneously support downscaling and upscaling in NREM sleep, and whether downscaling occurs in the hippocampus.
It is particularly interesting that electrical firing patterns that induce synaptic plasticity in hippocampal slices, such as theta burst stimulation and high frequency stimulation, occur endogenously during REM and NREM sleep. During exploration in wake and in REM sleep, hippocampal neurons express firing patterns characterized by theta rhythms (Figure 2; [41-43]). In contrast, in quiet waking and NREM sleep the same neurons fire in concentrated short bursts (approximately 120 ms) called sharp-wave ripple complexes, followed by low hippocampal firing rates between ripple events (Figure 2; [44,45]). Within the hippocampal subfield areaCA1, a portion of the hippocampus critical for episodic declarative memory, neuronal firing rates display a ‘sawtooth’-like pattern, with moderate increases in firing rate during slow wave sleep and reduced firing rates during subsequent REM sleep periods [46]. These increases during sharp-wave ripple events may reflect a local replay of newly encoded memories across ensembles of cells; a proposal discussed in detail in the following section
Field recordings of neuronal populations have also been used to determine how neuronal activity properties change after learning and consecutive episodes of sleep, and how they are impacted by sleep deprivation. Winson and Abzug in the 1970’s and 80’s reported that the excitatory postsynaptic potentials are reduced during slow wave sleep relative to alert wakefulness, with intermediate values observed during REM sleep [47-49]. Field recordings focusing on hippocampal CA1 Schaffer collaterals indicate that as little as 5-12 hours of total sleep deprivation, or sleep fragmentation for 24 hr, impairs long-lasting forms of LTP including those that require activation of the cAMP-PKA-CREB pathway and depend on protein synthesis (Figure 3C-E) [17,50-53]. Likewise, longer periods of REM sleep deprivation attenuates hippocampal LTP [19,54-58]. In vivo recordings in awake rats indicate that selective REM (but not NREM) sleep deprivation after LTP induction in the dentate gyrus significantly impairs the long-term maintenance of that induced LTP [59]. This conclusion is further strengthened by recent evidence indicating that hippocampal cAMP and CREB phosphorylation levels are elevated during REM sleep (Figure 2B) [16], although future work is needed to define the selective roles of NREM and REM sleep.
Glutamatergic signaling plays a critical role in the synaptic plasticity that underlies memory consolidation [60]. Several reports have investigated how such signaling is impacted by sleep loss. For example, extracellular glutamate levels in the cortex were found to steadily increase and remain elevated during the first few hours of total sleep deprivation, after which they start to decline [61], suggesting that loss of sleep perturbs glutamatergic signaling. Unfortunately, no studies have assessed whether hippocampal extracellular glutamate levels change during the course of sleep deprivation, although several clues suggest this may be the case. Work by Kopp et al. described alterations in NMDA receptor subunit composition in hippocampal CA1 with sleep deprivation [52]. Specifically, increases the number of NR2A subunits developed, thereby leading to alterations in calcium signaling and synaptic plasticity after 4 hours of total sleep deprivation [52]. However, others donot observe changes in hippocampal CA1 NMDA receptor function using whole-cell recordings after 5 hours of total sleep deprivation [17]. While the reasons for this difference remain unclear, one contributing factor may be the different handling methods used to impose the sleep deprivation in each study [62]; for detailed discussion see [12].
In addition to total sleep deprivation, long periods of selective REM sleep deprivation also result in decreased hippocampal NMDA receptor function, attributed to decreased surface expression of NR1 and NR2A subunits [55,56] (but see [63]), or NR2B subunits [63]. Interestingly, treating hippocampal slices with glycine, which facilitates NMDA receptor function, reversed the LTP deficit caused by long periods of REM sleep deprivation. This indicates that attenuated NMDA receptor activity appears to be a functional contributor to the deficits in hippocampal plasticity following longer periods of REM sleep deprivation [56]. Beyond NMDA receptors, AMPA receptors are another group of glutamatergic receptors that are important for memory. Twelve hours of total sleep deprivation and 75 hours of REM sleep deprivation both decrease AMPA receptor phosphorylation of the glutamate receptor 1 (GluA1) subunit PKA phosphorylation site [55,64](but see [21]). As a consequence, there is a reduction in the potentiation of GluA1 containing AMPA receptors [65]. In summary, the work described above suggests that glutamatergic signaling is attenuated by longer periods of sleep deprivation.
In addition to glutamate function, studies have examined how extended wakefulness and sleep also change extracellular adenosine, a degradation product of ATP whose levels increase with brain metabolism. Extracellular adenosine progressively increases during wakefulness and subsequently declines during sleep [66-68]. Researchers are working to identify the source of this extracellular adenosine, and one interesting idea is that it may derive from ATP released byglia [69]. To prevent neurotransmitter release from astrocytes (i.e., gliotransmission), mutant mice were engineered that express the cytosolic portion of the SNARE domain of synaptobrevin 2 (DN-SNARE) selectively in astrocytes (Figure 3F,G). DN-SNARE expression in astrocytes prevented the release of gliotransmitters from astrocytes attenuated the increase in extracellular adenosine that normally occurs under conditions of sleep loss [68,70]. Deficits in hippocampal LTP and memory consolidation normally seen after sleep deprivation were not observed when the release of gliotransmitters was blocked by DN-SNARE expression in astrocytes (Figure 3H) [51,71]. Furthermore, chronic infusion of the adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dimethylxanthine (CPT) into the brain also prevented sleep deprivation-induced deficits in the maintenance of hippocampal LTP (Figure 2I) [51], and the consolidation of both object-place and object identity memories [51,71]. Thus, astrocytes, via their ability to release ATP in a calcium-dependent fashion, may be one critical intermediary governing the impact of sleep deprivation on neuronal function.
Sleep, network activity and memory
Moving beyond the functioning of individual cells during sleep, classic work by Pavlides and Wilson [72] demonstrated that groups of “place” cells in the rodent hippocampus, which fire preferentially when an animal is in a specific spatial location, increase their firing rate specifically during subsequent NREM sleep after learning (Figure 4A). Furthermore, such latent activity was timelimited, returning to baseline levels after approximately 3 hours. From these findings emerged the proposal that experience-dependent neural activity during wake (here, place cells within the hippocampus), is re-instantiated during later NREM for finite time periods, potentially reflecting network-level memory (re)processing. Subsequent investigations [44,73] have demonstrated that both the coordinated spatial and temporal patterns of learning-related hippocampal place cell firing aresubsequently re-expressed during NREM sleep (and see [42] for a report of REM sleep-associated hippocampal replay). The speed of this replayed during NREM is as much as 20 times faster than during prior waking experience [74], the reasons for which remain largely unknown.
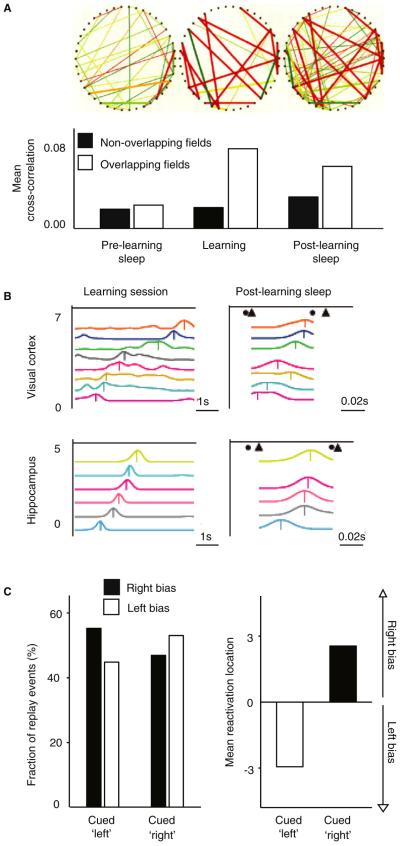
Figure 4. Intrinsic and Extrinsic Hippocampal Reactivation in Rodents.
A. Patterns of hippocampal place cell firing during sleep before learning (Pre-Learning Sleep), during wakeful learning (Learning), and after learning during sleep (Post-Learning Sleep). Top row: Effective connectivity matrix of 42 random hippocampal place cells from a single rat for each of the above three experimental phases, illustrating significantly increased effective connectivity during sleep after, relative to before, learning. Lines demonstrate significant (adjusted p<0.05) cross-correlation between cells (dots), with color of line reflecting strength of correlation (red – high; blue – low). Bottom row: Mean cross-correlation between hippocampal place cell pairs during each of the three experimental phases described above. Black bars indicate cell pairs that did not have overlapping (correlating) place fields during learning phase, while white bars indicate cell pairs that did have overlapping place fields during the learning phase that selectively express increased connectivity in sleep after learning. Adapted from Wilson and McNaughton, 1994. [44] B. Multiunit firing sequences during NREM sleep (right panels) demonstrating recapitulation of firing patters observed during prior learning while awake (left panels) in both the cortex (upper row) and hippocampus (lower row) cells. Note time scales, which reflect a compression of firing patterns in sleep, relative to learning during wake. Adapted from Ji, et al., 2007. [147] C. Extrinsic cued reactivation of previously learning memories during NREM, biasing NREM replay. During wake, rodents were trained to go to the left or right side of a track to seek a reward, dependent upon a specific paired auditory cue. During NREM sleep (left panel), a proportion of replay events could be manipulated to show a left or right bias as a function of which auditory cue was presented. Mean bias (right panel) of replay activity (left or right of center of the track) as a function of the specific auditory cue presented during sleep. Adapted from Bendor, et al. 2012. [82]
The reactivation of hippocampal ensembles during NREM sleep occurs in temporal synchrony with the hippocampal sharp wave ripple (SWR) complex (Figure 2). Moreover, the SWRs themselves show a temporal coupling with the neocortical depolarization (following periods of hyperpolarization), reflecting the up-state of the slow oscillation [75] (Figure 2). Interestingly, SWRs increase in number during NREM sleep after hippocampus-dependent learning [46,76-79]. Furthermore, abolishing SWRs during NREM after learning impairs subsequent offline consolidation [80]. Although the precise functional role that SWR events play in consolidation remains unclear, such findings support their causal involvementin sleep-dependent memory processing.
To characterize the dynamics of replay between the hippocampus and neocortex, offline spike-firing correlations between the visual cortex and hippocampus have been examined during sleep after spatial learning [81]. In NREM sleep episodes following learning, cell in both the visual cortex and hippocampus demonstrate a structured pattern of activity organized into periods or “frames” that arestatistically similar to task-related activity expressed during prior wake. Moreover, the frames in the visual cortex begin and end slightly ahead of those in hippocampus (by approximately 50 ms) (Figure 4B). Hippocampal replay during NREM sleep (co-occurring with SWRs) may therefore be preceded, and potentially driven, by cortical reactivation, rather than (or in addition to) spontaneous reactivity instigated within the hippocampus itself.
While neural replay in the hippocampus and cortex is a potential substrate for sleep-dependent memory consolidation, it should be recognized that a relatively small number of cells express such replay, and their strength of correlation with prior learning is somewhat low. There are several potential explanations for this. One reason is that the amount of recorded waking experience during the experiment (often just minutes in total) represents a small proportion of the animal’s total waking time and experience. Therefore, it may not be surprising that only a small number of cells and time windows during the ensuring sleep recordings (re)express this brief experimental pattern. Furthermore, replay studies only sample a relatively small number of cells of the total hippocampus or cortex involved in the experimental experience, making the probability of observing replay necessarily lower.
Recently, the intrinsic replay of network ensemble activity during NREM sleep has been manipulated experimentally using extrinsic triggers [82], offering novel causal evidence for replay of memories during sleep. Rodents performed a spatial learning task during wake that was associated with specific sound cues. When these same auditory cues were represented during sleep, both individual hippocampal place cells, as well as the ensemble set of hippocampal cells, demonstrated a replay-bias towards re-expressing prior cue-associated firing patterns (Figure 4C). Furthermore, such induced replay was observed only in NREM sleep, and not during subsequent offline periods of time awake or REM sleep. Reminiscent of the original studies by Pavlides and Wilson [72], the efficiency of these cues to induce patterned reactivation was also time limited, decaying in the later cycles of sleep.
In summary, considerable evidence now exists for learning-dependent ensemble reactivation within the hippocampus and cortex during sleep. Moreover, such coordinated replay may initially be instigated, in part, by activity within the cortex, one function of which may be to re-instantiate contextual (e.g., visual perceptual) signals necessary for accurate instructed replay within the hippocampus. That hippocampal reactivation can be experimentally induced using auditory cues, which are presumably first processed by the cortex, is consistent with this proposal. Following cortex-initiated reactivation, hippocampal replay may in turn send feedback to further influence cortical replay, ultimately promoting recurrent strengthening and hence long-term hippocampal-neocortical consolidation of select memory representation during NREM sleep.
Sleep and memory processing in the human brain
Sleep before learning for memory encoding
Building on early human behavioral studies [83][84], pioneering work by Drummond et al. established that sleep deprivation prior to learning disrupted encoding-related functional activity within the human medial temporal lobe [85]. Subsequent investigations have established that one night of sleep loss results in a significant impairment in encoding-related activity expressly within the bilateral posterior hippocampus [86], leading to the failure of participants to encode twice as many stimuli, relative to those in asleep-rested condition (Figure 5A). Moreover, selective slow wave sleep deprivation alone is sufficient to impair hippocampal encoding activity and associated memory formation [87]. These findings in the human brain are in considerable agreement with work discussed earlier in rodents, describing compromised cellular and molecular processes within the hippocampus and related learning impairments following sleep loss prior to encoding.
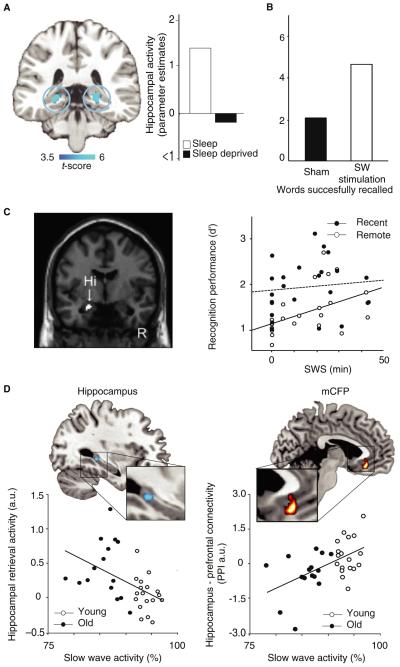
Figure 5. The impact of sleep on human memory encoding and memory consolidation.
A. Decreased hippocampal encoding activity following a night of prior sleep deprivation relative to a full night of sleep measured using functional magnetic resonance imaging (fMRI). Right panel bars reflects parameter estimates (effect size) of averaged hippocampal activity in each condition. Effects are significant at p <0.001; >5 contiguous voxels. Adapted from Yoo, et al., 2007. [86] B. Enhanced overnight declarative memory consolidation following transcranial direct current stimulation targeting NREM slow waves relative to sham stimulation. Plotted bars represent number of memory items (words) successfully recalled after relative to before sleep (white bar represents sham stimulation, black bar represents real stimulation). Adapted from Marshall, et al., 2006. [105] C. Progressive decrease in retrieval-related hippocampal activity measuring using fMRI for information learned before a nap, with intervening amount (min) of SWS demonstrating a significant relationship with recognition memory for items learned before the nap (“remote”; white circles, solid line) compared to items learned after the nap (“recent”; black circles, dotted line). Hippocampal effects are significant at p < 0.05, family-wise error (FWE)-corrected. Adapted from Takashima, et al., 2006 [118] D. Overnight hippocampal-neocortical transformation of episodic memory across young and older adults measured using fMRI, with a progressive independence of next-day retrieval-related hippocampal activity (left panel) and converse increase in hippocampal-medial prefrontal cortex connectivity correlating with the amount of prior SWA (white circles: young adults, black circles: older adults), Effects are significant at p < 0.05, FWE-corrected within Regions of Interest. Adapted from Mander, et al., 2013. [117]
Although such evidence establishes the detrimental impact of a lack of sleep on hippocampal encoding, a recent study has established the proactive benefit of sleep and specific sleep oscillatory activity in restoring learning ability [88]. Hippocampal encoding capacity was assessed twice across a 6hr daytime interval, with half of the participants remaining awake, while the other half obtained a 100-minute nap opportunity during this time. Learning capacity decreased across the day in those who remained awake. In contrast, participants who were allowed to sleep demonstrated a numeric enhancement in subsequent encoding ability. Furthermore, the degree of encoding restoration positively correlated with the duration of Stage 2 NREM sleep as well as the number of corresponding fast (13.5 – 15 Hz) sleep spindles over the left prefrontal cortex. Similar learning-related spindle associations have recently been reported across a full night of sleep [89]. Adding to the suggestion that NREM sleep aids in the restoration of encoding, prefrontal electrical stimulation targeting the enhancement of NREM slow wave activity also increases subsequent post-awakening episodic memory encoding ability [90]. While no associations were identified with spindle activity, fast-frequency sleep spindles were nevertheless organized by the up-phase of the slow wave-enhancing electrical stimulation, suggesting a possible co-operative relationship between these oscillations in promoting memory encoding.
This collection of findings describes an emerging role for sleep before learning, including specific NREM physiological oscillations, in renewing next-day hippocampal encoding capacity. They are consistent with a proposed framework of sleep-dependent memory processing that predicts declining episodic learning capacity with continued waking experience [91]. Consequently, NREM sleep spindles in the cortex, co-occurring with hippocampal sharp wave ripples [78], and possibly grouped by the slow oscillation [78,92-94], support a shift from hippocampal- to increasing cortical-dependence of previously encoded representations. The result is not only the consolidation of the original memory representations themselves, but synergistically, the post-sleep restoration and thus renewal of hippocampal encoding ability upon awakening.
Sleep after learning for memory consolidation
A robust literature has now described the necessary role of sleep after learning in the subsequent offline consolidation of memory in humans (and in animals), including hippocampus-dependent memory [1,91,95,96]. Moreover, this sleep benefit confers functional resilience beyond simply slowing the passive forgetting of information overtime. Instead, sleep is capable of rendering new memory representations more robust and therefore less vulnerable to the interfering influence of competing learning upon awakening [97]. Such overnight consolidation benefits on tasks of declarative episodic memory have commonly been attributed to early night sleep, rich in SWS [98-102]. Subsequent investigations have established a causal role for NREM slow waves in memory consolidation. Following learning of a word-pair list, transcranial direct current stimulation was applied over the prefrontal cortex (a region with monosynaptic connections to the hippocampus; [103]) during early night SWS. Stimulation increased EEG power within the slow wave range [104,105], consequently resulting in greater memory retention (Figure 5B) — a finding recently replicated using rhythmic auditory stimuli presented in NREM sleep to enhance slow wave activity [106].
Interestingly, electric stimulation not only elevated slow oscillation activity in the above report, but also increased sleep spindle activity, again suggesting a possible interaction between spindles and slow oscillations. Spindle activity increases following episodic learning in humans [107], indicative of a homeostatic response to encoding and thus potential demand for subsequent offline consolidation. It is of note that similar evidence has been reported in rodents following hippocampus-dependent learning, demonstrating increases in NREM cortical sleep spindles [108] as well as hippocampal SWRs [77]. Further, the density of human sleep spindles at night positively predicts episodic memory recall the next day [109][110]. A recent combined fMRI-EEG study reported increased functional connectivity between the hippocampus and select regions of the neocortex during the occurrence of sleep spindle events, further suggesting an underlying spindle-related mechanism capable of supporting memory consolidation [111]. While the relationship between spindles and consolidation may be especially strong for memories that are most dependent on the hippocampus [112], the impact of sleep and sleep loss on different functional subfields of the human hippocampus [113] remains largely unknown. Addressing this knowledge gap will provide important homologous evidence with work in rodents, described earlier.
Building on these past findings, emerging translational studies have begun to examine changes in sleep-dependent memory across the life span and in disease states. For example, in contrast to the abundance of SWS and related SWA in children and young adults, marked reductions in both are observed in older adults. Moreover, the extent of deficient NREM SWS predicts impaired overnight memory retention in middle-aged adults [114], healthy older adults and patients with amnestic mild cognitive impairment [115]. Most recently, it has been established that the degree of atrophy in the medial prefrontal cortex in older adults – a region known to be involved in the electrical source generation of slow waves [116] – predicts the extent of impaired SWA in the elderly, and with it, consequent impaired overnight episodic hippocampal memory consolidation (Figure 5D) [117]. Not only does this suggest the deterioration of sleep-dependent hippocampal memory consolidation with age, but at a clinical level, endorses the proposalthat improving SWA in older adults represents a novel treatment target for minimizing cognitive decline in later life [117].
As the physiological oscillations of sleep that promote memory consolidation have been increasingly well described, the underlying neural mechanisms supporting these benefits continue to be explored at the whole-brain human level. Several human neuroimaging reports have provided evidence that sleep plays a role in supporting the transformation of episodic memory from initial hippocampal- to increasingly neocortical-dependence. In the first such report, Takashima and colleagues examined the benefit of a daytime 90 min nap on episodic memory consolidation [118] (Figure 5C). It was demonstrated that the duration of NREM SWS during the intervening nap positively correlates with post-sleep recognition memory performance and negatively correlates with retrieval-related activity in the hippocampus. Beyond SWS, SWA over the prefrontal cortex similarly promotes the progressive independence of retrieval-related hippocampal activity, and positively predicts the extent of increased retrieval-related hippocampal-medial prefrontal cortex connectivity (Figure 5D) [117]. Extending these findings, Maquet and colleagues have demonstrated that one night of post-training sleep deprivation significantly compromises hippocampal-neocortical neural dynamics associated memory recollection [119].
Analogous to data described earlier in rodents, several studies have examined hippocampal reactivation during NREM sleep in humans. Following learning of a 3D navigation maze initially associated with hippocampal activity, increased regional cerebral blood flow within the hippocampus re-emerges during subsequent SWS (Figure 6A) [120]. Moreover, the degree of reactivation during SWS predicts the success of task performance the next day (Figure 6A). A recent report using simultaneous EEG and fMRI has furtherdemonstrated that patterns of prior wake encoding activity re-emerge in both the hippocampus and in the neocortex during NREM sleep. Importantly, these patterns of reactivation arephase-locked to the occurrence of NREM sleep spindle [121], with the extent of neural reactivation proportional to the degree of post-sleep memory retrieval success.
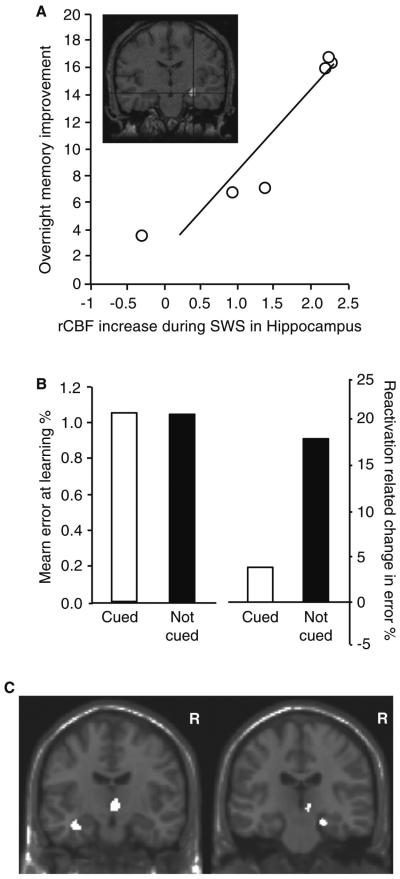
Figure 6. Intrinsic and Extrinsic Reactivation of Hippocampus-Dependent Memory in Humans.
A.Intrinsic reactivation (indexed using Positron Emission Tomography; PET) during Slow Wave Sleep (SWS) following prior spatial navigation learning, with the extent of reactivation positively predicting next-day task improvement (distance to target before sleep minus distance to target after sleep). Adapted from Peigneux, et al., 2004. [120] B. Extrinsic reactivation of memories during NREM sleep using auditory cues previously paired with items during encoding enhances overnight memory retention (white bars), relative to those items that were not reactivated during NREM sleep with their respective auditory cues (black bars). Adapted from Rudoy, et al., 2009. [124] C. The extent of hippocampal activity measured using fMRI during similar auditory cue reactivation during NREM SWS (as in B above) positively correlated the degree of cue-related overnight memory. Effects are significant at p < 0.05, FWE-corrected with Regions of Interest. Adapted from van Dongen, et al., 2012. [125]
Beyond intrinsic reactivation, several studies have extrinsically trigger memory reactivation during sleep in humans, and with it, enhanced post-sleep memory retrieval (for a detailed review, see [122]). An initial study [123] presented individuals with an odor during SWS that was previously presented during learning. Relative to a control condition (where the odor was not presented at night), re-exposure to the odor during SWS results in greater co-occurring hippocampal activation and significantly improved recall the following day. Similar NREM sleep-dependent cued reactivation has been accomplished using auditory rather than olfactory stimuli paired with learning (Figure 6B) [124]. As with odors, the representation of auditory cues aretemporally linked to increased hippocampal activation during sleep, as measured by fMRI (Figure 6C) [125]. Furthermore, post-sleep recollection of previously cued memory items areassociated with increased functional connectivity between the hippocampus and the neocortex — suggesting cued reactivation alters the post-sleep neural representation of consolidated information [125].
In summary, sleep after learning preferentially supports the offline processing and consolidation of episodic declarative memories in humans, the success of which correlates with NREM slow wave and sleep spindle oscillations. Mechanistically, these changes are reflected in coordinated patterns of neural reactivation that may be analogous to network replay observed in rodents. Moreover, the consequence of such processing may result in the neural transformation of episodic memories from an initially hippocampus-dependent state to an increasingly cortical-dependent and hence stabilized state, conferring long-term retention [117].
Discussion
Taken together, there is now clear evidence of, and emerging mechanistic pathways explaining, sleep-dependent memory processing and brain plasticity (summarized in Box 2). Sleep and sleep deprivation bi-directionally impact the molecular signaling pathways that regulate synaptic strength and control plasticity-related gene transcription and protein translation. Moreover, sleep loss impairs the excitability of neurons that is necessary for inducing synaptic potentiation, and leads to a more rapid decay of already established synaptic plasticity. Conversely, both NREM and REM sleep further enhance previously induced synaptic potentiation, although evidence for sleep-related synaptic de-potentiation has also been observed. At a network level, ensembles of hippocampal and cortical cells demonstrate coordinated replay of prior learning-related firing patterns during subsequent sleep, often in unison with specific NREM sleep oscillations. In humans, learning-related hippocampal activity has been shown to similarly re-emerge during subsequent NREM sleep. Furthermore, cortical NREM oscillations that coincide with replay in rodents also facilitate hippocampal memory consolidation in humans, and this consolidation benefit can be experimentally enhanced using exogenous reactivation cues. Finally, specific NREM sleep oscillations before encoding can restore next-day human hippocampal learning, while sleep loss conversely impairs subsequent hippocampal-dependent encoding, converging with molecular and cellular findings in rodents.
In addition to useful commonalities that emerge by uniting this cross-section of findings, so too do knowledge gaps and differences between data sets. These represent fruitful targets for future research programs, and we conclude with a select discussion of several such themes.
Relating sleep to behavior
Sleep and memory are defined perhaps most fundamentally as behaviors. Although neuronal firing properties and regional brain activation during specific sleep stages have been studied extensively, many of these studies, particularly in rodent models, have yet to link this physiology to learning and memory behavior, before and/or after sleep. Without such measures, it is more difficult to interpret changes in hippocampal neural function as expressly “memory” related. One potential novel innovation to bridge cellular and molecular levels with behavior is to use real-time brain activity maps in animals [126]. Beyond the inclusion of behavior, future studies can include more than one learning task, one of which depends on hippocampus function, the other that does not, thereby providing possible insights into interactions or dissociations (in the context of control tasks) in memory processing and underlying neural pathways. Early findings examining differences between hippocampus- and striatum-dependent tasks [127], or between declarative and procedural tasks [3], are already proving insightful.
The role of sleep in remote memories
The majority of sleep-dependent memory consolidation studies focus on the ability to recall “recent” memories that are only a few hours to a few days old. Such work has productively focused on the classical hippocampal-neocortical or “systems-level” model of consolidation, ultimately resulting in remote neocortical storage of memories that remain stable long-term, independent of the hippocampus. Evidence from human functional neuroimaging studies discussed above has described a profile of change in hippocampal activity and connectivity fitting these predictions across a single night of sleep, or even a nap. While some rodent studies have examined similar questions across wakefulness [128,129], congruent studies in rodent models of sleep have been as abundant. However, emerging work using extrinsic cueing shows promise in elucidating the mechanisms of dialogue between hippocampus and neocortex during sleep [82]. Furthermore, the role of sleep in memory processing is unlikely to have finished after a single night. Therefore, moving beyond the examination of sleep across one night and, instead, examining how sleep modulates memory across multiple nights over longer time durations will provide a more ecological understanding of long-term sleep-dependent memory processing. Such experiments will also enable the generation and testing of new models of consolidation that move beyond categorical distinctions between systems consolidation and synaptic consolidation [95]. The fact that changes at the level of the synapse are probably involved in systems consolidation suggests that such distinctions, and hence models, may not be the most accurate mechanistic conception of memory processing. Future theoretical work that aims to set forth a more holistic model of memory processing should provide valuable; one that perhaps unites, rather than segregates, forms of plasticity.
Sleep stages and types of memory
As with the need to examine distinct time courses of memory, understanding differences in the role of particular sleep stages continues to warrant deeper examination. The majority of studies in humans have indicated that NREM sleep is critical for both the consolidation and encoding of non-emotional hippocampus-dependent memory, potentially mediated by its stereotypical slow wave [104] and sleep spindle [88]oscillations. However, when compared to those studies, rodent findings at the cellular and molecular levels of analyses commonly indicate REM sleep as especially critical in modulating the mechanisms underlying memory encoding and consolidation. We currently lack an understanding of this discrepancy. One possibility is that animal memory paradigms involve emotion (punishment or reward) to incentivize each learning trial. In human studies, while subjects often receive monetary compensation for participation, they are not often incentivized on each trial, or with respect to their overall performance. Interestingly, when hippocampus-dependent paradigms in humans have been incentivized or are emotionally valenced, associations with REM sleep have frequently emerged (e.g. [130,131]). Future studies that manipulate demands of each memory task (for example emotional or reward-sensitive components) will clarify the specific roles of sleep stages and associated oscillations with greater homology across species. Furthermore, the field could usefully employ double dissociation experiments that look at two kinds of tasks and two kinds of sleep simultaneously, as has been done in classical studies on subjects with brain lesions [132,133]. Finally, dissociating unique sleep-stage mechanisms using optogenetic approaches in animals holds the promise of precise and selective deprivation of specific stages of sleep, without the need for gross pharmacological or handling manipulations [134]).
Finding common ground
A recurrent theme emerging from the above is that while large bodies of complementary work now emerge from rodents and from humans, across levels of analysis, further homology between studies is needed to directly infer common and causal mechanisms. There are a number of key areas where this homology can be achieved. First is the necessity of developing highly analogous hippocampus-dependent memory tasks in humans and rodents. Only by way of such task similarity can results from different species, and levels of analysis, more easily be compared. An example of such overlap may be achieved by using touch screen tasks that are equally suitable in both animal and human contexts. Second, tasks that are sensitive to common sub-fields of hippocampal function in rodents and humans already exist [113], yet have not been explored in the context of sleep-dependent memory, despite the powerful mechanistic insights they may provide. Third, and beyond behavior, parallel approaches to characterizing the physiology of sleep oscillations across species will strengthen links between separate bodies of knowledge, and potentially resolve inconsistencies in relation to memory processing. For example, standardized definitions of sleep spindles, slow waves, and sharp wave ripples (e.g. frequency ranges, amplitude, temporal dynamics) will improve comparability of datasets both within and between species.
Translational issues and future directions
Across the breadth of clinical diagnostic manuals, a wide range of neurological [135], neurodevelopmental [136]and psychiatric disorders [137]demonstrate comorbid sleep abnormalities - negatively impacting general health and life quality. Importantly, many of these same conditions also display co-occurring impairments in learning, memory and plasticity. Sufficient knowledge now exists to generate empirically informed hypotheses to test the causal contribution of sleep disruption to memory impairment in these conditions. Not only will this help explain mnemonic abnormalities comorbid with sleep disruption, but it will also allow clinicians and clinical researchers a path to develop the next generation of therapeutic approaches to treat memory deficits whose etiology is sleep related. Early work in conditions such as depression [138,139], schizophrenia [140], Parkinson’s disease [141] and cognitive aging [117] (and others) already signal significant interactions. Further, many of the medications used to treat psychiatric and neurological disorders can themselves alter sleep. For example, the frequently used antidepressant and norepinephrine reuptake inhibitor desipramine is a potent inhibitor of REM sleep and modulates sleep-dependent memory [127]. In addition, millions of people each night routinely self-administer prescribed and non-prescribed pharmacological agents in an attempt to initiate and/or maintain sleep. Nevertheless, we know remarkably little about their effects on sleep-dependent memory processes [142].
Finally, although the impact of sleep deprivation has been studied extensively, the time course and severity of the mnemonic impairments that develop with accruing sleep loss, and as important, the time course of recovery following sleep loss, remains poorly characterized. Studies that more formally develop a“dose-response curve” of the impact of sleep deprivationand sleep recovery on memory function are needed for several reasons. First, they will better inform potentially different underlying neural mechanisms associated with short-, medium- and long-term sleep loss, and the capacity of the biological system to recover from each. Second, they will more clearly elucidate what type (or types) of sleep physiology restore memory functions following these respective deprivation periods. Third, they will be of significant ecological validity: in industrialized nations, chronic sleep restriction is more common than acute sleep deprivation, yet we know far less about the former, and recovery from it, at the level of memory and brain plasticity. Finally, the vast array of clinical disorders that express comorbid sleep disruption and memory impairment commonly present with a history of long-term sleep loss. What the memory impact of such temporally sustained sleep deprivation is, what (if any) compensation mechanisms evolve, and the degree to which the neural systems of memory can and will recover when sleep is treated, all remain unanswered questions in the context of therapeutic expectations and interventions.
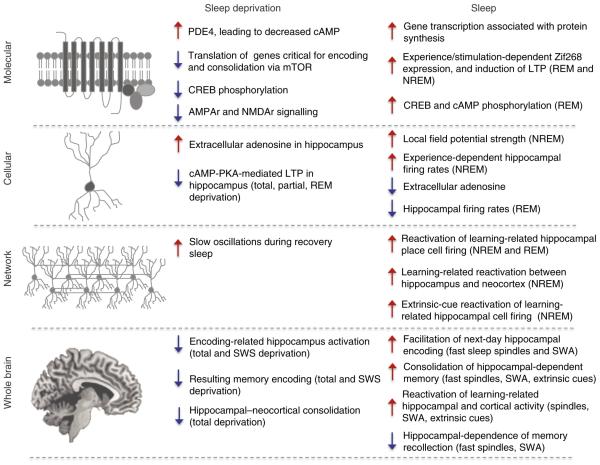
Figure 7. Impact of sleep and sleep deprivation on memory and brain plasticity across descriptive levels of analysis.
Summary of the impact of sleep deprivation (left column) and sleep (right column) on hippocampus-dependent learning and memory processes across descriptive levels. At each level of analysis (Rows [top-bottom]: Molecular, Cellular, Network, Whole Brain) effects that decrease functional outcomes are depicted with blue downward arrows, while effects that increase functional outcomes are depicted with red upward arrows.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nat. Neurosci. 2013;16:139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCoy JG, Strecker RE. The cognitive cost of sleep lost. Neurobiol. Learn. Mem. 2011;96:564–582. doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson EM. From creation to consolidation: a novel framework for memory processing. PLoS biology. 2009;7:e19. doi: 10.1371/journal.pbio.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giuditta A, Rutigliano B, Vitale-Neugebauer A. Influence of synchronized sleep on the biosynthesis of RNA in neuronal and mixed fractions isolated from rabbit cerebral cortex. J. Neurochem. 1980;35:1267–1272. doi: 10.1111/j.1471-4159.1980.tb08997.x. [DOI] [PubMed] [Google Scholar]
- 5.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 6.Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI. Macromolecule biosynthesis: a key function of sleep. Physiological genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 7.Vecsey CG, Peixoto L, Choi JH, Wimmer M, Jaganath D, Hernandez PJ, Blackwell J, Meda K, Park AJ, Hannenhalli S, et al. Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiological genomics. 2012;44:981–991. doi: 10.1152/physiolgenomics.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J. Neurochem. 2005;92:1150–1157. doi: 10.1111/j.1471-4159.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi H, Sun Y, Nakamura RK, Mori K, Ito M, Suda S, Namba H, Storch FI, Dang TP, Mendelson W, et al. Positive correlations between cerebral protein synthesis rates and deep sleep in Macaca mulatta. Eur. J. Neurosci. 1997;9:271–279. doi: 10.1111/j.1460-9568.1997.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 10.Ramm P, Smith CT. Rates of cerebral protein synthesis are linked to slow wave sleep in the rat. Physiol. Behav. 1990;48:749–753. doi: 10.1016/0031-9384(90)90220-x. [DOI] [PubMed] [Google Scholar]
- 11.Seibt J, Dumoulin MC, Aton SJ, Coleman T, Watson A, Naidoo N, Frank MG. Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr. Biol. 2012;22:676–682. doi: 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havekes R, Vecsey CG, Abel T. The impact of sleep deprivation on neuronal and glial signaling pathways important for memory and synaptic plasticity. Cell. Signal. 2012;24:1251–1260. doi: 10.1016/j.cellsig.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res. Brain Res. Rev. 1998;26:360–378. doi: 10.1016/s0165-0173(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 14.Xia Z, Storm DR. Role of signal transduction crosstalk between adenylyl cyclase and MAP kinase in hippocampus-dependent memory. Learn. Mem. 2012;19:369–374. doi: 10.1101/lm.027128.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Liu Y, Briesemann M, Yan J. Computational analysis of gene regulation in animal sleep deprivation. Physiological genomics. 2010;42:427–436. doi: 10.1152/physiolgenomics.00205.2009. [DOI] [PubMed] [Google Scholar]
- 16.Luo J, Phan TX, Yang Y, Garelick MG, Storm DR. Increases in cAMP, MAPK Activity, and CREB Phosphorylation during REM Sleep: Implications for REM Sleep and Memory Consolidation. J. Neurosci. 2013;33:6460–6468. doi: 10.1523/JNEUROSCI.5018-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li X-Y, Descalzi G, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagewoud R, Bultsma LJ, Barf RP, Koolhaas JM, Meerlo P. Sleep deprivation impairs contextual fear conditioning and attenuates subsequent behavioural, endocrine and neuronal responses. J. Sleep Res. 2010 doi: 10.1111/j.1365-2869.2010.00895.x. [DOI] [PubMed] [Google Scholar]
- 19.Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA. Sleep deprivation prevents stimulation-induced increases of levels of P-CREB and BDNF: protection by caffeine. Mol. Cell. Neurosci. 2011;46:742–751. doi: 10.1016/j.mcn.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z, Huang L, Wu H, Li Y, Zhang L, Yin Y, Xiang Z. Neuropeptide S mitigates spatial memory impairment induced by rapid eye movement sleep deprivation in rats. Neuroreport. 2010;21:623–628. doi: 10.1097/WNR.0b013e328339b5f9. [DOI] [PubMed] [Google Scholar]
- 21.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat. Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 22.Havekes R, Abel T. Genetic dissection of neural circuits and behavior in Mus musculus. Adv. Genet. 2009;65:1–38. doi: 10.1016/S0065-2660(09)65001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 24.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 25.Tiba PA, Oliveira MG, Rossi VC, Tufik S, Suchecki D. Glucocorticoids are not responsible for paradoxical sleep deprivation-induced memory impairments. Sleep. 2008;31:505–515. doi: 10.1093/sleep/31.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro S, Goyal V, Mello CV, Pavlides C. Brain gene expression during REM sleep depends on prior waking experience. Learn. Mem. 1999;6:500–508. doi: 10.1101/lm.6.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J. Neurosci. 2002;22:10914–10923. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauchs G, Desgranges B, Foret J, Eustache F. The relationships between memory systems and sleep stages. J. Sleep Res. 2005;14:123–140. doi: 10.1111/j.1365-2869.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 29.Chauvette S, Seigneur J, Timofeev I. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75:1105–1113. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30:1631–1642. doi: 10.1093/sleep/30.12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M, Tononi G. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30:1643–1657. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J. Neurosci. 2010;30:8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30:129–139. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- 37.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank MG. Why I am not shy: a reply to Tononi and Cirelli. Neural Plas. 2013;2013:394946. doi: 10.1155/2013/394946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tononi G, Cirelli C. Time to be SHY? Some comments on sleep and synaptic homeostasis. Neural Plas. 2012;2012:415250. doi: 10.1155/2012/415250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank MG. Erasing synapses in sleep: is it time to be SHY? Neural Plas. 2012;2012:264378. doi: 10.1155/2012/264378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buzsaki G, Csicsvari J, Dragoi G, Harris K, Henze D, Hirase H. Homeostatic maintenance of neuronal excitability by burst discharges in vivo. Cereb. Cortex. 2002;12:893–899. doi: 10.1093/cercor/12.9.893. [DOI] [PubMed] [Google Scholar]
- 42.Louie K, Wilson M. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 43.Montgomery S, Sirota A, Buzsaki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J. Neurosci. 2008;28:6731–6741. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 45.Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 46.Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsaki G. REM sleep reorganizes hippocampal excitability. Neuron. 2012;75:1001–1007. doi: 10.1016/j.neuron.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winson J, Abzug C. Neuronal transmission through hippocampal pathways dependent on behavior. J. Neurophysiol. 1978;41:716–732. doi: 10.1152/jn.1978.41.3.716. [DOI] [PubMed] [Google Scholar]
- 48.Winson J, Abzug C. Gating of neuronal transmission in the hippocampus: efficacy of transmission varies with behavioral state. Science. 1977;196:1223–1225. doi: 10.1126/science.193192. [DOI] [PubMed] [Google Scholar]
- 49.Bramham CR, Srebro B. Synaptic plasticity in the hippocampus is modulated by behavioral state. Brain Res. 1989;493:74–86. doi: 10.1016/0006-8993(89)91001-9. [DOI] [PubMed] [Google Scholar]
- 50.Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J. Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- 51.Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J. Neurosci. 2011;31:6956–6962. doi: 10.1523/JNEUROSCI.5761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kopp C, Longordo F, Nicholson JR, Lüthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J. Neurosci. 2006;26:12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tartar J, Ward C, McKenna J, Thakkar M, Arrigoni E, McCarley R, Brown R, Strecker R. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur. J. Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J. Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ravassard P, Pachoud B, Comte J-C, Mejia-Perez C, Scoté-Blachon C, Gay N, Claustrat B, Touret M, Luppi P-H, Salin PA. Paradoxical (REM) Sleep Deprivation Causes a Large and Rapidly Reversible Decrease in Long-Term Potentiation, Synaptic Transmission, Glutamate Receptor Protein Levels, and ERK/MAPK Activation in the Dorsal Hippocampus. Sleep. 2009;32:227–240. doi: 10.1093/sleep/32.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDermott C, Hardy M, Bazan N, Magee J. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- 58.Marks CA, Wayner MJ. Effects of sleep disruption on rat dentate granule cell LTP in vivo. Brain Res. Bull. 2005;66:114–119. doi: 10.1016/j.brainresbull.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 59.Ishikawa A, Kanayama Y, Matsumura H, Tsuchimochi H, Ishida Y, Nakamura S. Selective rapid eye movement sleep deprivation impairs the maintenance of long-term potentiation in the rat hippocampus. Eur. J. Neurosci. 2006;24:243–248. doi: 10.1111/j.1460-9568.2006.04874.x. [DOI] [PubMed] [Google Scholar]
- 60.Peng S, Zhang Y, Zhang J, Wang H, Ren B. Glutamate receptors and signal transduction in learning and memory. Mol. Biol. Rep. 2011;38:453–460. doi: 10.1007/s11033-010-0128-9. [DOI] [PubMed] [Google Scholar]
- 61.Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J. Neurosci. 2009;29:620–629. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vecsey CG, Wimmer ME, Havekes R, Park AJ, Perron IJ, Meerlo P, Abel T. Daily acclimation handling does not affect hippocampal long-term potentiation or cause chronic sleep deprivation in mice. Sleep. 2013;36:601–607. doi: 10.5665/sleep.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park HJ, Kang WS, Paik JW, Kim JW. Effect of valproic acid through regulation of NMDA receptor-ERK signaling in sleep deprivation rats. J. Mol. Neuro. 2012;47:554–558. doi: 10.1007/s12031-011-9673-5. [DOI] [PubMed] [Google Scholar]
- 64.Hagewoud R, Havekes R, Novati A, Keijser JN, Van der Zee EA, Meerlo P. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J. Sleep Res. 2010;19:280–288. doi: 10.1111/j.1365-2869.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 65.Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 66.Huston JP, Haas HL, Boix F, Pfister M, Decking U, Schrader J, Schwarting RK. Extracellular adenosine levels in neostriatum and hippocampus during rest and activity periods of rats. Neuroscience. 1996;73:99–107. doi: 10.1016/0306-4522(96)00021-8. [DOI] [PubMed] [Google Scholar]
- 67.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmitt LI, Sims RE, Dale N, Haydon PG. Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. J. Neurosci. 2012;32:4417–4425. doi: 10.1523/JNEUROSCI.5689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57:343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 71.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J. Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 74.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 75.Sirota A, Csicsvari J, Buhl D, Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eschenko O, Sara S. Learning-Dependent, Transient Increase of Activity in Noradrenergic Neurons of Locus Coeruleus during Slow Wave Sleep in the Rat: Brain Stem-Cortex Interplay for Memory Consolidation? Cereb. Cortex. 2008 doi: 10.1093/cercor/bhn020. [DOI] [PubMed] [Google Scholar]
- 77.Eschenko O, Ramadan W, Molle M, Born J, Sara S. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn. Mem. 2008;15:222–228. doi: 10.1101/lm.726008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mölle M, Eschenko O, Gais S, Sara SJ, Born J. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur. J. Neurosci. 2009;29:1071–1081. doi: 10.1111/j.1460-9568.2009.06654.x. [DOI] [PubMed] [Google Scholar]
- 79.Sara SJ. Reactivation, retrieval, replay and reconsolidation in and out of sleep: connecting the dots. Frontiers in behavioral neuroscience. 2010;4:185. doi: 10.3389/fnbeh.2010.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 81.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 82.Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat. Neurosci. 2012;15:1439–1444. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morris GP, Williams HL, Lubin A. Misperception and disorientation during sleep deprivation. Arch. Gen. Psychiatry. 1960;2:247–254. [Google Scholar]
- 84.Harrison Y, Horne JA. Sleep loss and temporal memory. Q. J. Exp. Psychol. A. 2000;53:271–279. doi: 10.1080/713755870. [DOI] [PubMed] [Google Scholar]
- 85.Drummond SP, Gillin JC, Brown GG. Increased cerebral response during a divided attention task following sleep deprivation. J. Sleep Res. 2001;10:85–92. doi: 10.1046/j.1365-2869.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 86.Yoo S, Hu P, Gujar N, Jolesz F, Walker M. A deficit in the ability to form new human memories without sleep. Nat. Neurosci. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 87.Van Der Werf YD, Altena E, Schoonheim MM, Sanz-Arigita EJ, Vis JC, De Rijke W, Van Someren EJW. Sleep benefits subsequent hippocampal functioning. Nat. Neurosci. 2009;12:122–123. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- 88.Mander BA, Santhanam S, Saletin JM, Walker MP. Wake deterioration and sleep restoration of human learning. Curr. Biol. 2011;21:R183–R184. doi: 10.1016/j.cub.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lustenberger C, Maric A, Durr R, Achermann P, Huber R. Triangular relationship between sleep spindle activity, general cognitive ability and the efficiency of declarative learning. PloS one. 2012;7:e49561. doi: 10.1371/journal.pone.0049561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Antonenko D, Diekelmann S, Olsen C, Born J, Molle M. Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur. J. Neurosci. 2013;37:1142–1151. doi: 10.1111/ejn.12118. [DOI] [PubMed] [Google Scholar]
- 91.Walker MP. The role of sleep in cognition and emotion. Ann. N. Y. Acad. Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 92.Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007;130:2868–2878. doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- 93.Siapas A, Wilson M. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 94.Clemens Z, Mölle M, Erőss L, Jakus R, Rásonyi G, Halász P, Born J. Fine-tuned coupling between human parahippocampal ripples and sleep spindles. Eur. J. Neurosci. 2010 doi: 10.1111/j.1460-9568.2010.07505.x. [DOI] [PubMed] [Google Scholar]
- 95.Diekelmann S, Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 96.Saletin JM, Walker MP. Nocturnal mnemonics: sleep and hippocampal memory processing. Frontiers in neurology. 2012;3:59. doi: 10.3389/fneur.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr. Biol. 2006;16:1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 98.Fischer S, Diekelmann S, Born J. Sleep's role in the processing of unwanted memories. J. Sleep Res. 2010 doi: 10.1111/j.1365-2869.2010.00881.x. [DOI] [PubMed] [Google Scholar]
- 99.Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nat. Neurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- 100.Wagner U, Fischer S, Born J. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosom. Med. 2002;64:627–634. doi: 10.1097/01.psy.0000021940.35402.51. [DOI] [PubMed] [Google Scholar]
- 101.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn. Mem. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gais S, Born J. Declarative memory consolidation: mechanisms acting during human sleep. Learn. Mem. 2004;11:679–685. doi: 10.1101/lm.80504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Synaptic influence of hippocampus on pyramidal cells of the rat prefrontal cortex: an in vivo intracellular recording study. Cereb. Cortex. 2003;13:782–792. doi: 10.1093/cercor/13.7.782. [DOI] [PubMed] [Google Scholar]
- 104.Marshall L, Mölle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J. Neurosci. 2004;24:9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 106.Ngo HV, Martinetz T, Born J, Molle M. Auditory Closed-Loop Stimulation of the Sleep Slow Oscillation Enhances Memory. Neuron. 2013;78:545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 107.Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eschenko O, Molle M, Born J, Sara S. Elevated sleep spindle density after learning or after retrieval in rats. J. Neurosci. 2006;26:12914–12920. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meier-Koll A, Bussmann B, Schmidt C, Neuschwander D. Walking through a maze alters the architecture of sleep. Percept Mot Skills. 1999;88:1141–1159. doi: 10.2466/pms.1999.88.3c.1141. [DOI] [PubMed] [Google Scholar]
- 110.Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 111.Andrade KC, Spoormaker VI, Dresler M, Wehrle R, Holsboer F, Samann PG, Czisch M. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J. Neurosci. 2011;31:10331–10339. doi: 10.1523/JNEUROSCI.5660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van der Helm E, Gujar N, Nishida M, Walker MP. Sleep-dependent facilitation of episodic memory details. PloS one. 2011;6:e27421. doi: 10.1371/journal.pone.0027421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14:336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam MM, Zee PC, Paller KA. Concurrent Impairments in Sleep and Memory in Amnestic Mild Cognitive Impairment. J Int Neuropsychol Soc. 2012:1–11. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murphy M, Riedner B, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernández G. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc. Natl. Acad. Sci. U. S. A. 2006;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gais S, Albouy G, Boly M, Dang-Vu T, Darsaud A, Desseilles M, Rauchs G, Schabus M, Sterpenich V, Vandewalle G, et al. Sleep transforms the cerebral trace of declarative memories. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18778–18783. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 121.Bergmann TO, Molle M, Diedrichs J, Born J, Siebner HR. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage. 2012;59:2733–2742. doi: 10.1016/j.neuroimage.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 122.Oudiette D, Paller KA. Upgrading the sleeping brain with targeted memory reactivation. Trends in cognitive sciences. 2013;17:142–149. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 123.Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 124.Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009;326:1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Dongen EV, Takashima A, Barth M, Zapp J, Schad LR, Paller KA, Fernandez G. Memory stabilization with targeted reactivation during human slow-wave sleep. Proc. Natl. Acad. Sci. U. S. A. 2012;109:10575–10580. doi: 10.1073/pnas.1201072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mukamel EA, Nimmerjahn A, Schnitzer MJ. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron. 2009;63:747–760. doi: 10.1016/j.neuron.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Watts A, Gritton HJ, Sweigart J, Poe GR. Antidepressant suppression of non-REM sleep spindles and REM sleep impairs hippocampus-dependent learning while augmenting striatum-dependent learning. J. Neurosci. 2012;32:13411–13420. doi: 10.1523/JNEUROSCI.0170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Frankland PW, Bontempi B. The organization of recent and remote memories. Nature reviews. Neuroscience. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 129.Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 130.Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb. Cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oudiette D, Antony JW, Creery JD, Paller KA. The Role of Memory Reactivation during Wakefulness and Sleep in Determining Which Memories Endure. J. Neurosci. 2013;33:6672–6678. doi: 10.1523/JNEUROSCI.5497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav. Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- 133.McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav. Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- 134.Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, de Lecea L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13305–13310. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gasanov RL, Gitlevich TR, Lesnyak VN, YaI L. Structure of nocturnal sleep in patients with cerebral insult. Neurosci. Behav. Physiol. 1998;28:325–329. doi: 10.1007/BF02462964. [DOI] [PubMed] [Google Scholar]
- 136.Reynolds AM, Malow BA. Sleep and autism spectrum disorders. Pediatr. Clin. North Am. 2011;58:685–698. doi: 10.1016/j.pcl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 137.Peterson MJ, Benca RM. Sleep in mood disorders. The Psychiatric clinics of North America. 2006;29:1009–1032. doi: 10.1016/j.psc.2006.09.003. abstract ix. [DOI] [PubMed] [Google Scholar]
- 138.Dresler M, Kluge M, Pawlowski M, Schussler P, Steiger A, Genzel L. A double dissociation of memory impairments in major depression. J. Psychiatr. Res. 2011;45:1593–1599. doi: 10.1016/j.jpsychires.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 139.Dresler M, Kluge M, Genzel L, Schussler P, Steiger A. Impaired off-line memory consolidation in depression. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2010;20:553–561. doi: 10.1016/j.euroneuro.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 140.Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol. Psychiatry. 2012;71:154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Terpening Z, Naismith S, Melehan K, Gittins C, Bolitho S, Lewis SJ. The contribution of nocturnal sleep to the consolidation of motor skill learning in healthy ageing and Parkinson's disease. J. Sleep Res. 2013 doi: 10.1111/jsr.12028. doi: 10.1111/jsr.12028. [DOI] [PubMed] [Google Scholar]
- 142.Rasch B, Pommer J, Diekelmann S, Born J. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat. Neurosci. 2009;12:396–397. doi: 10.1038/nn.2206. [DOI] [PubMed] [Google Scholar]
- 143.Battaglia FP, Benchenane K, Sirota A, Pennartz CM, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends in cognitive sciences. 2011;15:310–318. doi: 10.1016/j.tics.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 144.Hellman K, Hernandez P, Park A, Abel T. Genetic evidence for a role for protein kinase A in the maintenance of sleep and thalamocortical oscillations. Sleep. 2010;33:19–28. doi: 10.1093/sleep/33.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tsanov M, Manahan-Vaughan D. Visual cortex plasticity evokes excitatory alterations in the hippocampus. Frontiers in integrative neuroscience. 2009;3:32. doi: 10.3389/neuro.07.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ribeiro S, Goyal V, Mello C, Pavlides C. Brain gene expression during REM sleep depends on prior waking experience. Learn. Mem. 1999;6:500–508. doi: 10.1101/lm.6.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ji D, Wilson M. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]